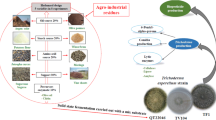
Abstract
During scaling of fermentations, choosing a bioreactor is fundamental to ensure the product's quality. This study aims to produce bioherbicides using Trichoderma koningiopsis fermentation, evaluating process parameters in an Airlift bioreactor. As a response, we quantified the production of enzymes involved in the bioherbicide activity (amylase, cellulase, laccase, lipase, and peroxidase). In addition, it evaluated the agronomic efficiency of the fermented extract optimized through tests that promoted soybean growth and nodulation, soybean seed germination, and in vitro phytopathogen control. As a result of optimizing the scaling bioprocess, it was possible to obtain an adequate fermentation condition, which, when applied to soybean seeds, had beneficial effects on their growth. It allowed the production of an enzyme cocktail. These results add a crucial biotechnological potential factor for the success of the optimized formulation in the Airlift bioreactor, in addition to presenting relevant results for the scientific community.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biotechnological products have always aroused great interest among consumers, scientists, and companies. Within this sector, sales of organic products have increased significantly due to the population's demand for healthier products obtained in a sustainable, ecological, and organic way [1].
The popularization of the use of products of biological origin is a matter of time. The growing demand for this type of product has stimulated the expansion of the agricultural bio-input market, for example. Bio-inputs are more beneficial for soil fertility than chemical-based inputs. In addition to helping in the sustainable management of crops, it is possible to reduce the use of agrochemicals [2].
Fermentation is the main bioprocess involved in obtaining valuable bioproducts. Submerged fermentation is the most promising technology for the recovery of liquid waste; in addition to enabling the development of a fully controlled bioprocess, these characteristics are relevant to study parameters in bioreactors, aiming at expanding the process scale [3].
During the scale-up of fermentations, monitoring pH, dissolved oxygen, temperature, cell concentration, and aeration is essential for a successful scale-up. The choice of the type of bioreactor is also fundamental to ensure the quality of the final product. This will also depend on the microorganism used to produce the expected product. The choices for this study were based on fungal strains [4].
In this sense, submerged fermentation with free cells can be favored by employing mechanical aeration. Airlift bioreactors fulfill this function very well, performing complete aeration inside the reactor by forming a column of bubbles. This is one of the most applied designs in fungal fermentation bioprocesses, as this type of aeration contributes to sporulation [5].
The optimization of obtaining bioproducts via bioreactors can be determined by approaches that consider the type of strain, the sporulation and growth process, the nutritional conditions of the culture medium, and the operating conditions of the bioreactor [4]. In Airlift type bioreactors, the ascending airflow is designed to promote constant circulation of the substrate as it is aerated; this is beneficial for developing filamentous fungi. In addition, they have other advantages, such as low energy consumption, mixing, oxygenation, and heat transfer efficiency [6].
As important as developing strategies for optimizing bioprocesses in a bioreactor, it is essential to study the influence of operating parameters (pH, temperature, dissolved oxygen, air flow, etc.) on the quality of the final product. It is possible to adjust the fermentation conditions according to what is expected, directing the composition of the final product via process optimization. Therefore, finding the optimal fermentation conditions that provide stability, maximize its potential, and minimize environmental damage, allowing these products to reach agricultural producers with high biological activity, is the key to expanding the market of farming bio inputs [5, 7].
Bioherbicides are formulated based on their biologically active compound, which will make the necessary interactions with the target plant, such as weeds [1]. Some fungi can form metabolites during the fermentation process, such as phytohormones, organic acids, alcohols, plant growth regulators, and enzymes (lipases, peroxidases, cellulases, amylases, among others) that can act by degrading plant cell walls [8]. The action of bioherbicides can be directed; more specifically, we can facilitate the formation of enzymes and other metabolites during fermentation, provided the conditions for this available [9,10,11].
In previous studies [9, 11], our research group first optimized the production of composites on a small scale via submerged fermentation using the fungus Trichoderma koningiopsis to control weeds in crops. In addition, we evaluated their phytotoxicity through applications in the E. heterophylla plant, and we determined the acute toxicity of these bioproducts using the microcrustacean Daphnia magna [9]. After that, we evaluate bioherbicide production from the same fungus by a fermentative process in a stirred tank bioreactor, its application on weeds, and determining its genotoxicity potential in model plants [11]. This represents a scale-up, but the bioreactor design was not the most effective for this process, which justifies the research presented here.
Now, this study aims to prospect the parameters of the fermentation process of a fungal extract using microalgal biomass as the substrate from the phytoremediation treatment of wastewater from biogas production in an Airlift bioreactor. At the same time, as a response, we quantified the production of enzymes involved in the bioherbicide activity (amylase, cellulase, laccase, lipase, and peroxidase) in the extracts obtained by different fermentation processes. Furthermore, the agronomic efficiency of the fermented extract optimized in Airlift was evaluated through tests on promoting soybean growth and nodulation, soybean seed germination, and in vitro phytopathogen control.
Material and methods
Microalgae biomass: fermentation substrate
The microalgae used as fermentative substrate belong to the genus Chlorella spp. and come from the phytoremediation treatment of wastewater from biogas production (digestate), implemented at EMBRAPA Swine and Poultry (Concordia, SC, Brazil) [12]. Microalgal biomass comprises 56.1% m/m of protein, 34.7% m/m of carbohydrate, 1.7% m/m of lipid, and 7.8% m/m of minerals [13].
Microbial strain: Trichoderma koningiopsis
The fungus used in this study was the Trichoderma koningiopsis (identification code in GenBank MK860714), which was isolated from the weed Digitaria ciliares and showed promising results for enzyme production and weed control in other studies [9, 14, 15].
Airlift bioreactor operating conditions
Preliminary small-scale fermentation tests were developed and provided subsidies for the conditions tested in scale-up [16]. The fermentations occurred in an Airlift benchtop bioreactor, model Bio-Tec-Pro-II (Tecnal, Brazil) (Fig. 1), with a practical volume of 3.0 L. Five different fermentations were carried out, in which adjustments in the operational parameters were made until an adequate condition for the development of the bioherbicide potential was obtained. The conditions tested can be seen in Table 1.
The fermentations occurred with a final volume of 2.0 L. The medium was composed of 200 g of wet microalgal biomass, aiming to meet the supplementation of a synthetic fermentative medium for bioherbicide production [9], and the remainder of the liquid portion was divided between distilled water, inoculum, and antifoam (see Table 1). Dissolved oxygen was monitored, aiming at its minimum maintenance of 75%. This value was determined to maintain the microorganism for enzymatic production.
During the study in a bioreactor, it was necessary to add antifoam, aiming at its control and prioritizing the preservation of the equipment and the process with minimal interventions. As a control, F1 was performed without inoculum, only with the substrate. In F2, an antifoam called Fermax, provided by the Centro de Tecnologia Canavieira (CTC) was used. In F3, F4, and F5 tests, the antifoam AFP 320 was used from FAXON Química. Table 1 also shows the total amounts of antifoam used in the fermentations, and the values are arranged as follows: Vi + Vf = total. F3, F4, and F5 were performed with the antifoam diluted in the fermentation medium, represented by the initial volume values (Vi). In addition, the antifoam was added during the process (Vf). The bioreactor was autoclaved at 120 °C for 30 min at 1 atm. After sterilization, they were inoculated with 106 spores/mL of Trichoderma koningiopsis suspension. During fermentation, samples (approximately 20 mL) were taken every 24 h for enzymatic verification.
In fermentations F4 and F5, at each sample withdrawal, the same volume of microalgal biomass diluted in distilled water (10% concentration) was added to the bioreactor to evaluate how the process would behave with the reintroduction of the substrate over time.
Fermentations remained until a plateau in enzyme production was verified. The collected samples and the final extract were filtered by manual pressing in synthetic fabric, the solid retained was sterilized and discarded, and the liquid permeate was centrifuged (NT 815-NovaTecnica, Brazil) at 2000 rpm, 4 °C for 30 min. The supernatant from the centrifugation was used for the subsequent steps.
Enzymatic activities
Some enzymes involved in the bioherbicide activity were quantified to evaluate the effects of the different tests (F1, F2, F3, F4, F5) carried out in the bioreactor. The activities of amylase, cellulose, lipase, and peroxidase were quantified using the fermented extract [9, 14,15,16,17,18,19,20,21,22,23,24]. For the determination of the amylase activity, the extract was initially reacted with starch-diluted acetate buffer (100 mM, pH 5.0) at 38 °C for 10 min for subsequent quantification of the total reducing sugars (TRS) at 540 nm in a spectrophotometer (UV-M51, Bel Photonics, Monza, Italy) using the DNS method. Cellulase activity was assayed using the reaction of the extract in acetate buffer (0.2 mM, pH 5.5) at 50 °C for subsequent TRS measurement. Enzymatic activities of amylase and cellulose were calculated based on the glucose standard curve and were expressed as U/mL.
Lipase activity was measured using titration according to the methodology using an emulsion composed of gum Arabic (5%, v/v. Degree of purity P.A. Dinâmica, Brazil) olive oil (10%, v/v. Gallo, Portugal) and phosphate buffer (100 mM, pH 6.0) was prepared, to which the fermented extract was added and maintained at 35 °C, 165 rpm for 32 min. The reaction was stopped with acetone/ethanol solution (1:1, v/v. Degree of purity 99,5%. Dinâmica, Brazil) and titrated to pH 11.0 with sodium hydroxide (NaOH, 0.050 M. Degree of purity 98%. Dinâmica, Brazil). A control containing the extract, emulsion, and acetone/ethanol solution (for non-reaction) was tested for each sample. Lipase activity was calculated using sodium hydroxide's molarity and expressed as U/mL.
To quantify peroxidase enzyme activity, the extract was added to the reaction medium composed of phosphate buffer (5 mM, pH 5.0), guaiacol (1%. Degree of purity ≥ 98,0%, Merck, Germany), hydrogen peroxide (0.08%. Degree of purity 35%, Dinâmica, Brazil) and distilled water, at 35 °C for 10 min. A control sample was tested using water instead of the fermented extract. The transmittance of the oxidized compounds was measured at 470 nm, and the enzymatic activity was estimated by the oxidation reaction of the substrate to tetraguaiacol and was expressed as U/mL.
Fermented extract produced by the fungus (bioherbicide) optimized in airlift: agronomic validation
Trials were carried out to validate the fermented extract’s activity in Airlift against promoting soybean growth and nodulation, soybean seed germination, and in vitro phytopathogen control, aiming to evaluate its agronomic efficiency.
The soybean growth and nodulation promotion trial was conducted in a greenhouse, using a completely randomized design, including four treatments:
-
1.
Control (containing only commercial inoculant based on Bradyrhizobium spp.);
-
2.
100% crude extract (EB100) + commercial inoculant;
-
3.
50% crude extract (EB50) + commercial inoculant;
-
4.
Trichoderma koningiopsis + commercial inoculant.
The 50% crude fermented extract was obtained by mixing the 100% crude extract and water in a 1:1 ratio. Six replications were performed per treatment. It is essential to highlight that the crude extract used in these tests refers to the extract considered optimized during the fermentation optimization study in the Airlift bioreactor.
Soybean seeds from the BMX Lança IPRO cultivar were previously disinfected by immersing them in 70% ethanol for one minute, followed by sodium hypochlorite (2.5% active chlorine) for two minutes, and washed in sterilized distilled water. Sowing was carried out by adding two soybean seeds per vase. Then, the bioproducts (treatments 1–4) were inoculated at 0.5 mL of bioproduct per seed−1 in Leonard-type vases. The vases contained 500 cm3 of a 1:2 (v/v) mixture of sand and sterile vermiculite in the upper part and 350 mL of modified autoclaved nutrient solution without nitrogen in the lower part [25]. Pure water was added daily to replace evapotranspiration, and the nutrient solution was renewed 15 days after sowing.
After 30 days, the number of nodules (NN), dry mass of nodules (DMN), dry roots mass (DRM), and dry mass of aerial part (DMAP) were determined. To determine the mass, the material obtained was dried in an oven with air circulation at 60 ºC until constant weight. Additionally, the nitrogen content in the shoot was determined using the semi-micro Kjedahl method [26]. The nitrogen accumulated in the aerial part was obtained by multiplying the N content with the air mass produced.
Aiming to verify the influence of the extracts on the germination of soybean seeds from the BMX Lança IPRO cultivar, they were treated with the three bioproducts (EB 100, EB 50, and Trichoderma) by adding 50 mL of each bioproduct to 100 seeds, which were subjected to stirring (140 rpm) for 15 min, at 25 ºC. A control treatment consisting of sterilized distilled water was also used. Subsequently, the pre-treated soybean seeds (n = 24) were distributed equidistantly on Germitest paper, applying sterilized water in an amount equivalent to 3 times the weight of the paper. Then, the seeds and Germitest paper were placed in plastic bags to maintain humidity and incubated at 25 ºC in an incubation chamber. The germination percentage was determined every 24 h for four days. Tests were performed in triplicate.
To evaluate the potential of the optimized fermented extract against the control of phytopathogens in vitro, the fungal isolate Rhizoctonia solani (Collection of Microorganisms of Interest for Agriculture of Embrapa Soja, nº 1796) was used as a target, which is known to be a pathogen for several crops of agricultural interest, including soybeans. Petri dishes (90 × 15 mm) were used as an experimental unit for the tests, with five replications per treatment. In addition to the treatments corresponding to seeds treated with bioproducts, the following treatments were tested:
-
a.
Positive control: soybean seeds treated with a commercial product based on Trichoderma asperellum, indicated for controlling the target of interest, as indicated for use by the manufacturing company
-
b.
Negative control: soybean seeds treated only with sterilized distilled water;
-
c.
Control: Petri dishes containing only the pathogen.
To evaluate the biofungicide potential, four soybean seeds treated with bioproducts were added to each plate. With the aid of a template, the central positioning of the seeds was defined concerning the center of the vessel, where a 5 mm diameter disc containing propagules of the target of interest was introduced. The evaluations were carried out when the growth of the target pathogen of the control treatment reached 100% of the plaque. The assessments consisted of determining the average diameter of the target colony (mm) using a digital caliper to determine the percentage of pathogen growth inhibition, according to Eq. 1.
Therefore, CP refers to the average colony diameter of the target pathogen obtained from two perpendicular measurements, and CT refers to the colony diameter of the control treatment.
Statistical analysis
The data were statistically treated by analysis of variance followed by Tukey’s test, considering a significance level of 95% (p < 0.05). Statistica 8.0 software (Statsoft Inc., Tulsa, OK, USA) was used for this. The agronomic validation data were subjected to normality analysis (Shapiro–Wilk) and homogeneity of variances (Bartlett). Considering the assumptions, the data were submitted to ANOVA with a 5% probability in the statistical program SISVAR, version 5.6 (Ferreira, 2011). The SNK mean separation test or regression analysis was carried out if there were significant differences. Graphs were generated with the Software Sigma-Plot v. 12.5 (Systat Corp., San Jose, USA).
Results and discussion
Use of antifoam
Between 72 and 120 h of fermentation, an intensification of foam formation in the medium was observed. This may be linked to the moment of more excellent metabolic activity of the fungus Trichoderma koningiopsis, a period in which the fungus is assimilating nutrients from the medium in its exponential phase, and this fact was also observed in other studies using a stirred tank bioreactor [11].
Foam formation demonstrated stability and persistence, which may indicate the presence of saponins, possibly originating from microalgae, in the fermentation medium. The main characteristic of the existence of saponins is the formation of foam when in contact with water and agitation, which was the situation observed when fermentations occurred in the Airlift bioreactor. Furthermore, as saponins are considered secondary metabolites, they can produce various agro-industrial products, including biopesticides [27,28,29]. Foam can also originate from protein–protein interaction and interaction with other molecules present in the medium, such as enzymes produced during fermentation [30, 31].
Antifoam did not negatively affect fungal development and enzyme production; however, it enabled fermentations to occur in this bioreactor, preserving the equipment and the bioprocess.
Enzymatic activities and airlift bioreactor operating conditions
The enzymatic activity results are represented graphically, as shown in Fig. 2 (A–E). The graphs show each fermentation's enzymatic production over time (F1, F2, F3, F4, and F5). Fermentations continued for 168 h until constancy in enzymatic quantifications was observed.
Results of enzymatic production of amylase (A), cellulase (B), laccase (C), lipase (D), and peroxidase (E), present in fermentations carried out in the Airlift bioreactor under different conditions: F1, F2, F3, F4 e F5.a,b,c Different lowercase letters in the graph bars indicate statistical differences by Tukey’s test, with a confidence level of 95% (p < 0.05). Equal letters do not differ significantly from each other
The highest enzymatic productions were concentrated between 120 and 168 h, reflecting the more excellent metabolic activity of the fungus, observed both by enzymatic activities and foam formation (previously reported). Among the conditions studied, fermentations 4 and 5 deserve to be highlighted in terms of enzymatic production, which can be linked to the reintroduction of the substrate during the fermentation process and also to the maintenance of dissolved oxygen (check Fig. 3). Other studies have already demonstrated that the reintroduction of the substrate increases enzyme production mainly because the bioprocess does not suffer adverse effects from the depletion of nutritional sources [31, 32].
Another relevant parameter for enzyme production was the pH of the fermentations. It is essential to highlight that no adjustment was made during the fermentations; the pH was only monitored to reproduce a fermentation system with minimal operational interventions. The pH of the medium increased with the addition of antifoam, which may be due to the accumulation of protein particles and changes in surface hydrophobicity [32]. Generally, pH values of antifoam vary between 6.5 and 8.3, which corroborates the medium's pH values increasing with antifoam’s addition [33]. Furthermore, other studies that investigated the enzymatic production of Trichoderma koningiopsis using microalgae biomass as substrate found that at pH 8.5, it was possible to obtain an enzymatic pool (amylase, cellulase, lipase, laccase, and peroxidase) and an ideal value of fungal biomass for application as a potential bioherbicide [13] results that corroborate the findings in our study.
Oxygen transfer is an essential operational parameter for process maintenance and enzyme production. As shown in Fig. 3, in fermentation 5 (F5), we could execute a more stable process compared to other fermentations. During F5, dissolved oxygen remained close to 100% throughout the process. We believe that the aeration of 8 (LPM), the reintroduction of a substrate, and the volume of defoamer enabled an optimized system, which benefited an excellent adaptation of the microorganism to the process, also providing a favorable environment for the production of enzymes.
Therefore, fermentation 5 was defined as an optimized condition considering the process's peculiarities, such as microalgal substrate, microorganism, and type of bioreactor. Finally, the optimized condition has the following characteristics: 200 g of wet microalgal biomass, aeration of 8 LPM, temperature 28 ºC, pH 8.3, 400 mL of antifoam diluted in the fermentation medium, and final volume of 2.0 L. The volume of antifoam used during the process may vary.
As in fermentation 5, there were no sudden drops in dissolved oxygen, and this may have been another factor favoring enzymatic production and greater robustness of the process. In contrast, in fermentations with sudden drops in oxygen, it could be that the effect of shear has negatively acted on fungal growth and, consequently, on enzyme production. This behavior probably resulted from the different operational configurations in fermentations, as they caused a change in the hydrodynamic performance of bioprocesses [33,34,35,36].
Therefore, we can associate the production of enzymes with fermentative conditions such as aeration, dissolved oxygen, antifoam, pH, microorganism, and substrate. Different optimization techniques will probably lead to different results. Still, in our study, it is essential to emphasize that the enzymes obtained are of industrial and agronomic interest, demonstrating the biotechnological potential of this study [37,38,39].
Agronomic validation tests with the fermented extract (bioherbicide) optimized in airlift
Figure 4 shows the results of the biometric and nutritional variables of soybeans treated with bioproducts after 30 days of sowing. The treatments had no significant effects on the DRM and DMAP variables nor the N content and N accumulation of soybeans. These tests were enhanced with the extract produced by fermentation 5, which was considered the optimized condition in this study.
Effect of bioproduct application on biometric (DRM and DMAP) and nutritional variables (N content and accumulation) of soybean, 30 days after sowing. Control (treatment containing only commercial inoculant based on Bradyrhizobium spp.; EB100: 100% crude extract + commercial inoculant; EB50: 50% crude extract + commercial inoculant; and Trichoderma: Trichoderma koningiopsis + commercial inoculant. Vertical bars represent the standard error of average (n = 6). There were no significant differences according to the F test (p > 0.05)
Regarding nodulation, it was verified that treatments had no significant effect on the number of soybean root nodules. However, it was found that bioproducts significantly reduced nodules' dry mass (Fig. 5).
Number (A) and dry mass of nodules (B) in soybeans after application of bioproducts. Control (treatment containing only commercial inoculant based on Bradyrhizobium spp.; EB 100: 100% crude extract + commercial inoculant; EB 50: 50% crude extract + commercial inoculant; and Trichoderma: Trichoderma koningiopsis + commercial inoculant. Vertical bars represent the error mean pattern (n = 6). There were no significant differences by the F test (p > 0.05) in Fig. 4A. In Fig. 4B, means followed by the same lowercase letter do not differ from each other by the SNK test (p < 0.05)
In Fig. 4, it is noticeable with the application of EB 100 that there was an increase in the dry mass of the roots and area of the plant, which corroborates the more significant number of nodulations after the application of the extract (Fig. 4). This may result from stimuli caused by the metabolic profile of this extract (microalgae, Trichoderma koningiopsis, enzymes, and other metabolites that may have been excreted), which increased the absorption of nutrients by the plant roots, favoring the growth of the aerial part the plant. This test's higher dry weight values of roots, leaves, and nodulation can observe this fact.
The improvement in nodulation obtained with the application of EB 100 is interesting in the sense that the characteristics of the extract were beneficial in the treatment of soybean seeds. We can understand that the mechanism involved in this context acted as a symbiosis, in which the bioproduct colonized the roots, and together with the decomposition of the soil, the nutrients could be more available for absorption [40].
The EB 100 test accumulated a significant amount of N. This may be linked to the presence of microalgae, the fermentative substrate used to obtain the bioproduct. Microalgae can potentially increase soil porosity and secrete metabolites that assist in this process. Furthermore, microalgae are already known for their potential as biofertilizers [41].
When the bioproduct based on Trichoderma koningiopsis is used, N levels are even higher than the control, which may be linked to the ability of this fungus to fix atmospheric N and act directly on photosynthesis, as well as improving vegetative growth and reproductive and plant development [41, 42].
The influence of nodulation and N fixation directly impacts soybean productivity and the performance of the applied bioproduct. However, performance will be affected by factors such as nutrients, soil pH, and climate. This must be considered for successful use of the product [43].
Table 2 presents the results of the germination of soybean seeds treated with bioproducts over 96 h of incubation. There was a significant effect of incubation time on seed germination, with quadratic regression adjustment. On the other hand, there was no significant effect of seed treatment with the tested bioproducts.
Trichoderma treatment demonstrated a higher germination rate than the fermented extract tests. This may be linked to the phytohormones it produces, which may not be present in the fermented extract, as it went through a fermentative process that may have modified its metabolite profile. Likewise, we believe the seed coat was protected by the applied bioproducts since there was no significant difference between the treatments, and they did not negatively affect germination. On the contrary, they allowed germination more healthily and safely, being able to offer pre-germination treatment [40]. Other studies concluded that some strains of Trichoderma specialized in the rhizosphere when applied, showing positive effects in promoting plant growth and nutrient absorption, increasing seed germination rate, and stimulating plant defense [41, 42].
Although there were no significant benefits for soybean growth and nodulation under the conditions tested, we understand that due to the origin and/or composition of the evaluated bioproducts, they can also act as biofertilizers or soil conditioners, contributing to plant growth in stages most advanced cultures. In this sense, studies involving the soil's physical, chemical, and biological attributes could better evaluate bioproducts' agronomic efficiency.
Figure 6 shows that all bioproducts used in the treatment of soybean seeds showed the ability to inhibit the target pathogen Rhizoctonia solani under in vitro conditions. The Trichoderma-based bioproduct, which presented an inhibition capacity close to 50%, similar to the positive control, deserves to be highlighted, and this presented inhibition approximately three times higher than the other bioproducts evaluated (EB 100 and EB 50).
In vitro antagonism test of soybean seeds treated with bioproducts on the growth of the target pathogen Rhizoctonia solani. Control (seeds treated with sterile distilled water); EB 100 (crude extract 100%); EB 50 (crude extract 50%); Trichoderma (Trichoderma koningiopsis). Vertical bars represent the standard error of the mean (n = 5). Means followed by the same letter do not differ by the SNK test (p < 0.05). Illustration of the antagonism potential of bioproducts on the target pathogen in vitro in the inferior part
In this line of investigation, the potential of bioproducts to act in the biological control of pathogens was highlighted, with emphasis on Trichoderma koningiopsis, which presented an ability to antagonize Rhizoctonia solani, similar to a commercial product registered for soybean cultivation. This phenomenon can be explained by the mycoparasitic potential of the genus Trichoderma, which occurs mainly through the secretion of cell wall-degrading enzymes, followed by the target fungus's penetration and inhibition or death. Other studies have already highlighted the potential of Trichoderma spp. for the biocontrol of R. solani, in which the metabolites provided by Trichoderma from different species formed a protective shield against rot, a disease caused by R. solani that can strongly affect crops of economic importance such as beans and soy [44].
Conclusion
The results obtained in this study expand our knowledge about the beneficial effects of bioherbicide production using Airlift bioreactors. Likewise, this study provides insights into how the type of bioreactor and the bioprocess's operational mode impact the bioproduct's productivity and quality.
Therefore, we believe that the bioproducts tested can control other plant pathogens of agronomic importance, the results of which can facilitate and encourage their introduction into the national bio-input market.
So, this study adds a crucial biotechnological potential factor for the success of the optimized formulation in the Airlift bioreactor and presents relevant results for the scientific community.
Availability of data and materials
The datasets generated for this study are available to the corresponding author on request.
References
Hasan M, Ahmad-Hamdani MS, Rosli AM, Hamdan H (2021) Bioherbicides: an eco-friendly tool for sustainable weed management. Plants 10:1212. https://doi.org/10.3390/plants10061212
Mohite BV, Koli SH, Borase HP et al (2019) New age agricultural bio-inputs. Microbial Interventions in Agriculture and Environment. Springer Singapore, Singapore, pp 353–380
Dutra TR, Guimarães VM, Varela EM et al (2023) A new approach for Chrysoporthe cubensis cellulolytic cocktail production using solid and submerged-state fermentation. Braz J Chem Eng. https://doi.org/10.1007/s43153-023-00309-y
Gupta VKZSSHBDI (2020) New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier, Amsterdam
Klaic R, Kuhn RC, Foletto EL et al (2015) An overview regarding bioherbicide and their production methods by fermentation. Fungal Biomolecules. John Wiley Sons, Ltd, Chichester, pp 183–199
Ferreira A, Rocha F, Mota A, Teixeira JA (2017) Characterization of industrial bioreactors (mixing, heat, and mass transfer). Current Developments in Biotechnology and Bioengineering. Elsevier, Amsterdam, pp 563–592
Harding DP, Raizada MN (2015) Controlling weeds with fungi, bacteria and viruses: a review. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00659
Adetunji CO, Oloke JK, Bello OM et al (2019) Isolation, structural elucidation and bioherbicidal activity of an eco-friendly bioactive 2-(hydroxymethyl) phenol, from Pseudomonas aeruginosa (C1501) and its ecotoxicological evaluation on soil. Environ Technol Innov 13:304–317. https://doi.org/10.1016/j.eti.2018.12.006
Bordin ER, Frumi Camargo A, Rossetto V et al (2018) Non-toxic bioherbicides obtained from trichoderma koningiopsis can be applied to the control of weeds in agriculture crops. Ind Biotechnol 14:157–163. https://doi.org/10.1089/ind.2018.0007
R Radhakrishnan AA Alqarawi EF Abd_Allah (2018) Bioherbicides: Current knowledge on weed control mechanism. Ecotoxicol Environ Saf 158:131–138. https://doi.org/10.1016/j.ecoenv.2018.04.018
Frumi Camargo A, Venturin B, Bordin ER et al (2020) A low-genotoxicity bioherbicide obtained from trichoderma koningiopsis fermentation in a stirred-tank bioreactor. Ind Biotechnol 16:176–181. https://doi.org/10.1089/ind.2019.0024
Michelon W, Da Silva MLB, Mezzari MP et al (2016) Effects of nitrogen and phosphorus on biochemical composition of microalgae polyculture harvested from phycoremediation of piggery wastewater digestate. Appl Biochem Biotechnol 178:1407–1419. https://doi.org/10.1007/s12010-015-1955-x
Stefanski FS, Camargo AF, Scapini T et al (2020) Potential use of biological herbicides in a circular economy context a sustainable approach. Front Sustain Food Syst. https://doi.org/10.3389/fsufs.2020.521102
Camargo AF, Stefanski FS, Scapini T et al (2019) Resistant weeds were controlled by the combined use of herbicides and bioherbicides. Environ Qual Manage 29:37–42. https://doi.org/10.1002/tqem.21643
Reichert Júnior FW, Scariot MA, Forte CT et al (2019) New perspectives for weeds control using autochthonous fungi with selective bioherbicide potential. Heliyon 5:e01676. https://doi.org/10.1016/j.heliyon.2019.e01676
Camargo AF, Dalastra C, Ulrich A et al (2023) The bioherbicidal potential of isolated fungi cultivated in microalgal biomass. Bioprocess Biosyst Eng 46:665–679. https://doi.org/10.1007/s00449-023-02852-x
Fuwa H (1954) A new method for microdetermination of amylase activity by the use of amylose as the substrate. J Biochem 41:583–603. https://doi.org/10.1093/oxfordjournals.jbchem.a126476
Pongsawadi P, Yagisawa M (1987) Screening and identification of a cyclomaltoxtrinv glucanotransferase-producing bacteria. J Ferment Technol 65:463–467. https://doi.org/10.1016/0385-6380(87)90144-0
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. https://doi.org/10.1351/pac198759020257
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Hou H, Zhou J, Wang J et al (2004) Enhancement of laccase production by pleurotus ostreatus and its use for the decolorization of anthraquinone dye. Process Biochem 39:1415–1419. https://doi.org/10.1016/S0032-9592(03)00267-X
Treichel H, Sbardelotto M, Venturin B et al (2017) Lipase production from a newly isolated aspergillus niger by solid state fermentation using canola cake as substrate. Curr Biotechnol. https://doi.org/10.2174/2211550105666151124193225
Khan AA, Robinson DS (1994) Hydrogen donor specificity of mango isoperoxidases. Food Chem 49:407–410. https://doi.org/10.1016/0308-8146(94)90013-2
Devaiah SP, Shetty HS (2009) Purification of an infection-related acidic peroxidase from pearl millet seedlings. Pestic Biochem Physiol 94:119–126. https://doi.org/10.1016/j.pestbp.2009.04.010
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. In: Arnon DI (ed) California Agricultural Experiment Station, 3rd edn. The College Of Agricultureuniversity Of California, Berkeley
Tedesco MJ, Gianello C, Bissani CA et al (1995) Análise de solo, plantas e outros materiais (boletim técnico de Solos, 5), 2nd edn. Departamento de Solos da Universidade Federal do Rio Grande do Sul, Porto Alegre
Bonilla H, Carbajal Y, Gonzales M et al (2019) Determination of the insecticide activity of the saponine of the quinua (chenopodium quinoa) in larvas of drosophila melanogaster. Sci Agrop 10:39–45. https://doi.org/10.17268/sci.agropecu.2019.01.04
Vélez Salazar YH, Velásquez Flórez MA (2020) Diseno conceptual de una planta de extracci´on de saponinas presentes en el jugo de fique. Ingen 25:50–67. https://doi.org/10.14483/23448393.15298
Sharma S, Kataria A, Singh B (2022) Effect of thermal processing on the bioactive compounds, antioxidative, antinutritional and functional characteristics of quinoa (chenopodium quinoa). LWT 160:113256. https://doi.org/10.1016/j.lwt.2022.113256
Singhania RR, Patel AK, Tseng Y-S et al (2022) Developments in bioprocess for bacterial cellulose production. Bioresour Technol 344:126343. https://doi.org/10.1016/j.biortech.2021.126343
Dineshkumar R, Sen R (2020) A sustainable perspective of microalgal biorefinery for co-production and recovery of high-value carotenoid and biofuel with CO2 valorization. Biofuels Bioprod Biorefin 14:879–897. https://doi.org/10.1002/bbb.2107
Zhan F, Zhou X, Jiang Y et al (2023) From an oil with “antifoaming” properties to stabilization for foam: a novel approach for establishing a long-term stable foam system. Food Hydrocoll 145:109086. https://doi.org/10.1016/j.foodhyd.2023.109086
Santos TP, Cunha RL (2020) Designing biotechnological processes to reduce emulsions formation and improve oil recovery: study of antifoams application. Biochem Eng J 163:107745. https://doi.org/10.1016/j.bej.2020.107745
Banks M, Johnson R, Giver L et al (2022) Industrial production of microbial protein products. Curr Opin Biotechnol 75:102707. https://doi.org/10.1016/j.copbio.2022.102707
Wang X-T, Wen Z-N, Luo Y et al (2021) Oxygen mass transfer intensification in an inner-loop rotor-stator reactor: production of sodium gluconate as an example. Chem Eng Process -Process Intensif 160:108290. https://doi.org/10.1016/j.cep.2020.108290
Aragão MS, Menezes DB, Ramos LC et al (2020) Mycoremediation of vinasse by surface response methodology and preliminary studies in airlift bioreactors. Chemosphere 244:125432. https://doi.org/10.1016/j.chemosphere.2019.125432
Camargo AF, Bonatto C, Scapini T et al (2023) Fungus-based bioherbicides on circular economy. Bioprocess Biosyst Eng. https://doi.org/10.1007/s00449-023-02926-w
Reichert Júnior FW, Mulinari J, Camargo AF et al (2023) Trichoderma sp: bioprodutcs and their main uses in agriculture. In: Michaels M (ed) Trichoderma: Taxonomy, Biodiversity and Applications. Nova Science Publishers Inc, Hauppauge
Tarafdar A, Sirohi R, Gaur VK et al (2021) Engineering interventions in enzyme production: Lab to industrial scale. Bioresour Technol 326:124771. https://doi.org/10.1016/j.biortech.2021.124771
Macena AMF, Kobori NN, Mascarin GM et al (2020) Antagonism of trichoderma-based biofungicides against Brazilian and north American isolates of sclerotinia sclerotiorum and growth promotion of soybean. Biocontrol 65:235–246. https://doi.org/10.1007/s10526-019-09976-8
Kumar M, Poonam AS, Singh RP (2022) Plant growth promoting microbes: diverse roles for sustainable and ecofriendly agriculture. Energy Nexus 7:100133. https://doi.org/10.1016/j.nexus.2022.100133
Reichert Júnior FW, Chiomento JLT, Tortelli B et al (2023) Trichoderma uses in agriculture: a multiprurpose tool for biological control and plant growth. In: Mouton MS (ed) Trichoderma: Taxonomy, Biodiversity and Applications. Nova Science Publishers Inc, Hauppauge
Nakei MD, Venkataramana PB, Ndakidemi PA (2022) Soybean-nodulating rhizobia: ecology, characterization, diversity, and growth promoting functions. Front Sustain Food Syst. https://doi.org/10.3389/fsufs.2022.824444
Dutta P, Mahanta M, Singh SB et al (2023) Molecular interaction between plants and Trichoderma species against soil-borne plant pathogens. Front Plant Sci. https://doi.org/10.3389/fpls.2023.1145715
Acknowledgements
The authors thank the Brazilian Funding Agencies: Brazilian National Council for Scientific and Technological Development (CNPq-302484/2022-1), Coordination of the Superior Level Staff Improvement (CAPES), the support of the Bioprocess and Biotechnology for Food Research Center (Biofood), which is funded through the Research Support Foundation of Rio Grande do Sul (FAPERGS-22/2551-0000397-4), Federal University of Fronteira Sul (UFFS) and Federal University of Santa Catarina (UFSC) for the financial support.
Funding
CAPES, CNPq and FAPERGS.
Author information
Authors and Affiliations
Contributions
Aline Frumi Camargo, Gislaine Fongaro, and Helen Treichel conceived and designed the study. Aline Frumi Camargo analyzed the data and drafted the manuscript. Charline Bonatto, Suzana Fátima Bazoti, Simone Kubeneck, Júlia Pieper Nerling, Gabriel Henrique Klein, and William Michelon helped with writing and carefully revised the manuscript. Aline Frumi Camargo, Sérgio L. Alves Jr., Altemir José Mossi, Gislaine Fongaro, and Helen Treichel critically reviewed and supervised the development of the paper. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Consent for publication
All authors agreed with this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Camargo, A.F., Kubeneck, S., Bonatto, C. et al. Trichoderma koningiopsis fermentation in airlift bioreactor for bioherbicide production. Bioprocess Biosyst Eng 47, 651–663 (2024). https://doi.org/10.1007/s00449-024-02991-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-024-02991-9