Abstract
Hyaluronic acid (HA), linear high-molecular-weight glycosaminoglycan produced from Streptococcus sp., has raised interest in the medical and cosmetics industries because of the various biological functions of HA. In this paper, we report on the optimization of medium components for HA production in Streptococcus sp. ID9102 (KCTC 11935BP) by two-step optimization (one-factor-at-a-time and taguchi orthogonal array design). In the first step, medium components, such as carbon, nitrogen, phosphate, and mineral sources, were selected for HA production in Streptococcus sp. ID9102 (KCTC 11935BP) using the one-factor-at-a-time method. In the second step, the concentration of the selected medium components was optimized using taguchi orthogonal array design. The design for medium optimization was developed and analyzed using MINITAB 14 software. In addition, the effect of amino acid and organic acid, such as glutamine, glutamate, and oxalic acid, was studied for HA production in Streptococcus sp. ID9102 (KCTC 11935BP). Through these processes, the optimum medium comprising 4% glucose, 0.75% yeast extract, 1.0% casein peptone, 0.25% K2HPO4, 0.05% MgCl2, 0.5% NaCl, 0.04% glutamine, 0.06% glutamate, and 0.02% oxalic acid was determined. We were able to produce HA with a molecular weight of 5.9 × 106 at a productivity of 6.94 g/l on pilot scale fermentation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hyaluronic acid (HA) is a uniformly repetitive, linear, high-molecular-weight glycosaminoglycan composed of 2,000–25,000 disaccharides of glucuronic acid and N-acetylglucosamine joined alternately by β-1-3 and β-1-4 glycosidic bonds [4]. Owing to its variety of biological functions, HA has a wide range of applications in the fields of medicine and cosmetics, including osteoarthritis treatment, ophthalmic surgery, plastic surgery, drug delivery, skin moisturizers, and wound healing [4, 12, 20].
It is well known that HA can be obtained commercially through three routes: human umbilical cords, rooster combs, and strains of group C Streptococcus. However, the first two routes have some disadvantages, such as relatively low yields, contamination, and risk of cross-species viral injections. Hence, strains of Streptococcus sp. have been used to produce HA industrially. Much work, such as improving the fermentation process [1, 3, 10, 13–16, 18, 19, 22], adding lysozyme [18, 24], the alkaline-stress strategy [21], adding hydrogen peroxide and ascorbate [23], and changing the medium composition [27, 30], has been done to increase the production yield of hyaluronic acid in Streptococcus sp. Although many studies have been performed on HA production, there are very few reports on general medium optimization for high-molecular-weight HA production and on economically efficient conditions for HA production.
In this study, we carried out optimization of medium components to produce high-molecular-weight HA in Streptococcus sp. ID9102 (KCTC 11935BP) that lacked hemolytic activity and hyaluronidase industrially. In the first step, the effects of medium components, such as carbon sources, nitrogen sources, phosphate sources, and mineral sources, on HA production were investigated by the one-factor-at-a-time method. In the second step, the concentration of selected medium components was optimized using taguchi orthogonal array design. In addition, the effects of various amino acids and organic acids were studied in order to improve the productivity and molecular weight of HA. The optimized medium was applied in a 75-l jar fermenter to produce high-molecular-weight HA on a pilot scale.
Materials and methods
Organism
Streptococcus sp. ID9102 designed as a nonhemolytic and hyaluronidase-negative mutant strain by N-methyl-N′-nitro-N-nitroso-guanidine (NTG) was used as the HA producer in this study. This strain was deposited with the Korean Collection for Type Cultures (KCTC) with the accession number KCTC 1139BP.
Inoculum, culture media, and conditions
The stock culture preserved in 20% glycerol solution at −72°C was plated in brain heart infusion (BHI, Difco) medium containing 1.5% agar and incubated at 37°C for 24 h. A loopful of cells from the agar slant was transferred to 40 ml of sterilized Todd Hewitt broth (THB, Difco) medium in a 250-ml Erlenmeyer flask as seed culture and incubated at 37°C on a rotary shaker at 120 rpm for 6 h. This was used as the inoculum for flask culture. For the production of HA, the flask culture was placed in 250-ml Erlenmeyer flasks, each containing 40 ml of HA production medium. The HA production medium was aseptically inoculated with 2 ml of 6-h-old seed culture. The inoculated flasks were incubated on a rotary shaker at 37°C and 120 rpm for 24 h. In order to produce HA and optimize medium components, the basal medium comprised 4.0% glucose, 0.5% yeast extract, 0.25% K2HPO4, 0.07% MgSO4, and 0.5% NaCl. The pH of the medium was adjusted to 7.0 using 0.1 N NaOH or HCl before sterilization. The medium was sterilized in an autoclave for 20 min at 121°C, except glucose. Glucose solution was sterilized by autoclave separately. All flask cultures were carried out in triplicate.
Batch fermentation in a 75-l jar fermenter
A total of 80 ml of the first seed culture was inoculated into a 2-l jar fermenter containing 1.8 l of THB medium. This was used as the second seed culture for the 75-l jar fermentation. The second seed culture was inoculated into the 75-l jar fermenter containing 45 l of production medium. The temperature was maintained at 36°C, and the aeration rate was 0.5 vvm. The agitation was provided by two six-bladed disk turbine impellers. The diameters of the impeller and vessel were 120 mm and 350 mm, respectively. The pH was controlled automatically at 7.0 by adding 10 N NaOH solution. Batch fermentation was performed for 24 h in a 75-l jar fermenter (Bioengineering, Switzerland) with a working volume of 45 l.
Statistical analysis
Taguchi orthogonal array design was carried out for optimization of selected medium components (yeast extract, casein peptone, MgCl2, and K2HPO4) on HA production by the strain Streptococcus sp. ID9102 (KCTC 11935BP). Four medium factors and four different levels were designed as a L16-orthogonal array. The design was developed and analyzed using MINITAB 14 software. Table 1 shows the cultivation conditions and the L16-orthogonal array design used in this study.
Analytical methods
Cell growth was observed by measuring the optical density of the culture broth at 600 nm using a spectrophotometer. The glucose concentration was determined using the glucose assay kit (Sigma). HA concentration in the culture broth was determined by the turbidimetric method [5, 11]. Broth samples were digested by an equal volume of 0.1% (w/v) sodium dodecyl sulfate (SDS) and incubated at room temperature for 10 min to release the capsular HA [5]. The samples were centrifuged at 14,000 rpm for 10 min. Of the cell-free supernatant, 200 µl was mixed with 200 µl of 0.2 M acetate buffer. The mixture was incubated at 37°C for 10 min, and then CTAB (cetyltrimethyl-ammonium bromide) buffer was added to the mixture sample. The finished mixture was measured at OD600. HA concentration was calculated by standard curve prepared at different concentrations of HA standard (Sigma). The viscosity of culture broth was measured using a viscometer (Brookfield, Middleboro, MA). The molecular weight of HA was determined by high-performance liquid chromatography (Spectra AS3000, Thermo Separation Products, Piscataway, NJ) equipped with a refractive index detector (RI-71, Shodex, Japan) and a gel permeation chromatography program. A column of TSK G6000 PW (Toyo Soda, Japan) was used. Polyethylene oxide (Sigma) was used as a reference standard for measuring the molecular weight of hyaluronic acid.
Results
Selection of medium components using the one-factor-at-a-time method
To study the effects of carbon sources on HA production, glucose in the basal medium was replaced with nine other carbon sources, such as fructose, galactose, mannose, lactose, maltose, sucrose, xylose, dextrin, and soluble starch. All carbon sources were used at 4% concentration. Figure 1 indicates the effect of carbon sources on HA production. The highest HA productivity (1.58 g/l) was achieved using glucose as a carbon source (Fig. 1). Hence, glucose in basal medium was maintained as the carbon source for HA production in Streptococcus sp. ID9102 (KCTC 11935BP).
To study the effects of nitrogen sources on HA production, yeast extract was replaced with other organic nitrogen sources, such as peptone, casein peptone, skim milk, casein, soytone, dry yeast, and soybean meal, and with inorganic nitrogen sources, such as ammonium sulfate, ammonium citrate, ammonium chloride, urea, glycine, potassium nitrate, and sodium nitrate at 0.5% concentration. The organic nitrogen sources in basal medium containing glucose resulted in an abundant growth and high yield of HA. However, Streptococcus sp. ID9102 (KCTC 11935BP) strain was not grown in all media containing inorganic nitrogen sources (data not shown). Figure 2 shows the effect of different organic nitrogen sources on HA production in Streptococcus sp. ID9102 (KCTC 11935BP). The highest HA production was achieved using yeast extract and casein peptone among the various organic nitrogen sources (Fig. 2). Yields of yeast extract and casein peptone were 1.65 and 1.64 g/l, respectively. The effect of the above two nitrogen sources was similar. Therefore, both of them were added in medium at 0.5% concentration, respectively. Addition of both of them enhanced HA productivity in Streptococcus sp. ID9102 (KCTC 11935BP) (Fig. 2). In this case, the yield of HA was 1.92 g/l. Both were selected as nitrogen sources for HA production in Streptococcus sp. ID9102 (KCTC 11935BP).
Effect of different nitrogen sources on HA production by Streptococcus sp. ID9102 (KCTC 11935BP). Each of the nitrogen sources was added at 0.5% concentration, respectively, in basal medium. Y + C means adding both yeast extract and casein peptone at 0.5% concentration, respectively. Cultivation was accomplished on a rotary shaker at 37°C and 120 rpm for 24 h
To study the effects of phosphate sources on HA production, K2HPO4 was replaced with other phosphate sources, such as (NH4)2HPO4, Na2HPO4, NaH2PO4, Na3PO4, and KH2PO4 at 0.25% concentration. All test cultures were carried out in medium containing 4% glucose, 0.5% yeast extract, 0.5% casein peptone, 0.05% MgSO4, and 0.5% NaCl. Of the different phosphate sources tested, K2HPO4 was the most effective for HA production in Streptococcus sp. ID9102 (KCTC 11935BP), and its yield was 1.94 g/l (Fig. 3). Glucose was used as the carbon source. Yeast extract and casein peptone were used as the nitrogen source. At this time the C/N ratio was 13.3. KH2PO4 was also effective for HA production, but not as effective as K2HPO4.
Effect of different phosphate sources on HA production by Streptococcus sp. ID9102 (KCTC 11935BP). Each of the phosphate sources was added at 0.25% concentration, respectively, in medium containing 4% glucose, 0.5% yeast extract, 0.5% casein peptone 0.05% MgSO4, and 0.5% NaCl. Cultivation was accomplished on a rotary shaker at 37°C and 120 rpm for 24 h
To study the effects of mineral sources on HA production, MgSO4 was replaced with 13 other mineral sources, MgCl2, CaCl2, KCl, FeCl2, FeSO4, MnSO4, BaCl2, CuCl2, CuSO4, CoSO4, ZnCl, ZnSO4, and NaF, at different concentrations (0.01% and 0.05%). All test cultures were carried out in medium containing 4% glucose, 0.5% yeast extract, 0.5% casein peptone, 0.25% K2HPO4, and 0.5% NaCl. Among the various mineral sources used, only MgSO4 and MgCl2 were found to be useful at 0.05% (data not shown). MnSO4 was a bit effective, and the others were not entirely effective. The Mg2+ source was necessary for HA production. MgCl2 supported the highest yield of 1.98 g/l HA, and MgSO4 resulted in a yield of 1.89 g/l HA at the end of 24 h. Accordingly, MgCl2 was determined as an optimum mineral source.
Optimization of selected medium components using taguchi orthogonal array
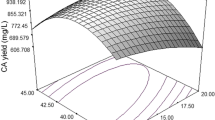
Taguchi orthogonal array design can plan the minimal number of experiments and supply outright information for all the factors. According to the results obtained, glucose, yeast extract, casein peptone, K2HPO4, and MgCl2 were finally selected as the best nutrient components. Among them, yeast extract, casein peptone, K2HPO4, and MgCl2 were optimized using L16-orthogonal array design. Glucose and NaCl were maintained in all media at 4 and 0.5%, respectively. The results are shown in Table 1. The data were analyzed using MINITAB. The response table for means (large is better) and S/N (signal-to-noise) ratio are summarized in Table 2. Rank and delta values shown in the last two rows in the tables assist in estimating the effect of factors. Delta measures the extent of the effect by calculating the difference between the highest and lowest data mean for a factor. The higher the delta value, the greater the suggested effect of that component. Rank arranges the factors to form the greatest effect to the least effect on the basis of the delta values. As presented in Table 2, the factors are ordered as follows: MgCl2 > yeast extract > casein peptone > K2HPO4. MgCl2 had the greatest effect and K2HPO4 the least effect on HA production by Streptococcus sp. ID9102 (KCTC 11935BP).
Figure 4 shows the main effect plot for the mean and for the S/N ratio. MINITAB creates the main effect plot by plotting the data mean for each factor level. These means are the same as those shown in Table 2. Lines connect the point for each factor. There is no main effect present when the line is flat. However, when the line is not flat, then there is a main effect present. In the present study, the main effect of each of the four factors (yeast extract, casein peptone, K2HPO4, and MgCl2) was at level 3, level 4, level 2, and level 2, respectively. Experiments were carried out in optimum medium in order to confirm the results obtained by taguchi orthogonal array design. HA production of final optimized medium was 2.51 g/l as compared with 2.56 g/l predicted using MINITAB for the same composition. Moreover, the final optimized medium produced 2.51 g/l HA at the end of 24 h as compared with 1.98 g/l before optimization. Hence, the optimum medium for HA production was determined as follows: 4% glucose, 0.75% yeast extract, 1.0% casein peptone, 0.25% K2HPO4, 0.05% MgCl2, and 0.5% NaCl.
Effect of amino acids and organic acids added on HA production
To study the effects of amino acids added on HA production, each of the 17 amino acids was added at different concentrations (0.02, 0.04, and 0.06%) in medium optimized by taguchi orthogonal array design. Among the various amino acids used, glutamine and glutamate enhanced more HA yield than the other amino acids (data not shown). Glutamine obtained maximum HA yield (2.65 g/l) at 0.04%, and glutamate obtained maximum HA yield (2.67 g/l) at 0.06% (Fig. 5). Both glutamine and glutamate were added in optimized medium at 0.04% and 0.06%, respectively. The yield of HA in the medium containing both glutamine (0.04%) and glutamate (0.06%) was higher at 2.76 g/l than the yield of a medium containing either glutamine (2.65 g/l) or glutamate (2.67 g/l) (Fig. 5).
Effect of glutamine and glutamate on HA production by Streptococcus sp. ID9102 (KCTC 11935BP). Amino acids were added in medium optimized by taguchi orthogonal array. Gln + Glu means adding both 0.04% glutamine and 0.06% glutamate. Cultivation was accomplished on a rotary shaker at 37°C and 120 rpm for 24 h
To study the effects of organic acids added to HA production, each of the 23 organic acids was added at 0.05% in optimized medium by taguchi orthogonal array design. Figure 6 shows the effects of organic acids on HA production. The highest HA yield (2.68 g/l) was obtained when oxalic acid was contained in the medium (Fig. 6). To determine the optimal concentration of oxalic acid, oxalic acid at different concentrations (0.01, 0.02, 0.05, 0.1, 0.2, 0.4, 0.6, 0.8, and 1.0%) was added to the above-mentioned medium. The optimal oxalic acid concentration for HA production was 0.02% to give a maximum yield of HA (2.75 g/l).
Glutamine, glutamate, and oxalic acid were added to the above-mentioned medium at 0.04, 0.06, and 0.02%, respectively. The yield of HA in the medium containing 0.04% glutamine, 0.06% glutamate, and 0.02% oxalic acid was higher at 2.96 g/l. At this time, the optimum C:N ratio was 7.5. From these results, it can be suggested that the addition of glutamine, glutamate, and oxalic acid enhances HA production.
Large-scale production of HA in a 75-l jar fermenter
Large-scale production of HA was carried out in a 75-l jar fermenter with 45 l of working volume. Bach fermentation was performed at 36°C, 0.5 vvm, and 400 rpm for 24 h. The pH was maintained at 7.0 by adding 10 N NaOH solution. Three-type media (basal medium, optimized medium without glutamine, glutamate, and oxalic acid and optimized medium with them) were used for HA production in a 75-l jar fermenter. Figure 7 shows the time course of HA production by Streptococcus sp. ID9102 (KCTC 11935BP) in a 75-l jar fermenter containing each medium. Through medium optimization using the taguchi orthogonal array design, the HA yield was increased from 3.27 g/l to 5.88 g/l, and the molecular weight of HA was also increased from 1.5 × 106 Da to 3.6 × 106 Da at the end of 24 h. The yield of HA in the medium with 0.04% glutamine, 0.06% glutamate, and 0.02% oxalic acid was higher at 6.94 g/l than that (5.88 g/l) of a medium without them, and the molecular weight of HA also increased from 3.6 × 106 Da to 5.9 × 106 Da. Also, HA in the medium with 0.04% glutamine, 0.06% glutamate, and 0.02% oxalic acid was produced earlier than the others. Through large-scale production of HA in a 75-l jar fermenter, we confirmed that medium optimized by taguchi orthogonal array design increased HA production, and the addition of amino acids and organic acids, including glutamine, glutamate, and oxalic acid, increased and stimulated the productivity and molecular weight of HA.
Time course of cell growth (a), glucose concentration (b), HA concentration (c), and molecular weight of HA (d) during HA fermentation by Streptococcus sp. ID9102 (KCTC 11935BP) in a 75-l jar fermenter. The 75-l jar fermentation was carried out at 36°C, agitation speed of 400 rpm, and aeration rate of 0.5 vvm for 24 h. Symbols: Filled circle, basal medium; filled square, optimized medium without glutamine, glutamate, and oxalic acid; filled triangle, optimized medium with glutamine, glutamate, and oxalic acid
Discussion
We carried out optimization of medium components for HA production in Streptococcus sp. ID9102 (KCTC 11935BP). In the first step, carbon, nitrogen, phosphate, and mineral sources for HA production were selected by the one-factor-at-a-time method. Sugar sources were used primarily as carbon sources. Since the precursors, UDP-glucuronic acid (UDP-GlcUA) and UDP-N-acetyl glucosamine (UDP-GlcNAc), are side reactions of the glycolytic pathway beginning from glucose-6-phosphate to fructose-6-phosphate, respectively [25], especially, glucose was used frequently for HA fermentation by Streptococcus sp. [1, 7, 14, 16, 18, 26, 29]. In our study, glucose was also determined to be the best carbon source for HA production by Streptococcus sp. ID9102 (KCTC 11935BP). Sucrose was used as the carbon source for HA fermentation [21, 22, 27]. It was reported that the HA production rate of maltose was higher than that of glucose, because the polymer conversion efficiency of maltose was higher than that of glucose [6]. Zhang et al. [30] used soluble starch as the carbon source for HA production by a S. zooepidemicus mutant designed by recursive generation. These reports suggest that Streptococcus sp. appears to favor simple sugars for HA production. Streptococcus sp. possesses fastidious nutrient requirements with respect to organic nitrogen [2, 9, 17, 25]. The high HA production was obtained in the media containing yeast extract, peptone, or casein peptone (Fig. 2). Figure 2 also shows that using a combination of two organic nitrogen sources (yeast extract and casein peptone) achieved higher HA production than using one organic nitrogen source. MgCl2 and K2HPO4 were selected as the best mineral source and phosphate source. Especially Mg2+ sources were used as cofactors to polymerize UDP-GlcUA and UDP-GlcNAc [8].
The nutrient sources selected by the one-factor-at-a-time method were optimized using taguchi orthogonal array design. HA yield and molecular weight in medium optimized by taguchi orthogonal array design were increased about 80 and 140%, respectively, compared to those in basal medium in a 75-l jar fermenter. In addition, glutamine, glutamate, and organic acid were added to medium optimized by taguchi orthogonal array design. Addition of glutamine affects HA production positively because of its use as the amido donor in amino sugar synthesis. We studied the effect of organic acids on HA production. Hence, we found oxalic acid. Oxalic acid was mentioned first for HA production. HA yield and molecular weight in the optimized medium with glutamine, glutamate, and organic acid were enhanced about 20% and 64% compared to those of the optimized medium without them. Although the enhancement of the HA yield was not high, the enhancement of the average molecular weight was high. These results suggest that the addition of amino/organic acid might affect the molecular weight of HA more than the yield of HA. In a previous report, tryptophan was used for enhancing the molecular weight (6.3 × 106 Da) of HA [28]; however, the HA yield was merely 0.8 g/l, which is very low. There have been several reports about increasing HA yield. Zhang et al. [30] obtained an HA yield of 6.7 g/l using medium optimization, and Liu et al. [21, 23] obtained an HA yield of 6.7 g/l and 6.5 g/l using the alkaline-stress strategy and the addition of hydrogen peroxide and ascorbate. However, these reports did not mention the molecular weight of HA or gaining a low-molecular-weight HA. Rangaswamy and Jain [27] obtained an HA yield of 6 g/l with a molecular weight of 4.0 × 106. We obtained an HA yield of 6.94 g/l with a molecular weight of 5.9 × 106. This suggested that we optimized medium components for high production of high-molecular-weight HA.
We supplied medium optimized by two-step processes (the one-factor-at-a-time method and taguchi orthogonal array design) and the addition of two amino acids (glutamine and glutamate) and one organic acid (oxalic acid) to produce high-molecular-weight HA in Streptococcus sp. ID9102 (KCTC 11935BP). A high yield of 6.94 g/l was produced in a 75-l jar fermenter containing optimum medium and was about 112% higher than the HA yield of the initial medium. Also, the average molecular weight of HA rose from 1.5 × 106 Da to 5.9 × 106 Da. These results indicated that our optimum medium can be applied for high-molecular-weight HA production on an industrial scale.
References
Armstrong DC, Johns MR (1997) Culture conditions affect the molecular weight properties of hyaluronic acid produced by Streptococcus zooepidemicus. Appl Environ Microbiol 63:2759–2764
Biotechnology General Corporation (1986) Method of producing high molecular weight sodium hyaluronate by fermentation of Streptococcus. World patent WO 8604355
Blank LM, McLaughlin RL, Nielsen LK (2005) Stable production of hyaluronic acid in Streptococcus zooepidemicus chemostats operated at high dilution rate. Biotechnol Bioeng 90:685–693
Chong BF, Blank LM, Mclaughlin R, Nielsen LK (2005) Microbial hyaluronic acid production. Appl Microbiol Biotechnol 66:341–351
Chong BF, Nielsen LK (2003) Amplifying the cellular reduction potential of Streptococcus zooepidemicus. J Biotechnol 100:33–41
Chong BF, Nielsen LK (2003) Aerobic cultivation of Streptococcus zooepidemicus and the role of NADH oxidase. Biochem Eng J 16:153–162
Cooney MJ, Goh LT, Lee PL, Johns MR (1999) Structured model-based analysis and control of the hyaluronic acid fermentation by Streptococcus zooepidemicus: physiological implications of glucose and complex-nitrogen-limited growth. Biotechnol Prog 15:898–910
DeAngelis PL (1999) Hyaluronan synthases: fascinating glycosyltransferases from vertebrates, bacterial pathogens, and algal viruses. Cell Mol Life Sci 56:670–682
Denki Kagaku Kogyo KK (1993) Manufacture of hyaluronic acid with Streptococcus equi. Japanese patent 9:3–195924
Duan XJ, Yang L, Zhang X, Tan WS (2008) Effect of oxygen and shear stress on molecular weight of hyaluronic acid produced by Streptococcus zooepidemicus. J Microbiol Biotechnol 18:718–724
Ferrante ND (1956) Turbidity measurement of acid mucopolysaccharides and hyaluronidase activity. J Biol Chem 220:303–306
Goa KL, Benfield P (1994) Hyaluronic acid: a review of its pharmacology and use as a surgical aid in ophthalmology and its therapeutic potential in joint disease and wound healing. Drug 47:536–566
Hasegawa S, Nagatsuru M, Shibutani M, Yamamoto S, Hasebe S (1999) Productivity of concentrated hyaluronic acid using a Maxblend® fermentor. J Biosci Bioeng 99:521–518
Huang WC, Chen SJ, Chen TL (2006) The role of dissolved oxygen and function of agitation in hyaluronic acid fermentation. Biochem Eng J 32:239–243
Huang WC, Chen SJ, Chen TL (2008) Production of hyaluronic acid by repeated batch fermentation. Biochem Eng J 40:460–464
Johns MR, Goh LT, Oeggerli A (1994) Effect of pH, agitation and aeration on hyaluronic-acid production by Streptococcus zooepidemicus. Biotechnol Lett 16:507–512
Khieokhachee T (1994) Kinetic studies of cheese starter cultures. Ph.D. thesis, University of New South Wales
Kim JH, Yoo SJ, Oh DK, Kweon YG (1996) Selection of a Streptococcus equi mutant and optimization of culture conditions for the production of high molecular weight hyaluronic acid. Enzyme Microb Technol 19:440–445
Kim SJ, Park SY, Kim CW (2006) A novel approach to the production of hyaluronic acid by Streptococcus zooepidemicus. J Microbiol Biotechnol 16:1849–1855
Kogan G, Soltes L, Stern R, Gemeiner P (2007) Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett 29:17–25
Liu L, Wang M, Du G, Chen J (2008) Enhanced hyaluronic acid production of Streptococcus zooepidemicus by and intermittent alkaline-stress strategy. Lett Appl Microbiol 46:383–388
Liu L, Wang M, Du G, Chen J (2008) Enhanced hyaluronic acid production by a two-stage culture strategy based on the modeling of batch and fed-batch cultivation of Streptococcus zooepidemicus. Bioresour Technol 99:8532–8536
Liu L, Du G, Chen J, Zhu Y, Wang M, Sun J (2009) Microbial production of low molecular weight hyaluronic acid by adding hydrogen peroxide and ascorbate in batch culture of Streptococcus zooepidemicus. Bioresour Technol 100:362–367
Ogrodowski CS, Hokka CO, Santana MHA (2005) Production of hyaluronic acid by Streptococcus: the effects of the addition of lysozyme and aeration on the formation and the rheological properties of the product. Appl Biochem Biotechnol 5:121–124
O’Regan M, Martini I, Crescenzi F, De Luca C, Lansing M (1994) Molecular mechanisms and genetics of hyaluronan biosynthesis. Int J Biol Macromol 16:283–286
Park MG, Jang JD, Kang WK (1996) Streptococcus zooepidemicus medium and process for preparing hyaluronic acid. US patent 5496726
Rangaswamy V, Jain D (2008) An efficient process for production and purification of hyaluronic acid from Streptococcus equi subsp. zooepidemicus. Biotechnol Lett 30:493–496
Stangohl S (2000) Methods and means for the production of hyaluronic acid. US Patent 6090596
Swann DA, Sukkivan BP, Jamieson G, Richardson KR, Singh T (1990) Biosynthesis of hyaluronic acid. US Patent 4897349
Zhang J, Ding X, Yang L, Kong Z (2006) A serum-free medium for colony growth and hyaluronic acid production by Streptococcus zooepidemicus NJUST01. Appl Microbiol Biotechnol 72:168–172
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Im, JH., Song, JM., Kang, JH. et al. Optimization of medium components for high-molecular-weight hyaluronic acid production by Streptococcus sp. ID9102 via a statistical approach. J Ind Microbiol Biotechnol 36, 1337–1344 (2009). https://doi.org/10.1007/s10295-009-0618-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-009-0618-8