Abstract
The lignocellulosic material bioconversion to bioproducts has received significant attention in recent years. Cellulases and hemicellulases catalyze the hydrolysis of lignocellulosic materials into fermentable sugars that are afterward converted to bioproducts by microorganisms. Chrysoporthe cubensis grown under solid-state fermentation (SSF) has produced more effective enzymatic extracts for sugarcane bagasse saccharification than commercial cellulolytic preparations. However, the investigation of new approaches for enzyme production by this fungus is still lacking. In this work, an enzyme cocktail (SSF-SmF-cocktail) was produced by extracting enzymes of C. cubensis grown under SSF using the extract produced by the same fungus under submerged fermentation (SmF). The total cellulase (FPase), carboxymethylcellulase (CMCase), cellobiohydrolase (CBH), β-glucosidase, xylanase, β-xylosidase, β-galactosidase, α-galactosidase, and α-arabinofuranosidase activities were evaluated in crude extracts obtained from C. cubensis cultivation under SSF, SmF, and also in the SSF-SmF-cocktail. The C. cubensis protein profiles cultivated under SSF and SmF were compared by SDS-PAGE. Extract produced by C. cubensis grown under SmF presented proteins with estimated molecular weights of 10.7, 29.3, 38.6, 46.0, and 170.0 kDa, respectively, but not in that produced by this fungus under SSF. When cultivated under SSF, C. cubensis produced an extract with greater protein diversity between 13 and 51 kDa than that obtained by this fungus under SmF. The 83.0 and 95.3 kDa protein bands were present in both C. cubensis cultures. The C. cubensis SSF-SmF-cocktail presented better efficiency in glucose release after 48 h of the alkali-pretreated sugarcane bagasse hydrolysis when compared to those produced by this fungus under either SSF or SmF. This extract showed the highest xylananase/FPase rate and the second highest CMCase/FPase and b-glucosidase/FPase rates among the evaluated extracts, suggesting that these enzymes are the main determinants of this cocktail the efficiency on the alkali pretreated sugarcane bagasse sacchariffication process. These results demonstrated that the enzymes produced by C. cubensis cultivated under SSF and SmF are complementary for the alkali-pretreated sugarcane bagasse enzymatic hydrolysis, since the SSF-SmF-cocktail was more efficient than other extracts produced by this fungus and that the commercial Accellerase®. Therefore, the SSF-SmF-cocktail is a promising alternative for industrial applications.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulases and hemicellulases are the main enzymes in the degradation of lignocellulosic polysaccharides to simple monomeric sugars, which are then converted to biofuels or other value-added products through microbial fermentation processes (Zhang et al. 2011).
Cellulose is degraded by the synergistic action of three types of enzymes: exoglucanase or cellobiohydrolase (EC 3.2.1.91), endoglucanase (EC 3.2.1.4) and β-glucosidase (EC 3.2.1.21) (Champreda et al. 2019; Olsen et al. 2016). The hemicellulose fraction, a more complex polymer, requires a more diverse enzyme group, such as β-1,4-endo-xylanase (EC 3.2.1.8), β-xylosidase (EC 3.2.1.37), and auxiliary enzymes, such as α-arabinofuranosidase (EC 3.2. 1:55), α-glucuronidase (EC 3.2.1.139), α-galactosidase (EC 3.2.1.22), acetylxylan esterase (EC 3.1.1.72) and ferulic acid esterase (Visser et al. 2013).
Since enzymatic saccharification is one of the most important bottlenecks to lignocellulosic bioconversion technologies consolidation, new approaches have been made to produce low-cost and efficient cellulolytic preparations (Vicari et al. 2012).
Solid-state fermentation (SSF) and submerged fermentation (SmF) are the main approaches for cellulase production by microorganisms. In the SSF, there is an absence or near absence of free water, while the SmF is carried out in an aqueous medium (Barrios-González 2012).
SSF presents advantages such as yielding concentrated products with high stability, lower contamination risks, and lower costs than SmF (Holker et al. 2004; de Almeida et al. 2013). Moreover, the production of enzymes in the SSF currently gains more attention due to its advantages such as higher volumetric productivity, less water usage, and the use of natural products, such as agro-industrial wastes (Mendonça et al. 2020).
SmF is mainly used in large-scale processes, where bioreactors are required (Fanaei and Vaziri 2009). The industrial cellulase production is mainly done under SmF conditions (Subramaniyam and Vimala 2004). Fungi cultivation under SSF presents some disadvantages including the build-up of gradients of temperature, pH, moisture, substrate concentration or pO2 during cultivation, which are difficult to control under limited water availability (Hölker et al. 2004).
The ascomycete fungus, Eucalyptus pathogen, Chrysoporthe cubensis grown under SSF conditions produced more efficient enzymatic extracts for sugarcane bagasse saccharification than commercial cellulolytic preparations as Multifect CL®, Multifect XL® and Accellerase® (Falkoski et al. 2013; Maitan-Alfenas et al. 2015; Dutra et al. 2017; de Albuquerque et al. 2021; Tavares et al. 2021). However, little is known about the hydrolytic capacity of the extracts produced by this fungus under submerged-state fermentation or by the extracts mixtures of SSF and SmF C. cubensis secreted enzymes.
In this work, cellulolytic cocktails were produced by C. cubensis cultivated under SSF and under SmF. Besides that, it was produced an enzyme cocktail by the extraction of the enzymes produced by C. cubensis under SSF cultivation with the extract produced by the same fungus under SmF condition (SSF-SmF-Cocktail). The C. cubensis cocktails efficiency was evaluated for the alkali-pretreated sugarcane bagasse hydrolysis capacity.
Methods
Microorganism
The fungus C. cubensis LPF-1 used in this study was obtained from the mycological collection of the Forest Pathology Laboratory of the Universidade Federal de Viçosa in Viçosa, Minas Gerais State, Brazil. The fungus was maintained on PDA (potato dextrose agar) plates at 28 °C and subcultured every 15 days.
Chrysoporthe cubensis enzyme production
All undermentioned extracts were produced with the same final volume (50 mL) for comparison in terms of enzymatic activity per volume (U mL−1). Wheat bran was used as the sole carbon source for cultivation conditions, since it is described as a strong inducer of lignocellulolytic enzyme production by C. cubensis (Falkoski et al. 2013; Maitan-Alfenas et al. 2015).
Submerged fermentation (SmF)
The C. cubensis cultivation under SmF was conducted in 125 mL Erlenmeyer flasks with 50 mL of culture medium composed of (g L−1): (NH4)2SO4, 1.4; urea, 0.3; KH2PO4, 2.0; MgSO4 7H2O, 0.3; CaCl2, 0.3; and yeast extract, 2.0. The wheat bran was added to the medium at the final concentration of 10 g L−1. The trace elements FeSO4 7H2O (1.0 mg L−1), ZnCl2 (3.5 mg L−1), MnSO4 H2O (1.0 mg L−1), CoCl 6H2O (1.0 mg L−1), CuSO45H2O (0.5 mg L−1) and 20MoO3 2H3PO4 48H2O (0.02 mg L−1) were also added. The flasks were autoclaved at 120 °C for 20 min, inoculated with 0.5 mL of a spore suspension (2.2 × 106 spores mL−1), and placed in a shaker for seven days at 180 rpm, and 28 °C. The samples were centrifuged at 10,000×g for 20 min and the supernatants were used as enzyme extracts.
Inoculum preparation for solid-state fermentation (SSF)
The inoculum was prepared by growing the fungus under SmF in 250 mL Erlenmeyer flasks containing 100 mL of medium with the following composition, in g L−1: glucose, 10.0; NH4NO3, 1.0; KH2PO4, 1.0; MgSO4, 0.5 and yeast extract, 2.0. Each flask was inoculated with 1.0 mL agar plugs cut from a 5-day-old colony of C. cubensis grown on PDA plates and incubated in a rotary shaker for 5 days, at 150 rpm and 28 °C. The culture obtained was used to inoculate the solid culture media.
Solid-state fermentation (SSF)
Chrysoporthe cubensis was cultured under SSF using wheat bran as the main carbon source. The fermentations were carried out in 125 mL Erlenmeyer flasks containing 5 g (dry weight) of the wheat bran moistened with culture media (final moisture of 60%), presenting the following composition, in g L−1: NH4NO3, 1.0; KH2PO4, 1.5; MgSO4, 0.5; CuSO4, 0.25 and yeast extract, 2. Furthermore, MnCl2 (0.1 mg L−1), H3BO3 (0.075 mg L−1), Na2MoO4 (0.02 mg L−1), FeCl3 (1.0 mg L−1) and ZnSO4 (3.5 mg L−1) also were added to the medium as trace elements. The flasks were autoclaved at 120 °C for 20 min and then inoculated with 3 mL (containing 1.5 × 107 spores mL−1) of the inoculum obtained as aforementioned. The flasks were maintained at 28 °C in a temperature-controlled chamber, and the enzymatic extraction was performed after seven days of fermentation. Enzymes secreted during SSF were extracted with sodium acetate buffer, 50 mM, pH 5, at a ratio of 10 mL from buffer to 1 g dry substrate, under the agitation of 150 rpm for 60 min at room temperature. Solids were separated by filtration through a nylon cloth followed by centrifugation at 15,000g for 10 min, and the clarified supernatants were frozen and stored for subsequent enzymatic analysis. Experiments were carried out with three replicates for each medium composition and each incubation time.
Chrysoporthe cubensis SSF-Smf-Cocktail production
For C. cubensis SSF-SmF-cocktail production this fungus was cultivated under solid state fermentation (SSF), as aforementioned, and the enzyme extraction was carried out using the the C. cubensis SmF extract (produced as previously described) instead of buffer solution at a ratio of 10 mL from the extract to 1 g dry substrate, under the agitation of 150 rpm for 60 min at room temperature. Solids were separated by filtration through a nylon cloth followed by centrifugation at 15,000g for 10 min, and the clarified supernatants were frozen and stored for subsequent enzymatic analysis. Experiments were carried out with three replicates for each medium composition and each incubation time.
Enzymatic assays
All enzymatic assays were carried out in sodium acetate buffer, 100 mM, pH 5, at 50 °C in triplicate, and the mean values were calculated. Relative standard deviations of measurements were below 5%. FPase and endoglucanase activities were determined using Whatman No. 1 filter paper and carboxymethylcellulose as substrates, respectively (Ghose 1987). The total reducing sugar liberated during the enzymatic assays was quantified by the dinitrosalicylic acid (DNS) method (Miller 1959) using glucose as a standard. Xylanase activity was determined using beechwood xylan (final concentration of 1% w/v) as substrate. The enzymatic reactions were initiated with the addition of 100 μL of enzyme extract diluted to 400 μL substrate solution with the polysaccharide prepared in a buffer. The reaction mixtures were incubated for 30 min and the amount of reducing sugars released was determined by the DNS method using glucose as standard. Cellobiohydrolase, β-galactosidase, β-glucosidase, β-xylosidase, α-galactosidase, and arabinofuranosidase activities were measured using 4-nitrophenyl β-d-cellobioside, 4-nitrophenyl β-d-galactopyranoside, 4-nitrophenyl β-d-glucopyranoside, 4-nitrophenyl β-d-xylopyranoside, 4-nitrophenyl α-d-galactopyranoside and 4-nitrophenyl α-l-arabinofuranoside as substrates, respectively. One enzyme activity unit (U) was defined as the amount of enzyme which released a μmol of the product (equivalent glucose and 4-nitrophenol) per minute under assay conditions used for all activities.
Protein quantification
Protein concentration in the enzymatic extracts was determined by the Coomassie Blue binding method using bovine serum albumin as the standard (Bradford 1976).
Electrophoresis
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) was performed using a 12% (w/v) polyacrylamide gel with a 5% stacking gel and the Mini-Protean II system (BioRad) according to the method previously described (Laemmli 1970), with some modifications. The gel obtained on SDS-PAGE was stained with G-250 colloidal Coomassie blue according to the method described (Dyballa and Metzger 2009). The molecular mass of the proteins was determined by linear regression obtained by correlating the logarithm of the molecular weight marker with the distance covered in the SDS-PAGE. The molecular weight markers were purchased from GE Healthcare Life Sciences.
Sugarcane bagasse alkaline pretreatment and saccharification
The ground sugarcane bagasse, particle size less than 1 mm, was submitted to alkaline pretreatment with NaOH 1% (w/v) solution (Dutra et al. 2017).
The enzymatic cocktails produced by C. cubensis and commercial Accellerase® 1500 purchased from Dupont/Genencor International Inc. (Rochester, NY, USA) were applied in a biomass saccharification experiment. The C. cubensis enzymatic cocktails were concentrated fivefold before the experiment using an Amicon Ultra-filtration system (Millipore Co. – Billerica, MA, USA) and a YM-10 (Cut-off Mr 10,000 Da) membrane filter. Enzymatic saccharification of alkali-treated sugarcane bagasse was performed in 25 mL Erlenmeyer flasks at an initial solid concentration of 2% dry matter (w/v) in 5.0 mL of 50 mM sodium acetate buffer at pH 4.5. Enzyme loading was specified as 10 FPase units per gram of biomass with the addition of sodium azide (10 mM) and tetracycline (40 μg mL−1) to the reaction mixture to inhibit microbial contamination. The reaction was carried out in an orbital shaker at 250 rpm and 50 °C for different time intervals up to 72 h. These samples were immediately heated to 100 °C to denature the enzymes, cooled, and then centrifuged for 5 min at 15,000 g. Products of the saccharification assays were analyzed by High-Performance Liquid Chromatography (HPLC) with a Shimadzu series 10 A chromatography. The HPLC was equipped with an Aminex HPX-87P column (300 × 7.8 mm) and refractive index detectors. The column was eluted with water at a flow rate of 0.6 mL min−1 and 80 °C.
Statistical analysis
The values of C. cubensis activities on different condition of cultivation were analyzed using Assistat 7.7 software, performing analysis of variance (ANOVA) followed by Tukey’s test at a significance level of 5% (α = 0.05). The standard deviation was also calculated for all assays.
Results and discussion
Electrophoretic profiles from C. cubensis extracts produced under SSF and SmF
SDS-PAGE showed different protein profiles for C. cubensis extracts produced under SSF and SmF (Fig. 1). Extract produced by C. cubensis grown under SmF presented proteins with estimated molecular weights of 10.7, 29.3, 38.6, 46.0 and 170.0 kDa, respectively, but not under SSF. Extract produced by this fungus cultured under SSF showed greater protein diversity between 13 and 51 kDa than under SmF. The differential protein secretion by C. cubensis under SSF and SmF agreed with reports from Aspergillus terreus CM20 and Aspergillus niger showing differentiated electrophoretic proteins patterns secreted for SSF and SmF cultivation (Venegas et al. 2013; Saritha et al. 2016; Acuña-Argüelles et al. 1995). Aspergillus terreus CM20 secreted multiple isoforms of endo-β-glucanase, β-glucosidase, and xylanase under SSF, but not under SmF, which was demonstrated by zymogram analysis (Saritha et al. 2016). SSF cultivation provides higher oxygenation and lower sugar supply than SmF cultivation, promoting morphological and physiological differences leading to differential protein secretion by fungi (Viniegra-González et al. 2003), justifying the results obtained for C. cubensis.
Chrysoporthe cubensis secreting 170.0 and 155.1 kDa proteins under SmF and SSF, respectively, agreed with the report of this fungus secreting some high molecular weight proteins (from 120 to 200 kDa), although the most predicted carbohydrate-active enzymes showed molecular weights from 15 to 120 kDa (Tavares et al., 2020). The 83.0 and 95.3 kDa protein bands were observed for both C. cubensis cultures. The production of 83.0 kDa protein by Chrysoporthe cubensis agreed with the report of this fungus secreting a glycosidase (85 kDa) able to improving sugarcane bagasse saccharification when added in cellulolytic cocktails as a supplement (Andrade et al. 2017).
Enzymatic production by C. cubensis
The enzymatic cocktail obtained by extracting the C. cubensis enzymes produced under SSF with the extract produced by the same fungus under SmF (SSF-SmF-Cocktail) showed the highest activity of FPase, endoglucanase, cellobiohydrolase, xylanase, and β-xylosidase, respectively (Table 1). This result was expected since this cocktail is composed of C. cubensis enzymes produced under both conditions. SSF enzyme extraction with the extract obtained under SmF has been described as a strategy for more concentrated enzymatic cocktail production than those obtained by simple culture under SSF or SmF (Visser et al 2013). It is expected that cocktails produced by this approach will be much more complete than those obtained under SSF or SmF, since they contain enzymes secreted under the two cultivation conditions (Visser et al. 2015). This is interesting for lignocellulosic biomass saccharification, which requires high hydrolases diversity for an efficient hydrolysis process (Dutra et al. 2017).
Chrysoporthe cubensis cultivated under SSF produced higher β-glucosidase and cellobiohydrolase activities (1.30 and 0.26 U mL−1, respectively) than those obtained by SmF (0.13 and 0.11 U mL−1, respectively). The extract produced by this fungus under SmF showed higher FPase, endoglucanase, and β-galactosidase activities under SmF (0.13, 4.84, and 0.22 U mL−1, respectively) than those obtained under SSF (0.12, 3.44 U mL-1 and not detected, respectively). These results are in agreement with those reported for the mutant Penicillium janthinellum NCIM 1171 that differentially secreted cellulolytic enzymes under SSF and SmF. Penicillium janthinellum NCIM 1171 secreted two β-glucosidases isoforms under SmF, but only one isoform under SSF, with wheat bran and Avicel as carbon sources (Singhvi et al. 2011).
Chrysoporthe cubensis enzyme profile and enzymatic saccharification
Enzymatic extracts produced by C. cubensis under SSF or SmF, and also the SSF-SmF-cocktail, were applied in the alkali-pretreated sugarcane bagasse saccharification process and compared with a commercial enzymatic cocktail.
To compare the extracts applied in saccharification assays, the enzymatic activities were normalized relative to FPase activity, which is the total cellulase activity of the enzymatic complexes (Table 2). The CMCase/FPase, β-glucosidase/FPase, cellobiohydrolase/FPase, xylanase/FPase, β-xylosidase/FPase, β-galactosidase/FPase, β-galactosidase/FPase, and β-arabinofuranosidase / FPase ratios of C. cubensis extracts produced under SSF or SmF, and in SSF-SmF-cocktail were higher than those found for the commercial extract Accellerase®. Chrysoporthe cubensis extract produced under SSF and the SSF-SmF-cocktail showed higher β-glucosidase/FPase ratios (10.85 and 6.07, respectively) than the extract produced by this fungus under SmF and the commercial extract (1.02 and 1.23, respectively). The highest CMCase/FPase and β-galactosidase/FPase ratios were observed in C. cubensis extract cultivated under SmF (37.20 and 1.73, respectively), whereas the highest ratios of β-glucosidase/FPase, cellobiohydrolase/FPase, α-galactosidase/FPase, and α-arabinofuranosidase/FPase were obtained for the extract produced by this fungus under SSF (10.85, 2.21, 0.48 and 0.08, respectively). The C. cubensis SSF-SmF-cocktail showed the highest xylanase/PFase rate (144.17). These results agree with C. cubensis previous reports, which also showed that this fungus produces much more complete cellulolytic extracts than many commercials preparations (Maitan-Alfenas et al. 2015; Dutra et al. 2017).
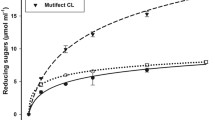
The C. cubensis SSF-SmF-cocktail released more glucose after 48 h of alkali-pretreated sugarcane bagasse saccharification (17.8 g L−1) than those produced by this fungus under SSF or SmF, and the commercial extract Accellerase®, 15.1, 12.7, and 11.6 g L−1, respectively (Fig. 2). These results demonstrated that the enzymes produced by this fungus under SSF and SmF complement themselves for a better alkali-pretreated sugarcane bagasse saccharification process. It can be justified by the fact of the sugarcane bagasse complexity, with cellulose, lignin, and hemicellulose in its composition, requiring a much more complete enzymatic cocktail for its efficient hydrolysis (Dutra et al. 2017). The low glucose release ratios in the alkali-pretreated sugarcane bagasse hydrolysis by the extract produced by C. cubensis under SmF and the commercial cocktail can be explained by the lower β-glucosidase/FPase ratios of these extracts compared to those found in the extract produced by this fungus under SSF and in the SSF-SmF-cocktail, respectively. Another factor that may justify the lower release of glucose by these extracts is their lower efficiency to hydrolyze the hemicellulosic sugarcane bagasse fraction, a barrier to the action of cellulases in the hydrolysis of cellulose observed by low levels of xylose release (Fig. 2).
Glucose and xylose production after 48 h of alkali pretreated sugarcane bagasse enzymatic saccharification using extracts produced by Chrysoporthe cubensis under solid-state fermentation (SSF) and submerged fermentation (SmF), cocktail produced by extracting of the enzymes from C. cubensis culture under SSF with the extract produced by this fungus under SmF (SSF-SmF-Cocktail) and the commercial extract Accellerase®
Another crucial factor for the higher efficiency of the C. cubensis extract produced under SSF and the SmF-SSF-cocktail in the glucose release is the higher xylanase/FPase ratio in comparison to the extract produced by this fungus under SmF and the commercial extract. Xylanases catalyze xylans hydrolysis facilitating the cellulases access to cellulose (Hu et al. 2011).
The C. cubensis extract produced under SSF released more xylose (14.0 g L−1) in alkali-pretreated sugarcane bagasse saccharification than the extract produced by this fungus under SmF, the SSF-SmF-cocktail, and the commercial extract, 12.0, 4.0, and 2.4 g L−1, respectively (Fig. 2). The higher xylose release from alkali-pretreated sugarcane bagasse saccharification by the C. cubensis extract produced under SSF can be justified by its higher β-xylosidase/FPase ratio compared to those obtained for the extract produced by this fungus under SmF, the SSF-SmF-cocktail, and the commercial extract, respectively, since β-xylosidases hydrolyze xylobiose and xylotriose to xylose (Inoue et al. 2016). The Chrysoporthe cubensis extract produced under SSF would be indicated when xylose is desired as a final product, as in the case of the production of xylitol from sugarcane bagasse (Rao et al. 2006; Hernández-Pérez et al. 2016).
The C. cubensis SSF-SmF-cocktail was more efficient for glucose release from alkali-pretreated sugarcane bagasse hydrolysis than the extracts produced by the same fungus under SSF or SmF. These results demonstrated that the enzymes produced under the two culture conditions were complementary for the hydrolysis of the alkali-pretreated sugarcane bagasse.
Conclusion
Enzyme extracts produced by C. cubensis under SSF and SmF showed different secreted protein and enzyme profiles. The enzyme cocktail obtained by the extraction of C. cubensis enzymes produced under SFF with the extract produced by the same fungus under SmF (SSF-SmF-cocktail) was more efficient for the glucose release from alkali-pretreated sugarcane bagasse saccharification than the other extracts produced by C. cubensis and also the commercial cellulolytic extract.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Acuña-Argüelles ME, Gutiérrez-Rojas M, Viniegra-González G, Favela-Torres E (1995) Production and properties of three pectinolytic activities produced by Aspergillus niger in submerged and solid-state fermentation. Appl Microbiol Biotechnol 43:808–814
Andrade LGA, Maitan-Alfenas GP, Morgan T, Gomes KS, Falkoski DL, Alfenas RF, Guimarães VM (2017) Sugarcane bagasse saccharification by purified β-glucosidases from Chrysoporthe cubensis. Biocatal Agric Biotechnol 12(199):205
Barrios-González J (2012) Solid-state fermentation: Physiology of solid medium, its molecular basis and applications. Process Biochem 47:175–185
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Champreda V, Mhuantong W, Lekakarn H, Bunterngsook B, Kanokratana P, Zhao X-Q, Zhang F, Inoue H, Fujii T, Eurwilaichitr L (2019) Designing cellulolytic enzyme systems for biorefinery: from nature to application. J Biosci Bioeng 128:637–654
de Albuquerque MFG, Guimarães VM, de Rezende ST (2021) Use of sugar beet flour and wheat bran as carbon source improves the efficiency of Chrysoporthe cubensis enzymes in sugarcane bagasse saccharification. Bioenerg Res 14:1147–1160
de Almeida MN, Falkoski DL, Guimarães VM, Ramos HJO, Visser EM, Maitan-Alfenas GP, De Rezende ST (2013) Characteristics of free endoglucanase and glicosydases multienzyme complex from Fusarium verticillioides. Bioresour Technol 143:413–422
Dutra TR, Guimarães VM, Varela EM, Fialho LS, Milagres AMF, Falkoski DL, Zanuncio JC, De Rezende ST (2017) A Chrysoporthe cubensis enzyme cocktail produced from a low-cost carbon source with high biomass hydrolysis efficiency. Sci Rep 7:3893
Dyballa N, Metzger S (2009) Fast and sensitive colloidal coomassie G-250 staining for proteins in polyacrylamide gels. J vis Exp 30:1431
Falkoski DL, Guimarães VM, De Almeida MN, Alfenas AC, Colodette JL, De Rezende ST (2013) Chrysoporthe cubensis: a new source of cellulases and hemicellulases to application in biomass saccharification processes. Bioresour Technol 130:296–305
Fanaei MA, Vaziri BM (2009) Modeling of temperature gradients in packed-bed solid-state bioreactors. Chem Eng Process 48:446–451
Ghose TK (1987) Measurement of cellulose activities. Pure Appl Chem 59:257–268
Hernández-Pérez AF, Arruda PV, Felipe MGA (2016) Sugarcane straw as a feedstock for xylitol production by Candida guilliermondii FTI 20037. Braz J Microbiol 47:489–496
Holker U, Hofer M, Lenz J (2004) Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl Microbiol Biotechnol 64:175–186
Hu J, Arantes V, Saddler JN (2011) The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: is it an additive or synergistic effect? Biotechnol Biofuels 4:36
Inoue H, Kitao C, Yano S, Sawayama S (2016) Production of β-xylosidase from Trichoderma asperellum KIF125 and its application in efficient hydrolysis of pretreated rice straw with fungal cellulase. World J Microbiol Biotechnol 32:186
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Maitan-Alfenas GP, Visser EM, Alfenas RF, Nogueira BRG, Campos GG, Milagres AF, De Vries RP, Guimarães VM (2015) The influence of pretreatment methods on saccharification of sugarcane bagasse by an enzyme extract from Chrysoporthe cubensis and commercial cocktails: a comparative study. Bioresour Technol 192:670–676
Mendonça EHM, Avanci NC, Romano LH, Branco DL, Pádua AX, Ward RJ, Neto AB, Lourenzoni MR (2020) Recombinant xylanase production by Escherichia coli using a non-induced expression system with different nutrient sources. Braz J Chem Eng. https://doi.org/10.1007/s43153-019-00004-x
Miller GL (1959) Use of dinitrosalicycilic acid reagent for determination of reducing sugars. Anal Chem 31:426–430
Olsen PO, Alasepp K, Kari J, Cruys-Bagguer N, Borch K, Westh P (2016) Mechanism of product inhibition for Cellobiohydrolase Cel7A during hydrolysis of insoluble cellulose. Biotechnol Bioeng 113:6
Rao RS, Jyothi CP, PrakashamRS SPN, Rao LV (2006) Xylitol production from corn fiber and sugarcane bagasse hydrolysates by Candida tropicalis. Bioresour Technol 97:1974–1978
Saritha M, Singh S, Tiwari R, Goel R, Nain L (2016) Do cultural conditions induce differential protein expression: Profiling of extracellular proteome of Aspergillus terreus CM20. Microbiol Res 192:73–83
Singhvi MS, Adsul MG, Gokhale DV (2011) Comparative production of cellulases by mutants of Penicillium janthinellum NCIM 1171 and its application in hydrolysis of Avicel and cellulose. Bioresour Technol 102:6569–6572
Subramaniyam R, Vimala R (2004) Solid state and submerged fermentation for the production of bioactive substances: a comparative study. Int J Sci Nat 3:480–486
Tavares MP, Morgan T, Gomes RF, Rodrigues MQRB, Castro-Borges W, de Rezende ST, Mendes TAO, Guimarães VM (2021) Secretomic insight into the biomass hydrolysis potential of the phytopathogenic fungus Chrysoporthe cubensis. J Proteom 236:104121
Venegas IM, Fuentes-Hernández J, Garcia-Rivero M, Martinez-Treujillo A (2013) Characteristics of Aspergillus niger xylanases produced on ricehusk and wheat bran in submerged culture and solid-statefermentation for an applicability proposal. Int J Food Sci Technol 48:1798–1807
Vicari KJ, Tallam SS, Shatova T, Joo KK, Scarlata CJ, Humbird D, Wolfrum EJ, Beckham GT (2012) Uncertainty in techno-economic estimates of cellulosic ethanol production due to experimental measurement uncertainty. Biotechnol Biofuels 5(1):1-12
Viniegra-gonzález G, Favela-Torres E, Aguilar CN, Rómero-Gomez SJ, Díaz-Godínez G, Augur C (2003) Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem Eng J 13:157–167
Visser EM, Falkoski DL, De Almeida MN, Maitan-Alfenas GP, Guimarães VM (2013) Production and application of an enzyme blend from Chrysoporthe cubensis and Penicillium pinophilum with potential for hydrolysis of sugarcane bagasse. Bioresour Technol 144:587–594
Visser EM, Leal TF, De Almeida MN, Guimarães VM (2015) Increased enzymatic hydrolysis of sugarcane bagasse from enzyme recycling. Biotechnol Biofuels 8:5
Zhang X, Tu M, Paice MG (2011) Routes to potential bioproducts from lignocellulosic biomass lignin and hemicelluloses. Bioenergy Res 4:246–257
Acknowledgements
We thank Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico (CNPq) for the financial support and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for providing scholarships. We would like to thank Thamires Felisberto Pereira Dutra for cooperating in the experiments execution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dutra, T.R., Guimarães, V.M., Varela, E.M. et al. A new approach for Chrysoporthe cubensis cellulolytic cocktail production using solid and submerged-state fermentation. Braz. J. Chem. Eng. 40, 359–366 (2023). https://doi.org/10.1007/s43153-023-00309-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-023-00309-y