Abstract
In this research, we examined the production of hyaluronic acid (HA) by Streptococcus zooepidemicus strain MW26985 using different substrates and potato peel waste (PPW) as an affordable substrate. First, culture medium components, including carbon and nitrogen sources, were optimized for bacterial HA production. Five different carbon sources (glucose, sucrose, lactose, sago starch, and potato starch, at a concentration of 30 g/L) and three distinct nitrogen sources (peptone, yeast extract, and ammonium sulfate, at a concentration of 10 g/L) were investigated. Glucose, among the carbon sources, and yeast extract, among nitrogen sources, produced the most HA which was determined as 1.41 g/L. Afterward, potato peel sugars were extracted by dilute acid and enzymatic hydrolysis and then employed as a cost-effective carbon source for the growth of S. zooepidemicus. Based on the results, the fermentation process yielded 0.59 g/L HA from potato peel sugars through acid hydrolysis and 0.92 g/L HA from those released by enzymatic hydrolysis. The supplementation of both hydrolyzates with glucose as an additional carbon source enhanced HA production to 0.95 g/L and 1.18 g/L using acidic and enzymatic hydrolyzates, respectively. The cetyltrimethylammonium bromide (CTAB) turbidimetric method was used to evaluate the concentration of HA in the fermentation broth using the colorimetric method. Also, the peaks observed by Fourier transform infrared (FTIR) spectroscopy confirmed that the exopolysaccharide (EPS) was composed of HA. These observations demonstrate that potato peel residues can be a novel alternative as a carbon source for the economical production of HA by S. zooepidemicus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In past decades, numerous procedures have been developed to produce many valuable products through fermentation as a result of advancements in the field of biotechnology [1,2,3]. Biopolymers, antibiotics, organic acids, and biofuels are some of these products. Many of these substances are typically produced through chemical processes that are undesirable and rely on the use of non-renewable resources. Biopolymers known as an important family of biochemicals can act similarly to conventional polymers in a wide scope of applications. However, the production costs of many biopolymers are significantly higher than those of their chemical counterparts. Therefore, recent studies have concentrated on expanding the scale and efficacy of these processes [4, 5]. Hyaluronic acid (HA) is a vital mucopolysaccharide with widespread functions that is frequently used in medical and pharmaceutical applications [6]. Generally, HA biopolymer can be commercially produced on a large scale through two main methods: isolation from vertebrate animal tissue, commonly from rooster combs, or microbial fermentation [7, 8]. HA was traditionally extracted from animal tissues, primarily rooster combs. However, several challenges associated with HA extraction from animal sources, such as ethical concerns, the inconsistency of animal tissue quality, and high costs, resulted in the expansion of microbial production processes [8]. Owing to considerable advancements in biotechnology, it is now possible to produce HA through bacterial fermentation, particularly by employing S. zooepidemicus. Streptococcus species Group C, which secretes HA as a protective capsule to elude the immunological reactions of the host, is currently the most widely used microorganism for producing HA [6, 9]. Due to the pathogenic nature of Streptococci, there are concerns regarding using the bacteria to produce HA. Although this microorganism can cause infections in animals, and the strains applied for HA production are typically non-pathogenic for humans; nevertheless, it can cause a severe co-infection where it infects people through contact with horses [10]. This has prompted continuous efforts to find safer alternatives through genetic engineering. Lactobacillus lactis is a bacterial species frequently used to produce HA. However, it has been noted that the synthesis of lactic acid lowers the molecular weight and HA output. The molecular weight of HA produced by these modified strains is significantly lower. This observation also applies to strains of E. Coli that have S. pyogenes genes involved in the synthesis of HA [8, 11]. Engineering techniques, like gene editing, can have off-target effects, modifying unintended regions of the genome and potentially introducing harmful mutations. Ethical concerns arise with genetically modified organisms, particularly in terms of potential environmental impact or unintended consequences on human health in specific applications [12].
Furthermore, economic obstacles remain to replacing animal-derived hyaluronic acid with microbial production methods. S. zooepidemicus has high nutritional requirements for producing HA on large scales. In particular, the sugars and proteins needed in the growth media account for over 80% of the total production costs. Decreasing the nutritional expenses associated with sugars and proteins will be key for microbial HA production to become an economically feasible alternative to animal-based extraction on a commercial scale [13]. To address these challenges, scientists have been exploring more sustainable and cost-effective approaches, such as utilizing agro-industrial derivatives like fishing by-products [14], carob pods [15], molasses and corn steep liquor [16], marine by-products [13], soya-based peptone [17], cashew apple juice [9], and agricultural resource derivatives [18], as substrates for microbial fermentation. In this regard, Amado et al. [16] conducted an experiment where they developed a low-cost process fermentation medium for HA production, corn steep liquor (CSL) as a nitrogen source instead of tryptone. In this medium, the HA production reached 3.48 g/L, which was comparable to the production of 3.60 g/L in the control medium. In another study by Arslan and Aydogan [19], the capacity of molasses and sheep wool peptone (SWP) as sources of carbon and nitrogen was evaluated, respectively, for HA production through microbial fermentation. They found that culture media supplemented with SWP yielded higher HA levels (3.54 g/L) compared to media including tryptone peptone (2.58 g/L) and protease peptone (2.47 g/L). Ghodke et al. [20] investigated the potential of sugar and soya peptone of palmyra palm (PJ) as alternative media components for HA production by Streptococcus zooepidemicus. Fermentations using PJ-based media yielded higher HA (0.41 g/L) and specific growth rates (0.54 h−1) compared to media with pure sucrose. After optimizing the initial PJ at 30 g/L, S. zooepidemicus produced 1.22 g/L HA. Pires et al. [9] evaluated cashew apple juice as an alternative substrate in the media for HA fermentation. Using cashew apple juice, S. zooepidemicus produced 0.89 g/L of HA.
At present, the concept of ‘circular economy’ has garnered significant attention. This approach is about converting ecological waste from one industry into a valuable source of raw materials for other industries [21]. Over the years, owing to a swift surge in population, urbanization, and economic progress, copious quantities of kitchen waste (KW) are generated daily by households, restaurants, and hotels. Agricultural waste and KW are not only rich in vital substances, especially carbohydrates, lipids, and proteins, but also include abundant bioactive compounds. As a result, these residues can be used as substrates for microbial production [22]. Potato peel waste (PPW) is an affordable and numerous by-product rich in starch, non-starch polysaccharides, lignin, polyphenols, proteins, and small quantities of lipids. Due to this diverse biochemical composition, PPW serves as an economical and useful raw material for extracting high-value products as well as for fermentation processes [23]. Arapoglou et al. [24] produced ethanol using leftover potato peel. In this study, a variety of collected potato peels were hydrolyzed with various acids and enzymes, and Saccharomyces cerevisiae var. bayanus was used for the fermentation to assess its capacity for fermentation and ability to produce ethanol. The three enzymes combined for enzymatic hydrolysis release 18.5 g/L of reducing sugars, and fermentation results in 7.6 g/L of ethanol. Abdelraof et al. [25] conducted extensive research on the eco-friendly conversion of PPW into bacterial cellulose (BC). The first study used Gluconacetobacter xylinus to produce BC from PPW. The result of this study showed that PPW hydrolyzate using nitric acid can be considered a worthwhile choice for the production of BC in an environmental friendly method.
As mentioned above, HA is one of the most significant microbial EPS and plays an extremely important role in the culinary, pharmaceutical, medical, and tissue engineering industries. The major problem in producing this biopolymer is the high cost of the final product. Therefore, finding an inexpensive and economically efficient substrate is vital for solving this challenge. In this regard, the present study’s ultimate aim was to investigate the feasibility of the production of HA from PPW as a cheap source of carbon for S. zooepidemicus. To improve the effectiveness of the fermentation process, some factors, including the type of carbon and nitrogen sources, were first evaluated. Then the fermentation medium containing potato peel sugars released by acid and enzymatic hydrolysis was applied as an alternative nutrient source to produce microbial HA.
Materials and methods
Microorganism and inoculum preparation
In this study, Streptococcus zooepidemicus MW269858 was used. The strain was isolated from horse mucus at the Faculty of Veterinary Medicine of the University of Tehran (Tehran, Iran) and was registered with GenBank code MW269858 at the National Center for Biotechnology Information (NCBI). It was grown on brain heart infusion (BHI) agar plate (Ibresco, Iran, containing (g/L): brain extract 12.5, heart extract 5, peptone 10, dextrose 2, sodium chloride 5, di-sodium phosphate 2.5, and agar 15) at 37 ℃ for 24 h. To prepare the inoculum, a loopful of cells taken from the BHI agar plate was transferred into 100 mL of BHI broth. To achieve optimal growth conditions, the pH of the medium was adjusted to 7.4 and then autoclaved at 121 °C for 15 min. It was then placed at 37 ℃ in an orbital shaking incubator set at 180 rpm for 24 h. The strain was stored for long-term storage at − 20 °C in BHI broth with 20% glycerol.
Preparation of potato peel waste
Potato peel was collected as kitchen waste from households by hand peeling using a manual peeler. Sand and dirt are typically found in potato peels. Therefore, it was first cleaned with tap water to remove impurities. The remaining potato material was dried in an oven at 60 ℃ for 48 h. The dried waste was ground into powder by a home grinder (AGB130W-APPEX) and then screened through a fine sieve (60 mesh) to obtain a homogeneous powder. It was then stored in a dark cool place until further use. Some of its basic characteristics, including moisture, protein, carbohydrate, and ash contents, were assessed. The ash content was measured by incinerating dried samples in a furnace at a high temperature of 550 ℃ for 3 h (NABERTHERM Germany Controller B170). The carbohydrates in the sample were analyzed using the phenol–sulfuric acid method described by Dubois et al. [26]. To estimate protein content, the Kjeldahl approach was used, which requires multiplying residual nitrogen (N) by 6.25 [27].
Optimization of HA production in synthetic medium
In this study, simple carbon sources (glucose, sucrose, and lactose) and two complex carbohydrates (potato starch and sago starch) were used at 30 g/L to investigate the effect of diverse carbon sources on the production of HA by S. zooepidemicus. The fermentation medium was prepared by dissolving 1.5 g sugar, 0.5 g yeast extract, 0.1 g potassium dihydrogen phosphate, 0.15 g sodium dihydrogen phosphate, and 25 mg magnesium sulfate heptahydrate in 50 mL of distilled water. In each trial, only the carbon source was changed and the remaining elements and their values were regarded as constant. Every experiment was run batch wise in 250-mL Erlenmeyer flasks with a 50 mL working volume. Based on the studies that applied S. zooepidemicus to produce HA, the temperature and pH values in the present study were set at 37 ℃ and 7, respectively, as the optimum values [28, 29]. After adjusting the initial pH to 7, cultures were autoclaved at 121 °C for 20 min. The sugar source was autoclaved distinctly and then added to the remaining culture media before inoculation to avoid the Maillard reaction. The activated suspension (10% v/v) was aseptically inoculated into each fermentation medium after sterilization and cooling of the culture media. Finally, the flasks were incubated at 37 °C and 180 rpm in a shaker incubator, and the pH levels, HA production, and cell growth were monitored at 24 h intervals for 96 h. Each experiment was performed three times, and the average of the results is shown. Following the same procedure used for carbon sources, different nitrogen sources were evaluated. Different nitrogen sources were considered, including ammonium sulfate, peptone, and yeast extract at 10 g/L, and glucose as a carbon source at 30 g/L. The other compounds required for culturing were similar to those described above. After sterilizing and cooling to room temperature, about 10% v/v of seed broth was added to each fermentation medium under sterile conditions. All batch experiments were performed in 250-mL conical flasks, utilizing a working volume of 50 mL.
Acidic and enzymatic hydrolysis of potato peel
Due to the insufficient amount of fermentable sugars in potato peels, direct fermentation is not practical. Therefore, a hydrolysis step is mandatory to enhance the sugar content and enable efficient fermentation, which releases fermentable sugars and makes them available to microorganisms [24, 30]. In the present study, acidic and enzymatic treatments were investigated to improve the use of PPW as feedstock for HA production. Acid hydrolysis of PPW was performed using hydrochloric acid, similar to the process explained by Arapoglou et al. [24]. Briefly, 5 g of potato peel powder was added to a 250-mL Erlenmeyer flask containing 4.1 mL of 0.5 M HCl and then topped up to a total volume of 100 mL with distilled water. The mixture was autoclaved at 121 °C for 15 min, then cooled to room temperature. Acid hydrolysis during sterilization converted the carbohydrates in potato peel into fermentable sugars. The mixture was adjusted to the desired pH value of 7 for S. zooepidemicus using 1 M NaOH. The 3,5-dinitrosalicylic acid (DNS) assay was used to estimate the reduced sugar content [31].
Enzymatic hydrolysis of PPW was conducted in three main steps: gelatinization, liquefaction, and saccharification. In the first step, gelatinization was applied as an alkali pretreatment to disrupt proteins (which act as a barrier to the digestion of starch) and increase the availability of starch content of PPW for subsequent enzymatic action [32]. For this purpose, 5 g of powdered potato peel was mixed with 45 mL of 0.1 M NaOH solution in an Erlenmeyer flask by stirring on a hot plate magnetic stirrer at 60 ℃ for 1 h. After the intended time, 55 mL of potassium hydrogen phthalate buffer (0.1 M) was added to the flask. After measuring the pH, the mixture was heated to 105 ℃, after which the gelatinization process was initiated. The slurry was cooled to 95 ℃, and 0.076 g of heat-stable α-amylase was added. The flask was kept at 95 ℃ for 2 h to complete the liquefaction stage. The pH of the mixture was corrected to 4.5 with phosphoric acid before the saccharification process. The third step was initiated by the addition of 0.048 g of amyloglucosidase enzyme to the liquefied starch. The reaction was conducted for 96 h at 60 ℃ and 120 rpm. To determine the quantity of reducing sugars released throughout the enzymatic hydrolysis of PPW, samples were taken at specific intervals. Also, it should be mentioned that heat-stable α-amylase and amyloglucosidase enzymes were supplied by Serva, Germany. The activities of these enzymes were 30 and 120 units/mg (U/mg), respectively.
Hyaluronan fermentation of PPW hydrolyzates
Hydrolyzates resulting from both acid and enzymatic hydrolysis were investigated as cost-effective culture media for HA production. Fermentation was performed in 250-mL Erlenmeyer flasks with a 50 mL working volume. To enhance the nutritional content of PPW hydrolyzates, 10 g/L of yeast extract and 0.5 g/L of magnesium sulfate heptahydrate were added. After adjusting the culture medium to a pH of 7.0, it was autoclaved at 121 ℃ for 20 min. Once cooled, it was inoculated with 5 mL of a pure culture of S. zooepidemicus, followed by incubation at 37 ℃ and 180 rpm for 96 h. Samples were collected at 12 h intervals for measurement of HA content, cell growth, sugar consumption, and medium pH. The fermentation conditions were explored by removing the carbon source, replacing the hydrolyzates of PPW, and evaluating HA production. Furthermore, the amount of HA produced was assessed as an auxiliary source of glucose for the hydrolyzates of PPW, for adjusting the total sugar content to 30 g/L.
Analytical procedures
The fermentation broth was analyzed using several methods. Reducing sugar levels were determined by the dinitrosalicylic acid (DNS) assay [31] by comparison to a glucose standard curve. Optical density at 600 nm (OD600) was measured by a spectrophotometer that monitored cellular biomass. A cetyltrimethylammonium bromide (CTAB) turbidimetric method estimated hyaluronic acid concentration based on color developed by the reaction between HA and CTAB, which was quantified spectrophotometrically at 580 nm. Cell-free supernatants were prepared by centrifugation and mixed with ethanol to precipitate HA. The precipitate was redissolved, reacted with CTAB reagent, and absorbance was measured against a standard curve to determine HA [33, 34]. In addition, microbial growth was assessed by cell dry weight (CDW) measurements. Biomass weights were obtained by centrifuging culture samples, washing, and drying the cell pellets. An OD600 versus biomass concentration calibration curve enabled the conversion of optical density to cell mass. HA was also precipitated from supernatants using ethanol, recovered by centrifugation, and dried. Each experiment was conducted in triplicate, and the results were averaged.
Fourier transform infrared (FTIR) spectroscopy
The HA structure was verified by Fourier transform infrared (FTIR) spectroscopy (WQF-510A, China). First, the polysaccharide sample extracted from the supernatant using ethanol was dried. It was then prepared according to the potassium bromide (KBR) technique [35], in which the sample was compressed into a pellet at high pressure. The infrared spectrum of the HA sample was obtained in the range of 4000–400 cm−1 with a resolution of 2.0 cm−1. Finally, the recorded peaks were compared with the peaks of the HA standard reported in the literature.
Results and discussion
Characteristics of potato peel
Potato peel waste is 15–40% of the original tuber weight, depending on the peeling efficiency. This substantial by-product has a potential for value-added utilization rather than disposal [36]. Furthermore, the features of potato peel waste are influenced by several other factors including potato cultivar, light exposure, irradiation treatment, storage conditions, and mechanical damage [37]. The composition of potato peel waste is demonstrated in Table 1, which presents the findings of some chemical composition parameters analyzed in the samples. These findings align with the results found in other studies. For instance, the PPW used by Khawla et al. [38] to produce bioethanol had the following compositions (% on dry basis): moisture 6.87, protein 15.21, starch 48.46, and ash 7.23.
HA production in synthetic medium
Effect of carbon source
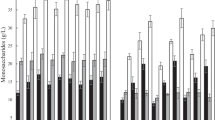
In the present study, S. zooepidemicus sp. MW269858 was examined for its ability to utilize five different sugars (monosaccharides, disaccharides, and polysaccharides) and to produce HA biopolymer. In this way, synthetic culture media containing glucose, lactose, sucrose, sago starch, and potato starch at 30 g/L were separately prepared and the rate of cell growth, HA, and the change in pH values were evaluated at regular intervals. The results are shown in Fig. 1 (a–e). As can be observed from this figure, in all the experiments, HA and biomass production first increased and then gradually decreased or remained constant. An increase in viscosity in the culture broth due to EPS and other metabolites synthesis, followed by a decrease in mixing performance and oxygen transfer limitation, can explain the reduction in HA production. The pH value also decreases, which can be due to the production of HA and other acidic products [39, 40]. The main metabolite that results from glucose catabolism in S. zooepidemicus is lactic acid which explains the pH drops observed in media [9]. The pH values decreased in both media; however, in the complex sugar medium (sago starch and potato starch), the decrease was less pronounced. One possible explanation for this observation is that when starch was used instead of glucose in the medium, lactic acid production decreased [41]. Besides, drops in pH levels below 5 may lead to a decline in microbial biomass content. The results of maximum biomass, maximum HA production, and time to reach them are summarized in Table 2. Among all the carbon sources exanimated, glucose resulted in the highest amount of HA, 1.41 g/L, in comparison to maximum HA concentrations of 0.09, 0.13, 0.93, and 0.16 g/L attained for sago starch, potato starch, sucrose, and lactose, respectively. Furthermore, a maximum biomass of 4.20 g/L was obtained for glucose, and the culture with glucose presented a rapid production of HA (within 24 h) than other substrates. This is because the other sugars must first be decomposed into glucose so that they can be used by bacteria [42]. As a result, in this research, glucose was chosen as the most suitable carbon for the synthesis of HA via S. zooepidemicus sp. and applied for the subsequent experiments. This finding aligns with the results previously conveyed by Im et al. [40] who examined ten carbon sources including fructose, glucose, galactose, maltose, dextrin, mannose, xylose, lactose, and soluble starch at 40 g/L for HA production by Streptococcus sp. ID9102. Hyaluronan production reached the highest value of 1.58 g/L when glucose was employed as the carbon substrate. HA synthesis in streptococci demands significant energy and contests bacterial cell growth for glucose, both as an energy source and as a UDP-sugar precursor. In fact, in the presence of abundant glucose, maximal bacterial growth was detected under optimal cultivation circumstances [43].
Effect of nitrogen source
The culture condition is a significant aspect of the composition of media for HA synthesis. S. zooepidemicus is a nutrient-demanding bacterium, nitrogen is one of the most important nutrients for it [39, 44]. Nitrogen sources are a factor that affects microbial metabolism [45, 46]. To study the effect of nitrogen sources, synthetic culture media containing peptone, yeast extract, and ammonium sulfate at 10 g/L were used. The experimental findings demonstrated that the strain was capable of using three nitrogen sources (organic and inorganic) and produced HA. The maximum amount of HA and biomass produced from the three distinct nitrogen sources is compared in Fig. 2. According to the data, the medium containing peptone and yeast extract produced the most HA after 24 h, at 1.41 and 0.89 g/L, respectively. The highest HA concentration in the ammonium sulfate-containing solution after 36 h was 0.74 g/L. Ammonium sulfate is an inorganic nitrogen source, whereas peptone and yeast extract are organic nitrogen sources. The utilization of organic nitrogen sources is more suitable for S. zooepidemicus and the production of HA. This has been confirmed by other experiments. In a study by Im et al. [40], the effects of different nitrogen sources on HA production by Streptococcus sp. ID9102 were examined. The yeast extract in the basal medium was replaced with various organic nitrogen sources, as well as inorganic sources each at 0.5% concentration along with glucose. Media containing the organic nitrogen sources supported plentiful bacterial growth and high HA yields. Khue and Vo [47] assessed the level effects of various factors like peptone, meat extract, yeast extract, glucose, Tween-80, potassium phosphate, sodium acetate, ammonium citrate, magnesium sulfate, and manganese sulfate to estimate their influence. The most significant impact on the yield of hyaluronic acid was observed with yeast extract. However, the strain failed to grow in media containing only the inorganic nitrogen sources. This demonstrated the requirement of an organic nitrogen source for HA synthesis by this strain. The fermentation culture supplemented with yeast extract exhibited more rapid HA production compared to cultures with other nitrogen sources. Maximum HA levels were achieved within 24 h. This is because yeast extract is a great source of nutrients like amino acids, vitamins, nucleosides, peptides, and minerals. This complex nutritional profile makes yeast extract an excellent growth medium for culturing both laboratory and industrial microorganisms [48]. In addition, yeast extract contains essential nutrients like amino acids and vitamins that are critical for S. zooepidemicus fermentation. The amino acids present in yeast extract provide the necessary precursors and building blocks for HA synthesis and biomass generation [49]. Purines, pyrimidine bases, and vitamin B complex are the main contributors to HA production in yeast extract. It is also important to control the amount of acetate to optimize HA production because acetate can compete with HA for the same precursors. This competition, along with the accumulation of by-products in the production media, can inhibit HA production. [8, 9, 14].
HA production using potato peel as a carbon source
Although potato peel contains abundant starch, its content of reducing sugars fermentable by microorganisms is low (0.6% dry weight). As a result, direct microbial fermentation of untreated potato peels is not feasible. Therefore, to enable fermentation, an initial hydrolysis step (either enzymatic or acidic) is necessary to break down the carbohydrates [24]. For this purpose, sugar is extracted from potato peel residue using acid and enzymatic hydrolysis. The structure of potato peel residue is disrupted during a hydrolysis process that converts polysaccharides into monosaccharides, which can then be metabolized by microorganisms. In comparison to enzymatic and acidic hydrolysis, each hydrolysis offers some advantages. Enzymatic breakdown of starch requires an initial gelatinization pretreatment, where starch is heated in water to unravel its granular structure. After cooling, this gelatinized starch forms a porous gel network that enables enzymes to readily permeate and access the starch polymers, facilitating more efficient enzymatic catalysis. In contrast, acid hydrolysis does not require this gelatinization step, as the acidic conditions directly cleave glycosidic bonds in starch without needing to first disrupt the granular morphology. Thus, acid hydrolysis provides a more direct breakdown of starch without the additional pretreatment. In addition to the more direct breakdown of starch, acid hydrolysis also has shorter reaction times and simpler pretreatment needs compared to enzymatic hydrolysis. Acid hydrolysis also has several key drawbacks. The acidic conditions generate degradation by-products such as furfural, 5-(hydroxymethyl)-2-furaldehyde (HMF), and acetic acid, which persist in the glucose solutions after the hydrolysis process. These particular compounds inhibit the growth of microorganisms and thus, need to be regulated to non-toxic levels to facilitate subsequent fermentation. Other disadvantages include the need to neutralize the acidic medium after hydrolysis, dispose of the resulting calcium sulfate waste and meet process energy requirements. Additional supplementary equipment is also required for neutralization and filtration steps, with some equipment components needing acid resistance. Thus acid hydrolysis, while directly cleaving polysaccharide bonds, requires extensive downstream processing to remove inhibitory by-products and adjust conditions for fermentation [50, 51]. Enzymatic hydrolysis of biomass, as in comparison with acid hydrolysis, happens at moderate conditions of the reaction. In addition, the highly specific nature of enzyme-catalyzed reactions enables complete and targeted deconstruction of the biomass into glucose [38]. Therefore, enzymatic hydrolysis provides the advantages of moderate conditions and high specificity for effective biomass saccharification into fermentable sugars. As a result, both acidic and enzymatic hydrolysis methods were employed in this study to evaluate fermentable reducing sugars released after enzymatic and acidic hydrolysis of PPW. To monitor the release of fermentable sugars over time, samples were collected from the enzymatic hydrolysis solutions every 24 h during the hydrolysis period. The DNS assay was used to reducing sugar release for acidic and enzymatic hydrolysis. Table 3 summarizes the research conducted.
Enzymatic and acidic hydrolyzates from PPW
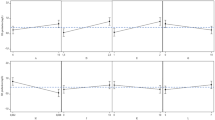
In this study, potato peel was selected as a cheap and economical substrate to evaluate the possibility of producing HA utilizing a low-cost carbon source. Figure 3 illustrates the results of comparing the amounts of glucose, cell growth, pH, and HA produced in the media containing acid hydrolyzate from potato peel and the PPW enzymatic hydrolyzate. A decrease in nutrition supplies, inhibitors produced during the process, by-products such as lactic acids and acetic acids, dissolved oxygen in the environment, a decrease in pH, and the strain’s subsequent transition into the stationary phase can all contribute to a decrease in hyaluronan levels. S. zooepidemicus requires a slightly neutral to slightly basic environment for optimal hyaluronic acid synthesis [8, 9]. The S. zooepidemicus strain MW269858 utilized the sugars released from enzymatic hydrolysis more readily, producing 19.68 g/L initially. This greater availability of fermentable sugars enabled increased bacterial growth and HA production over the first 24 h. However, as sugar levels decreased (total consuming sugar by strain 10.89 g/L) due to consumption by S. zooepidemicus and pH dropped, growth and HA synthesis began to plateau after 24 h. The greatest amount of HA reached was 0.92 g/L at 24 h with enzymatic hydrolysis. In comparison, the lower initial sugar release of 16.20 g/L from acid hydrolysis resulted in delayed bacterial growth (total consuming sugar by strain 8.04 g/L). The highest HA production was 0.59 g/L at 48 h with acid hydrolysis, likely due to the strain taking longer to adapt to this environment and the limitations of acid hydrolysis. To increase HA acid yields, glucose was supplemented as an additional carbon source to both the enzymatic and acid hydrolyzates. In this study, glucose was selected based on its previously being identified as the optimal carbon source for the growth of S. zooepidemicus MW269858, as mentioned above. The addition of a certain amount of glucose to the hydrolyzate enhanced the available sugar levels. Figure 4 depicts the variation of different parameters in the PPW hydrolyzates medium supplemented with glucose. This supplementation resulted in improved maximum HA production in the enzymatic and acid hydrolyzate, respectively, of 1.18 and 0.95 g/L. Figure 5 compares the amounts of cell proliferation and HA produced during enzymatic and acid hydrolysis of potato peel. Acid hydrolysis is less effective than enzymatic hydrolysis for different reasons. First, enzymatic hydrolysis is more incisive and environmentally friendly, without requiring harsh chemicals. Second, the sugars released from enzymatic hydrolysis were more compatible and accessible to the bacterial strain, facilitating rapid growth and HA synthesis that peaked faster at 24 h. In contrast, acid hydrolysis released lower fermentable sugars and required a longer adaptation time, reaching maximum HA levels only after 48 h. Based on our knowledge, PPW hydrolyzate has not yet been used as a source of carbon for HA production. The comparison between the production of HA achieved in this study and that attained in previous investigations is presented in Table 4. It is evident that the quantity of HA obtained in this study was marginally greater than some studies, disregarding the fact that other reports employed a higher initial concentration of sugars. This observation highlights the potential of PPW in the production of HA. Numerous other waste biomass materials have been explored as feedstocks which are summarized in Table 4.
FTIR spectrum of HA
The FTIR spectrum confirms the chemical structure of HA and can act as a validation that the exopolysaccharide obtained in this work is mainly composed of HA. The resemblance between the peaks representing the most significant functional groups in HA produced in this study is depicted in Fig. 6. The output of the FTIR spectrum is summarized in Table 5 and it matched the standard HA well. A prominent absorption band was observed at approximately 3432.67 cm−1 owing to the stretching oscillation of O–H and N–H hydrogen in the N-acetyl side chain. A cluster of overlapping bands at 2925.48 cm−1 was caused by aliphatic C–H stretching vibrations. Absorption bands detected at 1621.84 cm−1 and 1411.84 cm−1 can be assigned to the asymmetric and symmetric stretching vibrations of the carboxylate [57]. According to Ref. [58] after protonation, the C = O peak shifted up to 1735 cm−1. The C–O peak shifted down to 1255 cm−1. The infrared absorption bands at around 1621.84, 1563.98, and 1322.92 cm−1 relate to the amide I, amide II, and amide III vibrations of the peptide bond, respectively. The band at 1151.29 cm−1 can be attributed to the C–O–C stretching vibration of the O-bridge ether linkage. The bands at 1074.15 cm−1 and 1043.30 cm−1 correspond to the C–O stretching vibration of the exocyclic ether and the C–OH stretching of the secondary alcohol moiety, respectively [59]. The infrared absorption band observed at 948.80 cm−1 can be correlated to an asymmetric out-of-phase stretching vibration of the pyranose ring of carbohydrates [60].
Conclusion
The cost of the feedstock has a major effect on the overall economic feasibility and commercial viability of fermentative HA production. PPW has the advantages of being widely accessible and providing a “green” substrate option for fermentation processes. In this study, HA production by the S. zooepidemicus MW269858 was examined in two different culture media: one with a synthetic carbon source and the other with a low-cost carbon source. According to the data, glucose proved to be the ideal carbon substrate and yeast extract was selected as the optimal nitrogen source, giving high HA yields. The use of potato peel hydrolyzate as a substrate can provide a cost-effective option for fermentative HA production from S. zooepidemicus. Both acidic and enzymatic hydrolysis of the potato peels were assessed for fermentable sugars. Enzymatic hydrolysis was recognized to be better than acidic due to the selective cleavage of starch and cellulose polymers in the potato peel biomass. This demonstrates the potential for developing economical and sustainable HA manufacturing processes utilizing agricultural residues.
Availability of data and materials
All data included in this study are available upon request by contact with the corresponding author.
References
Patra P, Disha B, Kundu P, Das M, Ghosh A (2022) Recent advances in machine learning applications in metabolic engineering. Biotechnol Adv 108069 https://doi.org/10.1016/j.biotechadv.2022.108069
Nielsen J, Tillegreen CB, Petranovic D (2022) Innovation trends in industrial biotechnology. Trends Biotechnol 40(10):1160–1172. https://doi.org/10.1016/j.tibtech.2022.03.007
Mal N, Satpati G, Raghunathan S, Davoodbasha M (2022) Current strategies on algae-based biopolymer production and scale-up. Chemosphere 289:133178. https://doi.org/10.1016/j.chemosphere.2021.133178
Rahman MZ, Rahman M, Mahbub T, Ashiquzzaman M, Sagadevan S, Hoque ME (2023) Advanced biopolymers for automobile and aviation engineering applications. J Polym Res 30(3):106. https://doi.org/10.1007/s10965-023-03440-z
Hurst JR, Shannon BA, Craig HC, Rishi A, Tuffs SW, McCormick JK (2022) The Streptococcus pyogenes hyaluronic acid capsule promotes experimental nasal and skin infection by preventing neutrophil-mediated clearance. PLoS Pathog 18(11):e1011013. https://doi.org/10.1371/journal.ppat.1011013
Zamboni F, Wong CK, Collins MN (2023) Hyaluronic acid association with bacterial, fungal and viral infections: Can hyaluronic acid be used as an antimicrobial polymer for biomedical and pharmaceutical applications? Bioactive Materials 19:458–473. https://doi.org/10.1016/j.bioactmat.2022.04.023
Ucm R, Aem M, Lhb Z, Kumar V, Taherzadeh MJ, Garlapati VK, Chandel AK (2022) Comprehensive review on biotechnological production of hyaluronic acid: status, innovation, market and applications. Bioengineered 13(4):9645–9661. https://doi.org/10.1080/21655979.2022.2057760
Serra M, Casas A, Toubarro D, Barros AN, Teixeira JA (2023) Microbial hyaluronic acid production: a review. Molecules 28(5):2084. https://doi.org/10.3390/molecules28052084
Oliveira AH, Ogrodowski CC, Macedo ACd, Santana MHA, Gonçalves LR (2013) Cashew apple juice as microbial cultivation medium for non-immunogenic hyaluronic acid production. Braz J Microbiol 44:1097–1104. https://doi.org/10.1590/S1517-83822014005000017
Timoney J (2004) The pathogenic equine streptococci. Vet Res 35(4):397–409. https://doi.org/10.1051/vetres:2004025
Yu H, Stephanopoulos G (2008) Metabolic engineering of Escherichia coli for biosynthesis of hyaluronic acid. Metab Eng 10(1):24–32. https://doi.org/10.1016/j.ymben.2007.09.001
Liu Y Cao Y Du G Liu L (2020) Systems and synthetic metabolic engineering: challenges and prospects. In: Systems and synthetic metabolic engineering, pp 237–264https://doi.org/10.1016/B978-0-12-821753-5.00010-1
Vázquez JA, Montemayor MI, Fraguas J, Murado MA (2010) Hyaluronic acid production by Streptococcus zooepidemicus in marine by-products media from mussel processing wastewaters and tuna peptone viscera. Microb Cell Fact 9(1):1–10. https://doi.org/10.1186/1475-2859-9-46
Vázquez JA, Montemayor MI, Fraguas J, Murado MA (2009) High production of hyaluronic and lactic acids by Streptococcus zooepidemicus in fed-batch culture using commercial and marine peptones from fishing by-products. Biochem Eng J 44(2–3):125–130. https://doi.org/10.1016/j.bej.2008.11.007
Ozcan A, Germec M, Turhan I (2022) Optimization and kinetic modeling of media composition for hyaluronic acid production from carob extract with Streptococcus zooepidemicus. Bioprocess Biosyst Eng 45(12):2019–2029. https://doi.org/10.1007/s00449-022-02806-9
Amado IR, Vázquez JA, Pastrana L, Teixeira JA (2017) Microbial production of hyaluronic acid from agro-industrial by-products: Molasses and corn steep liquor. Biochem Eng J 117:181–187. https://doi.org/10.1016/j.bej.2016.09.017
Benedini LJ, Santana MHA (2013) Effects of soy peptone on the inoculum preparation of Streptococcus zooepidemicus for production of hyaluronic acid. Biores Technol 130:798–800. https://doi.org/10.1016/j.biortech.2012.12.161
Pires AM, Macedo AC, Eguchi SY, Santana MH (2010) Microbial production of hyaluronic acid from agricultural resource derivatives. Biores Technol 101(16):6506–6509. https://doi.org/10.1016/j.biortech.2010.03.074
Arslan NP, Aydogan MN (2021) Evaluation of sheep wool protein hydrolysate and molasses as low-cost fermentation substrates for hyaluronic acid production by Streptococcus zooepidemicus ATCC 35246. Waste and Biomass Valorization 12:925–935. https://doi.org/10.1007/s12649-020-01062-w
Rohit SG, Jyoti PK, Subbi RRT, Naresh M, Senthilkumar S (2018) Kinetic modeling of hyaluronic acid production in palmyra palm (Borassus flabellifer) based medium by Streptococcus zooepidemicus MTCC 3523. Biochem Eng J 137:284–293. https://doi.org/10.1016/j.bej.2018.06.011
Singh A, Singhania RR, Soam S, Chen CW, Haldar D, Varjani S et al (2022) Production of bioethanol from food waste: status and perspectives. Bioresour Technol 127651 https://doi.org/10.1016/j.biortech.2022.127651
Sharma P, Gaur VK, Kim S-H, Pandey A (2020) Microbial strategies for bio-transforming food waste into resources. Biores Technol 299:122580. https://doi.org/10.1016/j.biortech.2019.122580
Sepelev I, Galoburda R (2015) Industrial potato peel waste application in food production: A review. Res Rural Dev 1:130–136
Arapoglou D, Varzakas T, Vlyssides A, Israilides C (2010) Ethanol production from potato peel waste (PPW). Waste Manage 30(10):1898–1902. https://doi.org/10.1016/j.wasman.2010.04.017
Abdelraof M, Hasanin MS, El-Saied H (2019) Ecofriendly green conversion of potato peel wastes to high productivity bacterial cellulose. Carbohyd Polym 211:75–83. https://doi.org/10.1016/j.carbpol.2019.01.095
DuBois M, Gilles KA, Hamilton JK, Pt R, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. https://doi.org/10.1021/ac60111a017
Cunniff PA, Jee M (1995) Official methods of analysis of AOAC international (16th edn). Trends Food Sci Technol 6(11):382
Johns MR, Goh L-T, Oeggerli A (1994) Effect of pH, agitation and aeration on hyaluronic acid production by Streptococcus zooepidemicus. Biotech Lett 16:507–512. https://doi.org/10.1007/BF01023334
Badle SS, Jayaraman G, Ramachandran K (2014) Ratio of intracellular precursors concentration and their flux influences hyaluronic acid molecular weight in Streptococcus zooepidemicus and recombinant Lactococcus lactis. Biores Technol 163:222–227. https://doi.org/10.1016/j.biortech.2014.04.027
Arapoglou D, Vlyssides A, Varzakas T, Haidemenaki K, Malli V, Marchant R, Israilides C (2009) Alternative ways for potato industries waste utilisation. In: Proceedings of the 11th international conference on environmental science and technology, Chaina, Crete, Greece. pp 3–5
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428. https://doi.org/10.1021/ac60147a030
Farahi A, Najafpour G, Ghoreyshi A, Mohammadi M, Esfahanian M (2012) Enzymatic production of reducing sugars from broomcorn seed (sorghum vulgare): process optimization and kinetic studies. World Appl Sci J 18(4):568–574
Chen Y-H, Wang Q (2009) Establishment of CTAB Turbidimetric method to determine hyaluronic acid content in fermentation broth. Carbohyd Polym 78(1):178–181. https://doi.org/10.1016/j.carbpol.2009.04.037
Oueslati N, Leblanc P, Harscoat-Schiavo C, Rondags E, Meunier S, Kapel R, Marc I (2014) CTAB turbidimetric method for assaying hyaluronic acid in complex environments and under cross-linked form. Carbohyd Polym 112:102–108. https://doi.org/10.1016/j.carbpol.2014.05.039
Cheng K-C, Demirci A, Catchmark JM (2011) Evaluation of medium composition and fermentation parameters on pullulan production by Aureobasidium pullulans. Food Sci Technol Int 17(2):99–109. https://doi.org/10.1177/1082013210368719
Schieber A, Stintzing FC, Carle R (2001) By-products of plant food processing as a source of functional compounds—recent developments. Trends Food Sci Technol 12(11):401–413. https://doi.org/10.1016/S0924-2244(02)00012-2
Friedman M (2004) Analysis of biologically active compounds in potatoes (Solanum tuberosum), tomatoes (Lycopersicon esculentum), and jimson weed (Datura stramonium) seeds. J Chromatogr A 1054(1–2):143–155. https://doi.org/10.1016/j.chroma.2004.04.049
Khawla BJ, Sameh M, Imen G, Donyes F, Dhouha G, Raoudha EG, Oumèma N-E (2014) Potato peel as feedstock for bioethanol production: A comparison of acidic and enzymatic hydrolysis. Ind Crops Prod 52:144–149. https://doi.org/10.1016/j.indcrop.2013.10.025
Liu L, Liu Y, Li J, Du G, Chen J (2011) Microbial production of hyaluronic acid: current state, challenges, and perspectives. Microb Cell Fact 10(1):1–9. https://doi.org/10.1186/1475-2859-10-99
Im J-H, Song J-M, Kang J-H, Kang D-J (2009) Optimization of medium components for high-molecular-weight hyaluronic acid production by Streptococcus sp. ID9102 via a statistical approach. J Ind Microbiol Biotechnol 36(11):1337. https://doi.org/10.1007/s10295-009-0618-8
Zhang J, Ding X, Yang L, Kong Z (2006) A serum-free medium for colony growth and hyaluronic acid production by Streptococcus zooepidemicus NJUST01. Appl Microbiol Biotechnol 72:168–172. https://doi.org/10.1007/s00253-005-0253-x
Chen Y-H, Li J, Liu L, Liu H-Z, Wang Q (2012) Optimization of flask culture medium and conditions for hyaluronic acid production by a streptococcus equisimilis mutant nc2168. Braz J Microbiol 43:1553–1561. https://doi.org/10.1590/S1517-83822012000400040
Cooney M, Goh LT, Lee P, Johns M (1999) Structured model-based analysis and control of the hyaluronic acid fermentation by Streptococcus zooepidemicus: Physiological implications of glucose and complex-nitrogen-limited growth. Biotechnol Prog 15(5):898–910. https://doi.org/10.1021/bp990078n
Armstrong D, Cooney M, Johns M (1997) Growth and amino acid requirements of hyaluronic-acid-producing Streptococcus zooepidemicus. Appl Microbiol Biotechnol 47:309–312. https://doi.org/10.1007/s002530050932
de Oliveira MR, da Silva RSSF, Buzato JB, Celligoi MAPC (2007) Study of levan production by Zymomonas mobilis using regional low-cost carbohydrate sources. Biochem Eng J 37(2):177–183. https://doi.org/10.1016/j.bej.2007.04.009
Xu K, Xu P (2014) Efficient production of L-lactic acid using co-feeding strategy based on cane molasses/glucose carbon sources. Biores Technol 153:23–29. https://doi.org/10.1016/j.biortech.2013.11.057
Khue NTH, Vo PTM (2013) Study of complex nutrients, temperature and salts on hyaluronic acid production in Streptococcus zooepidermicus ATCC 43079. J Appl Pharm Sci 3(5):012–015. https://doi.org/10.7324/JAPS.2013.3503
Tao Z, Yuan H, Liu M, Liu Q, Zhang S, Liu H et al (2023) Yeast extract: characteristics, production, applications and future perspectives. J Microbiol Biotechnol 33(2):151. https://doi.org/10.4014/jmb.2207.07057
Bayarjargal M, Munkhbat E, Ariunsaikhan T, Odonchimeg M, Uurzaikh T, Gan-Erdene T, Regdel D (2011) Utilization of spent brewer’s yeast Saccharomyces cerevisiae for the production of yeast enzymatic hydrolysate. Mong J Chem 12:88–91. https://doi.org/10.5564/mjc.v12i0.179
Guerra-Rodríguez E, Portilla-Rivera OM, Ramírez JA, Vázquez M (2012) Modelling of the acid hydrolysis of potato (Solanum tuberosum) for fermentative purposes. Biomass Bioenerg 42:59–68. https://doi.org/10.1016/j.biombioe.2012.03.019
Guerra-Rodríguez E, Portilla-Rivera OM, Jarquín-Enríquez L, Ramírez JA, Vázquez M (2012) Acid hydrolysis of wheat straw: A kinetic study. Biomass Bioenerg 36:346–355. https://doi.org/10.1016/j.biombioe.2011.11.005
Pan NV, Vignoli JA, Baldo C, Pereira HCB, Silva RSSF, Celligoi MA (2015) Agroindustrial byproducts for the production of hyaluronic acid by Streptococcus zooepidemicus ATCC 39920. Int J Sci Technol Res 4(4):114–118
Vázquez JA, Pastrana L, Piñeiro C, Teixeira JA, Pérez-Martín RI, Amado IR (2015) Production of hyaluronic acid by Streptococcus zooepidemicus on protein substrates obtained from Scyliorhinus canicula discards. Mar Drugs 13(10):6537–6549. https://doi.org/10.3390/md13106537
Mohan N, Balakrishnan R, Sivaprakasam S (2016) Optimization and effect of dairy industrial waste as media components in the production of hyaluronic acid by Streptococcus thermophilus. Prep Biochem Biotechnol 46(6):628–638. https://doi.org/10.1080/10826068.2015.1128446
Amado IR, Vázquez JA, Pastrana L, Teixeira JA (2016) Cheese whey: A cost-effective alternative for hyaluronic acid production by Streptococcus zooepidemicus. Food Chem 198:54–61. https://doi.org/10.1016/j.foodchem.2015.11.062
Flores-Méndez DA, Ramos-Ibarra JR, Toriz G, Arriola-Guevara E, Guatemala-Morales G, Corona-González RI (2021) Bored coffee beans for production of hyaluronic acid by Streptococcus zooepidemicus. Fermentation 7(3):121. https://doi.org/10.3390/fermentation7030121
Pan NC, Pereira HCB, da Silva MdLC, Vasconcelos AFD, Celligoi MAPC (2017) Improvement production of hyaluronic acid by Streptococcus zooepidemicus in sugarcane molasses. Appl Biochem Biotechnol 182:276–293. https://doi.org/10.1007/s12010-016-2326-y
Gilli R, Kacuráková M, Mathlouthi M, Navarini L, Paoletti S (1994) FTIR studies of sodium hyaluronate and its oligomers in the amorphous solid phase and in aqueous solution. Carbohyd Res 263(2):315–326. https://doi.org/10.1016/0008-6215(94)00147-2
Choi J-I, Kim J-K, Kim J-H, Kweon D-K, Lee J-W (2010) Degradation of hyaluronic acid powder by electron beam irradiation, gamma ray irradiation, microwave irradiation and thermal treatment: A comparative study. Carbohyd Polym 79(4):1080–1085. https://doi.org/10.1016/j.carbpol.2009.10.041
Wu Y (2012) Preparation of low-molecular-weight hyaluronic acid by ozone treatment. Carbohyd Polym 89(2):709–712. https://doi.org/10.1016/j.carbpol.2012.03.081
Acknowledgements
The authors extend their sincere appreciation to the Babol Noshirvani University of Technology, Faculty of Chemical Engineering (Biochemical), for funding the project (grant number: BNUT/974115052/1400), and Dr. Iradj Ashrafi Tamai (University of Tehran) for their valuable help in this research work.
Funding
This research funding is supported by the Babol Noshirvani University of Technology, Faculty of Chemical Engineering (grant number: BNUT/974115052/1400).
Author information
Authors and Affiliations
Contributions
Seyedali Mousavi: investigation, software, methodology, formal analysis, validation, writing—original draft. Razieh Esfandiar: investigation, methodology, formal analysis, validation, writing—original draft, writing—review and editing. Ghasem Najafpour Darzi: supervision, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors report that there are no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mousavi, S., Esfandiar, R. & Najafpour-Darzi, G. Hyaluronic acid production by Streptococcus zooepidemicus MW26985 using potato peel waste hydrolyzate. Bioprocess Biosyst Eng 47, 1003–1015 (2024). https://doi.org/10.1007/s00449-024-03007-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-024-03007-2