Abstract
This study evaluated the bioherbicidal potential of wild fungi grown on microalgal biomass from the digestate treatment of biogas production. Four fungal isolates were used and the extracts were evaluated for the activity of different enzymes and characterized by gas chromatography coupled with mass spectrometry. The bioherbicidal activity was assessed by application on Cucumis sativus, and the leaf damage was visually estimated. The microorganisms showed potential as agents producing an enzyme pool. The obtained fungal extracts presented different organic compounds, most acids, and when applied to Cucumis sativus, showed high levels of leaf damage (80–100 ± 3.00%, deviation relative to the observed average damage). Therefore, the microbial strains are potential biological control agents of weeds, which, together with the microalgae biomass, offer the appropriate conditions to obtain an enzyme pool of biotechnological relevance and with favorable characteristics to be explored as bioherbicides, addressing aspects within the environmental sustainability.
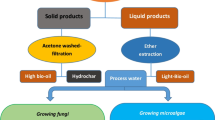
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the agricultural sector, there are activities aimed at providing food to meet the needs of a constantly developing population. Over the years, agricultural productivity has suffered successive decreases due to different factors, among which weeds are responsible for significant losses and can affect food security [1]
Herbicides have been widely used as a strategy to ensure crop safety and also to prevent weeds from increasing rapidly in these environments. This control strategy has been used since the late 1960s. Still, its continued use leads to plant resistance and negatively affects the environment and human and animal health, resulting in reduced yields and high costs to sustain healthy food production [2, 3].
Ending hunger, achieving food security, improving nutrition, and promoting sustainable agriculture are targets for achieving the Sustainable Development Goals by 2030; an exciting strategy to ensure the availability of healthy and more sustainable food is the use of bioherbicides as a way to reduce the use of synthetic herbicides [4].
Using natural products as a form of weed management offers some advantages over synthetic herbicides, such as specificity of action, rapid degradation, and low risk of environmental contamination. Bioherbicides are products based on biologically active organisms or secondary metabolites and enzymes produced by them. Although several natural products have been tested for bioherbicidal activity, few can be obtained commercially due to the need for rigorous validation to assess their efficacy and reliability in weed control [5,6,7].
The essence of bioherbicide formulation lies in defining the biologically active compound, which will make the necessary interactions with the target plant. The environment harbors many microorganisms that can be applied in various biotechnological areas. In this sense, bioprospecting microbial strains are fundamental to discovering new relevant species in this scenario. Fungi of the genera Fusarium and Trichoderma are already widely used as biological control agents; they act by forming metabolites (phytohormones, 2-(hydroxymethyl) phenol, organic acids, alcohols, plant growth regulators, among others) and enzymes (lipases, peroxidases, cellulases, amylases, among others) that may act degrading the cell wall of plants [8,9,10,11,12].
The production of a bioherbicide is still expensive, which does not make it competitive with other chemicals available in the market. To overcome this bottleneck, an alternative would be using a cheaper fermentation medium, adding value to the final product. To this end, microalgae resulting from the treatment of swine manure present a possible application as a readily available substrate rich in carbohydrates and proteins, which would make it possible to use them as a raw material to produce bioherbicides. This would be a sustainable solution to add value to microalgae and reduce the final cost of bioherbicide [13, 14].
Thus, this study evaluated the potential of wild fungi cultivated on microalgae biomass from the digestate treatment of biogas production, aiming to obtain fungal bioherbicides.
Materials and methods
Microalgae biomass: raw material for bioherbicide production
The microalgae used as fermentative substrate belong to the genus Chlorella spp. and come from the phytoremediation treatment of wastewater from biogas production (digestate), implemented at EMBRAPA Swine and Poultry (Concordia, SC, Brazil). The biochemical of microalgae (dry weight basis) is composed of 58.90 ± 1.30% protein (proportional to the nitrogen content), 25.20 ± 0.90% carbohydrates, 3.00 ± 0.50% lipids, 12.8 ± 0.60% minerals [15, 16] and has a moisture content of 88.99 ± 0.50%.
Microbial strains
Four fungal isolates were used to produce extracts with bioherbicidal potential. The first of them, from the species Trichoderma koningiopsis (identification code in GenBank MK860714), was isolated from the weed Digitaria ciliaris and showed promising results for enzyme production and weed control in other studies [8, 11, 17].
Three other microbial strains were isolated from the gut of military caterpillars (Spodoptera frugiperda). For this, caterpillars were subjected to the dissection process. Subsequently, the intestines were placed in Erlenmeyer flasks containing synthetic minimal YNB medium (6.7 g/L yeast nitrogenous base—SIGMA-Aldrich) plus 10 g/L xylose and 0.2 g/L chloramphenicol. The flasks were kept at 30 °C under 145 rpm until turbidity resulting from microbial growth could be perceived [18, 19]. Next, depletions were made in Petri dishes with the same culture medium described above to obtain isolated colonies containing 20 g/L of agar. Among the isolated strains, two cultures showed potential and were molecularly identified (Neoprospecta, Florianópolis-Brazil) as Fusarium sp. and two fungi with associated growth, Fusarium denticulatum and Mucor circinelloides. The DNA sample (extracted from mycelium grown in a culture medium) was subjected to a polymerase chain reaction (PCR) to amplify the rDNA internal transcribed spacer (ITS) region. The primers for the ITS region were: ITS1 (5'-TCCGTAGGTGAACCTGCGG-3') and ITS4 (5'-TCCTCCGCTTATTATTGATATGC-3'). The fragments were sequenced by chain termination analysis with the Big Dye 3.1 reagent (Applied Biosystems) in an automated capillary sequencer 3500 XL (Applied Biosystems). After, the sequences obtained for the isolated fungi were compared with the GenBank database using the BLAST (Basic Local Alignment Search Tool) [20].
The strains were repotted and kept in Petri plates containing Potato Dextrose Agar (PDA) culture medium (Merck, Germany) for 7 days at 28 ºC for fungal growth.
Submerged and solid-state fermentation
The fermentations to obtain extracts with bioherbicidal potential were performed in a submerged and solid state.
The submerged fermentation (SF) process was performed in 300 mL Erlenmeyer with a proper volume of 100 mL. The medium was composed of 10 g of wet microalgal biomass, aiming to meet the supplementation of a synthetic fermentative medium for bioherbicide production [8] and 90 mL of distilled water. The inoculated Erlenmeyers were kept on an orbital shaker (New Brunswick TM, Germany) at 120 rpm for 72 h and 28 °C.
Solid-state fermentation (SSF) was performed in polypropylene beakers (250 mL) containing a medium composed of 10 g of wet microalgal biomass without supplementation. The fermentation occurred in a B.O.D type incubator (Solab, Brazil) for 72 h at a fixed temperature of 28 ºC and relative humidity of 70%. After fermentation, 90 mL of distilled water was added to the fermented samples to perform the extraction. This solution was kept under 200 rpm agitation for 60 min at 28ºC.
The flasks were autoclaved at 120 ºC for 20 min at 101.325 kPa, to ensure that the rupture of microalgal biomass cells occurred, which led to the release of intracellular lipids, in addition to complete sterilization of the culture medium. After sterilization, the media were inoculated with a suspension of 106 spores/mL of each microorganism: Trichoderma koningiopsis, Fusarium sp., Fusarium denticulatum, and Mucor circinelloides. After fermentation, the extracts were filtered by manual pressing in synthetic fabric, the solid retained was sterilized and discarded, and the liquid permeate was centrifuged (NT 815-NovaTecnica, Brazil) at 2000 rpm, 4 ºC for 10 min. The supernatant from the centrifugation was used for the subsequent steps.
Enzymatic activity
The following enzymes were selected to evaluate the presence of an enzymatic pool in the extracts: amylase, cellulase, laccase, lipase, and peroxidase. The enzyme assays were performed in triplicate and always contained a reaction control without the enzyme extracts.
The quantification of the enzyme’s amylase [21, 22] and cellulose [23] occurred by quantifying the release of total reducing sugars, measured by the DNS (3,5 dinitrosalicylic acid) method [24]. One unit of enzyme activity (U) was defined as the amount of enzyme capable of releasing 1 µmol of glucose per minute under the reaction conditions.
To determine laccase activity, 2,2′-azino-di-3-ethylbenzotialozin-6-sulfonic acid (ABTS) was used as the substrate for the enzyme reaction [25]. One unit of laccase activity (U) was defined as the amount of enzyme capable of forming 1 µmol of ABTS + per minute under the reaction conditions.
Lipase quantification was performed by preparing an emulsion [26]. One unit of lipase activity (U) was defined as the enzyme able to hydrolyze 1 µmol of substrate per minute under the reaction conditions.
Peroxidases were quantified by one unit of peroxidase activity (U), defined as the amount of enzyme capable of causing an absorbance unit increase of 0.001 per minute in the reaction conditions [27, 28].
Bioherbicide activity
The evaluation of the bioherbicidal activity of the extracts produced by the fungi through submerged and solid fermentations and the combinations of extracts was performed through the application in Cucumis sativus; the choice for this species is due to its potential as a model plant because it is used in tests with chemical herbicides [29].
For the Cucumis sativus trials, each treatment was composed of 36 plants and 12 controls (no treatment), all with 4 repetitions. The extracts were applied in a greenhouse with controlled temperature and humidity conditions (26 ºC and 70%). The seeds were grown for 10 days, and when they reached three leaves, approximately 5 mL of the extracts were manually applied to the plant's leaf surface.
For the application tests, the control samples were: control A consisted of the culture medium (microalgae + distilled water) used in the submerged fermentations without the addition of microorganisms; control B consisted of the culture medium (microalgae) used in the solid fermentations, without the addition of microorganisms and after the extraction process; control C consisted of distilled water.
The extracts' bioherbicidal potential was evaluated 7 and 14 days after application. Leaf damage was visually estimated as a percentage reduction in growth compared to controls. The percentage of leaf damage was established according to the scale recommended by the Brazilian Weed Science Society (SBCPD) [30].
Characterization of compounds present in the extracts
The compounds are characterized in the fermented extracts through the analysis of the products generated from the liquid–liquid extraction of the fermented extracts via submerged and solid processes of the fungi. In addition, the fermentation medium of each fermentation was evaluated and considered a control.
For this, ethyl acetate (Et2O) was used as an organic solvent, mixed with the extracts in a separation funnel at a ratio of 1:1 (v/v). The samples were followed to a rotary evaporator (Rotary Evaporator—Q344B, QUIMIS, Brazil) until the reduction of the sample volume was observed due to the evaporation of the organic solvent. The conditions of the evaporation process were: bath at 30 ºC and agitation of 120 rpm (under vacuum). The aqueous phase was discarded, and the organic phase was filtered and dried with ammonium sulfate.
The reaction products were identified by gas chromatography coupled to mass spectrometry (GC/MS—QP2010, Shimadzu, Japan) using an NST 05 ms—3,025,025 column (30 m × 0.25 mm × 0.25 µm). The column temperature followed the schedule: 80 °C/3 min, 6 °C/min up to 218 °C, 2 °C/min up to 240 °C, and 7 °C/min up to 300 °C. The carrier gas was helium (He). The injector and detector temperature were set to 280 °C and 1.0 µL of the solution (Derivatization:1:1:1 (extract: ethyl acetate: BSTFA (N, O-bis(trimethylsilyl)trifluoroacetamide) + TMCS (Trimethylchlorosilane) (1%) at 60 °C for 1 h) was injected into the GC/MS system with a split ratio of 1:10. The apparatus operated at a flow rate of 19.1 mL/min in 70 V electronic impact mode and with the column pressure at 100 kPa. The compounds were identified by comparing the mass spectra with those from the Wiley library and by comparing the GC retention time of standard compounds. The products were partially quantified by the percentage of the area of each peak relative to the total peak area [31].
Statistical analysis
The data were statistically treated by analysis of variance followed by Tukey's test, considering a significance level of 95% (p < 0.05). Statistica 8.0 software (Statsoft Inc., Tulsa, OK, USA) was used for this.
Results and discussion
Evaluation of enzyme production
Table 1 shows the results of the enzymatic quantification of the extracts from the submerged and solid fermentations.
The microorganisms showed potential as agents for producing an enzyme pool via submerged and solid fermentation. The microalgae biomass used as substrate provided a suitable culture medium for the microorganisms to produce some enzymes of interest in the biological control of weeds.
As for the results regarding submerged fermentation, the extract fermented by the fungi Fusarium denticulatum and Mucor circinelloides showed the highest values of lipase activity, 1.80 ± 0.34 (U/mL), probably related to the fact that the fungus Mucor circinelloides is known to accumulate high lipid content in its metabolism and, consequently, to be a potential producer of microbial lipases [32]. Peroxidase activities were the highest in the extracts obtained in submerged fermentation. Previous studies using the fungus Trichoderma koningiopsis and species of the Fusarium already showed that these fungi are good producers of peroxidases [8, 11]. Using the associated fungi Fusarium denticulatum and Mucor circinelloides, an enzyme activity of 37.15 ± 0.01 (U/mL) was reached, the maximum obtained among the enzyme extracts produced by submerged fermentation. This fact may be related to the fungi's joint action, establishing synergism and potentiating enzymatic production [33].
Among the extracts obtained by solid fermentation, the extract fermented by the microorganism Fusarium sp. reached the highest enzyme concentrations of amylase, cellulase, and peroxidase, demonstrating that this fungus is an exciting producer of enzymes in the fermentative conditions that were made available. The metabolism of species of the genus Fusarium is related to the nutritional source used; it is versatile, adapting quickly to different fermentative conditions to which it is submitted [34]. Hydrolytic enzymes such as amylase and cellulase can be detected when their metabolism is associated with nitrogen. On the other hand, exposure to mild stress can produce oxidative enzymes such as peroxidases [35].
The enzyme lipase was not produced via solid-state fermentation, which may be related to the composition of the culture medium since when you have fermentative media with the presence of some oil, the fungus Fusarium sp. becomes a potential producer of microbial lipases [36]. On the other hand, when Fusarium denticulatum associated with Mucor circinelloides were subjected to submerged fermentation, they produced lipase, probably because, during the production of this enzyme, in environments with high lipid content and low amount of water (solid-state fermentation), the formation of phospholipid-based reverse micelles that capture water occurs, and may result in enzyme inactivation. In this sense, the higher the water content available in the substrate, the greater the possibility of lipase production; perhaps, for this reason, the submerged medium (water availability and low agitation) was the most suitable for these fungi to achieve microbial lipase production [37, 38].
The fungus Trichoderma koningiopsis obtained the lowest values of enzyme activity when compared to the other fungi. Although T. koningiopsis presents itself in a versatile way, to its performance observed in other studies, possibly this fungus adapts better in submerged fermentations [8, 11, 17].
The laccase enzyme was not quantified in the extracts obtained by submerged and solid fermentation. Generally, fungal laccases are difficult to obtain because they require substrates that contain chemical inducers such as phenolic compounds, aromatics, or copper ions; this makes laccase production only detected after a more complex and optimized fermentation [39]. In addition, the tested fungi may have produced distinct forms (isoforms) of this enzyme, which could be detected after an enzyme purification process [40].
The low production of cellulase obtained in the different fermentative processes may be linked to the composition of the microalgae biomass since the carbohydrate fraction may be formed by polysaccharides such as starch and cellulose. The (possibly low) content of these polysaccharides may limit enzyme production since the presence of these compounds in the biomass used as a substrate is not specific [41].
Analysis of bioherbicidal activity
Recent studies consider microalgae as potential biological control agents because they produce biologically active compounds, such as secondary metabolites [13]. Table 2 shows the values in the percentage of leaf damage observed 7 and 14 days after the applications, according to the scale recommended by the SBCPD. These values may be related to a higher presence of free water in the extract obtained by submerged fermentation (control A), which would facilitate the entry of metabolites in the microalgae on the leaf surface, causing minor damage. Control A, which refers to the culture medium of fermentation in a submerged, showed low foliar damage of 4.00 ± 0.06% after 14 days of application, which may be linked to a low bioherbicidal potential in the microalgal biomass. No foliar damage was observed for control B (solid-state fermentation medium) Table (3).
Using the fungi Trichoderma koningiopsis and Fusarium sp., complete control of the crops was obtained, with only a few living plants, according to the scale recommended by the SBCPD. The extracts produced by submerged fermentation showed a higher damage percentage than those produced by solid fermentation extract made by T. koningiopsis that showed the production of the peroxidase enzyme in significant concentrations; this enzyme is one of the responsibilities for eliminating reactive oxygen species (ROS), which are indicators of stress in the plant. When applied to the plant, this extract rich in peroxidases increases the concentration of superoxide radicals and hydrogen peroxide, damaging DNA and cell membranes. The genus Fusarium exhibits controlling characteristics in invasive plants, acting mainly on ethylene biosynthesis, stimulating ripening and yellowing leaves [6, 11]. These effects can be visualized in Fig. 1, in which the extract of Trichoderma koningiopsis acted to inhibit plant growth, causing necrosis on the leaf surface, and the extract produced by the fungus Fusarium sp. caused early leaf ripening (Table 4).
Phytotoxic effect of extracts obtained by submerged fermentation: control A (a) and of the fungi Trichoderma koningiopsis (b), Fusarium denticulatum in consortium with Mucor circinelloides (c), and Fusarium sp (d). Phytotoxic effect of extracts obtained by solid fermentation: control B (e) and of the fungi Trichoderma koningiopsis (f), Fusarium denticulatum in consortium with Mucor circinelloides (g) and Fusarium sp. (h) (after 14 days of application on Cucumis sativus)
Fusarium sp. in which the presence of the enzymes cellulase, peroxidase, and amylase was identified, the last two may have acted in the degradation of leaf structures together with cellulase responsible for serving as a gateway for the fungus into the leaf structures, working in the degradation of cellulose present in the cell wall of plants [10, 11]. The symptoms caused by the extracts produced by solid fermentation could already be identified 7 days after application on Cucumis sativus, different from those produced by submerged fermentation. These symptoms (Fig. 1) may be associated with specific characteristics of the enzymes in these extracts, such as those produced by the fungus Fusarium denticulatum in consortium with Mucor circinelloides (Table 5).
The application of the crude microalgae demonstrates that even if the microalgae present a mild level of phytotoxicity, the phytotoxic potential can be amplified when it is used as a substrate for fungal bioherbicide production. This can be observed in the bioherbicide activities of the extracts applied alone from the submerged and solid fermentations. Thus, it can be stated that the microalgae alone do not have the same potential for biological control as when applied in consortium with the fungi and metabolites produced by them.
Characterization of compounds present in the fermented extracts
About 40 compounds (Tables 3, 4, 5, 6, 7, 8, 9, 10) were identified in the submerged fermentation and 26 in the solid fermentation control. In contrast, for the fungal extracts, the values were reduced between 21 and 17 identified compounds (see supplementary material). This fact may be related to fermentation, in which the fungi can synthesize and/or assimilate some compounds [42, 43]. Among the compounds identified in the submerged fermentation control, 48.6% referred to hexadecanoic (22.9%), octadecanoic (14.93%), and propanoic (10.76%) acids. These same acids were identified in different percentages in the fermented extracts. The hexadecanoic acid compound is more observed in the extracts obtained from the submerged fermentations (41.65% in the ethanolic extract derived from Trichoderma koningiopsis and 35.36% in the extract derived from Fusarium sp.). In the derivatized extract of Fusarium denticulatum in consortium with Mucor cicinelloides of submerged fermentation, ethylene glycol was the primary compound with 36.98%. In the solid-state fermentation control, the main compounds identified were: ethylene glycol (26.96%), hexadecanoic acid (20.22%), octadecanoic acid (12.18%), and propanoic acid (4.71%). These compounds were also observed mainly in the extracts resulting from solid-state fermentation. Hexadecanoic acid is the most observed compound presenting up to 40.46% in the extract derived from Trichoderma koningiopsis. In addition to these compounds, linolenic acid was detected in all extracts of the solid fermentations, presenting up to 16.54% in the extract derived from Fusarium sp.
The fraction of fatty acids present in the extracts, such as pentanoic acid, hexadecanoic acid, octanoic acid, and linolenic acid, among others, when applied to Cucumis sativus plants, may have acted on the permeability of metabolic exchanges in the cell wall, resulting in membrane destruction and even altering photosynthesis processes [44]. Fatty acids can present phytotoxic effects on weeds and, for this reason, are considered compounds with allelopathic properties, defined as the condition in which plants have a competitive advantage, and through secondary metabolites produced by microorganisms or plants influence in a way that stimulates or regulates the development of other plants or organisms [45, 46].
On the other hand, organic and volatile compounds may be responsible for some phytotoxic effects in plants. One has only a few indications of possible phytotoxic compounds, such as eicosane [47] and 2-phenylmethyl [48]. The action of each of the identified compounds is not sure since the bioherbicidal activity is possibly the result of the interactions between all the phytotoxins present in each extract [49, 50] since new fungal molecules may present different mechanisms of action that may even be unknown until now [51].
Among the identified compounds, linolenic acid is a compound that, if applied at lower concentrations, can stimulate plant growth. This is a positive point for a selective bioherbicide if its formulation can reduce the growth of invasive plants and promote the development of crops of agronomic interest [14, 52, 53]. In this scenario, we would encompass a healthier form of production if compared to conventional techniques, using a product that is both a biostimulant and a bioregulator for plants.
Linolenic acid is one of the main precursors in the biosynthetic pathway that leads to plant jasmonates. The jasmonate group of plant hormones acts mainly in plant defense processes and is essential concerning the development and growth of plants, serving in several methods such as the induction of ethylene biosynthesis; retard or inhibit plant growth by blocking glucose incorporation; stimulating stomatal closure; induce or inhibit seed germination due to altered sensitivity; under stress conditions, it can increase plant tolerance to pathogens and pests and attract natural enemies because they are volatile compounds. For the synthesis of jasmonates, the presence of linolenic acid is essential. For this reason, plants with concentrations of this substance may have greater efficiency in the signaling system, increasing tolerance to stress [54, 55].
Linolenic acid and other fatty acids can be incorporated into structural elements in the lipid bilayers of the cell membrane and cuticular wax, helping plants to reduce water loss and pathogen invasion. Studies have shown that linolenic acid concentrations may indicate increased lipid biosynthesis in plants. Furthermore, the study demonstrated that all treated plants exhibited higher total concentrations of fatty acids, meaning the biostimulating effects of microalgae extracts on the lipid profile of the plants [54, 55].
Conclusion
Microalgae biomass can be considered an alternative culture medium to synthetic ones for fermentative purposes, rich in nutrients and with added value, taking into account its obtaining and recycling process.
The fungi used in this study highlight the relevance of research in bioprospecting new microbial strains with biotechnological potential. The microorganisms demonstrated pertinent characteristics, such as good enzyme producers and ease in adapting to different fermentative conditions and an alternative substrate. The enzyme production from the fungal strains and the microalgae biomass offered suitable conditions to obtain an enzyme cocktail for bioherbicidal purposes.
Through the preliminary tests performed on Cucumis sativus, it can be inferred that the microbial strains are potential biological control agents. Studies still need to be outlined regarding the impact of the permanence of these bioherbicides in the soil and the survival period of residues from the application of these extracts in the environment, besides the adaptation of these applications to the field.
With the characterization of the extracts, it was possible to obtain important information about the fermented extracts, identify some compounds, and associate their probable phytotoxicity effects to understand better the mechanisms of action involved in the final bioherbicidal activity.
Finally, some challenges were overcome in turning successful wild strains into viable bioprocesses. Results suggest that fungal extracts can be applied in weed control as bioherbicides, presenting the possibility of the full-scale application shortly, providing farmers and scientists with tools to control weeds innovatively and more sustainably.
Data availability
The datasets generated for this study are available on request to the corresponding author.
References
Macías-Rubalcava ML, Garrido-Santos MY (2022) Phytotoxic compounds from endophytic fungi. Appl Microbiol Biotechnol 106:931–950. https://doi.org/10.1007/s00253-022-11773-w
Perotti VE, Larran AS, Palmieri VE et al (2020) Herbicide resistant weeds: a call to integrate conventional agricultural practices, molecular biology knowledge and new technologies. Plant Sci. https://doi.org/10.1016/j.plantsci.2019.110255
Verma D, Banjo T, Chawan M, et al (2020) Microbial Control of Pests and Weeds. In: Natural Remedies for Pest, Disease and Weed Control. Elsevier
FAO (2022) Sustainable Development Goals. https://www.fao.org/sustainable-development-goals/goals/goal-2/en/. Accessed 12 Apr 2022
Cordeau S, Triolet M, Wayman S et al (2016) Bioherbicides: Dead in the water? a review of the existing products for integrated weed management. Crop Prot 87:44–49. https://doi.org/10.1016/j.cropro.2016.04.016
Radhakrishnan R, Alqarawi AA, AbdAllah EF (2018) Bioherbicides: Current knowledge on weed control mechanism. Ecotoxicol Environ Saf 158:131–138. https://doi.org/10.1016/j.ecoenv.2018.04.018
Hasan M, Ahmad-Hamdani MS, Rosli AM, Hamdan H (2021) Bioherbicides: an eco-friendly tool for sustainable weed management. Plants 10:1212. https://doi.org/10.3390/plants10061212
Bordin ER, Frumi Camargo A, Rossetto V et al (2018) Non-Toxic bioherbicides obtained from trichoderma koningiopsis can be applied to the control of weeds in agriculture crops. Ind Biotechnol 14:157–163. https://doi.org/10.1089/ind.2018.0007
Adetunji CO, Oloke JK, Bello OM et al (2019) Isolation, structural elucidation and bioherbicidal activity of an eco-friendly bioactive 2-(hydroxymethyl) phenol, from Pseudomonas aeruginosa (C1501) and its ecotoxicological evaluation on soil. Environ Technol Innov 13:304–317. https://doi.org/10.1016/j.eti.2018.12.006
Aita BC, Spannemberg SS, Schmaltz S et al (2019) Production of cell-wall degrading enzymes by solid-state fermentation using agroindustrial residues as substrates. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2019.103193
Reichert Júnior FW, Scariot MA, Forte CT et al (2019) New perspectives for weeds control using autochthonous fungi with selective bioherbicide potential. Heliyon. https://doi.org/10.1016/j.heliyon.2019.e01676
Camargo AF, Venturin B, Bordin ER et al (2020) A low-genotoxicity bioherbicide obtained from trichoderma koningiopsis fermentation in a stirred-tank bioreactor. Ind Biotechnol 16:176–181. https://doi.org/10.1089/ind.2019.0024
Costa JAV, Freitas BCB, Cruz CG et al (2019) Potential of microalgae as biopesticides to contribute to sustainable agriculture and environmental development. J Environ Sci Health B 54:366–375. https://doi.org/10.1080/03601234.2019.1571366
Michelon W, da Silva MLB, Matthiensen A et al (2022) Amino acids, fatty acids, and peptides in microalgae biomass harvested from phycoremediation of swine wastewaters. Biomass Convers Biorefin 12:869–880. https://doi.org/10.1007/s13399-020-01263-2
Michelon W, Da Silva MLB, Mezzari MP et al (2016) Effects of nitrogen and phosphorus on biochemical composition of microalgae polyculture harvested from phycoremediation of piggery wastewater digestate. Appl Biochem Biotechnol 178:1407–1419. https://doi.org/10.1007/s12010-015-1955-x
Stefanski FS, Camargo AF, Scapini T, et al (2020) Potential Use of Biological Herbicides in a Circular Economy Context: A Sustainable Approach. Front Sustain Food Syst 4:. https://doi.org/10.3389/fsufs.2020.521102
Camargo AF, Stefanski FS, Scapini T et al (2019) Resistant weeds were controlled by the combined use of herbicides and bioherbicides. Environ Qual Manage 29:37–42. https://doi.org/10.1002/tqem.21643
Cadete RM, Melo-Cheab MA, Dussán KJ et al (2017) Production of bioethanol in sugarcane bagasse hemicellulosic hydrolysate by Scheffersomyces parashehatae, scheffersomyces illinoinensis and Spathaspora arborariae isolated from Brazilian ecosystems. J Appl Microbiol 123:1203–1213. https://doi.org/10.1111/jam.13559
Barretto DA, Avchar R, Carvalho C et al (2018) Blastobotrys bombycis sp. nov., a d-xylose-fermenting yeast isolated from the gut of the silkworm larva Bombyx mori. Int J Syst Evol Microbiol 68:2638–2643. https://doi.org/10.1099/ijsem.0.002890
Schmitz A, Riesner D (2006) Purification of nucleic acids by selective precipitation with polyethylene glycol 6000. Anal Biochem 354:311–313. https://doi.org/10.1016/j.ab.2006.03.014
Fuwa H (1954) A new method for microdetermination of amylase activity by the use of amylose as the substrate. J Biochem 41:583–603. https://doi.org/10.1093/oxfordjournals.jbchem.a126476
Pongsawadi P, Yagisawa M (1987) Screening and identification of a cyclomaltoxtrinv glucanotransferase-producing bacteria. J Ferment Technol 65:463–467. https://doi.org/10.1016/0385-6380(87)90144-0
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. https://doi.org/10.1351/pac198759020257
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Hou H, Zhou J, Wang J et al (2004) Enhancement of laccase production by Pleurotus ostreatus and its use for the decolorization of anthraquinone dye. Process Biochem 39:1415–1419. https://doi.org/10.1016/S0032-9592(03)00267-X
Treichel H, Sbardelotto M, Venturin B et al (2017) Lipase production from a newly isolated aspergillus niger by solid state fermentation using canola cake as substrate. Curr Biotechnol. https://doi.org/10.2174/2211550105666151124193225
Khan AA, Robinson DS (1994) Hydrogen donor specificity of mango isoperoxidases. Food Chem 49:407–410. https://doi.org/10.1016/0308-8146(94)90013-2
Devaiah SP, Shetty HS (2009) Purification of an infection-related acidic peroxidase from pearl millet seedlings. Pestic Biochem Physiol 94:119–126. https://doi.org/10.1016/j.pestbp.2009.04.010
Todero I, Confortin TC, Luft L et al (2020) Concentration of exopolysaccharides produced by Fusarium fujikuroi and application of bioproduct as an effective bioherbicide. Environ Technol 41:2742–2749. https://doi.org/10.1080/09593330.2019.1580775
Brazilian Society of Weed Science (1995) Procedures for Installation, Evaluation and Analysis of Experiments with Herbicides
Lerin et al (2010) Microorganisms screening for limonene oxidation. Ciênc Tecnol Aliment 30:399–405
Kamoun O, Muralitharan G, Belghith H et al (2019) Suitable carbon sources selection and ranking for biodiesel production by oleaginous Mucor circinelloides using multi-criteria analysis approach. Fuel 257:1–13. https://doi.org/10.1016/j.fuel.2019.116117
Rodrigues P, De O, Gurgel LVA, Pasquini D et al (2020) Lignocellulose-degrading enzymes production by solid-state fermentation through fungal consortium among Ascomycetes and Basidiomycetes. Renew Energy 145:2683–2693. https://doi.org/10.1016/j.renene.2019.08.041
Preczeski KP, Dalastra C, Czapela FF et al (2020) Fusarium oxysporum and aspergillus sp. as keratinase producers using swine hair from agroindustrial residues. Front Bioeng Biotechnol 8:1–8. https://doi.org/10.3389/fbioe.2020.00071
da Rosa-Garzon NG, Laure HJ, Rosa JC, Cabral H (2019) Fusarium oxysporum cultured with complex nitrogen sources can degrade agricultural residues: evidence from analysis of secreted enzymes and intracellular proteome. Renew Energy 133:941–950. https://doi.org/10.1016/j.renene.2018.10.100
de Oliveira BH, Coradi GV, de Oliva-Netodo Nascimento VMG, P (2020) Biocatalytic benefits of immobilized Fusarium sp. (GFC) lipase from solid state fermentation on free lipase from submerged fermentation. Ind Crops Prod 147:1–10. https://doi.org/10.1016/j.indcrop.2020.112235
Amoah J, Ho S-H, Hama S et al (2016) Converting oils high in phospholipids to biodiesel using immobilized Aspergillus oryzae whole-cell biocatalysts expressing Fusarium heterosporum lipase. Biochem Eng J 105:10–15. https://doi.org/10.1016/j.bej.2015.08.007
Pessôa MG, Paulino BN, Mano MCR et al (2017) Fusarium species—a promising tool box for industrial biotechnology. Appl Microbiol Biotechnol 101:3493–3511. https://doi.org/10.1007/s00253-017-8255-z
Rodríguez RD, Heredia G, Siles JA et al (2019) Enhancing laccase production by white-rot fungus Funalia floccosa LPSC 232 in co-culture with Penicillium commune GHAIE86. Folia Microbiol (Praha) 64:91–99. https://doi.org/10.1007/s12223-018-0635-y
Li W, Yu J, Li Z, Yin W-B (2019) Rational design for fungal laccase production in the model host Aspergillus nidulans. Sci China Life Sci 62:84–94. https://doi.org/10.1007/s11427-017-9304-8
Kumar AN, Chatterjee S, Hemalatha M et al (2020) Deoiled algal biomass derived renewable sugars for bioethanol and biopolymer production in biorefinery framework. Bioresour Technol 296:1–7. https://doi.org/10.1016/j.biortech.2019.122315
Llamas M, Magdalena JA, Tomás-Pejó E, González-Fernández C (2020) Microalgae-based anaerobic fermentation as a promising technology for producing biogas and microbial oils. Energy 206:1–8. https://doi.org/10.1016/j.energy.2020.118184
Chu R, Li S, Zhu L et al (2021) A review on co-cultivation of microalgae with filamentous fungi: efficient harvesting, wastewater treatment and biofuel production. Renew Sustain Energy Rev 139:1–17. https://doi.org/10.1016/j.rser.2020.110689
Alamsjah MA, Hirao S, Ishibashi F et al (2008) Algicidal activity of polyunsaturated fatty acids derived from Ulva fasciata and U. pertusa (Ulvaceae, Chlorophyta) on phytoplankton. J Appl Phycol 20:713–720. https://doi.org/10.1007/s10811-007-9257-5
Wu J-T, Chiang Y-R, Huang W-Y, Jane W-N (2006) Cytotoxic effects of free fatty acids on phytoplankton algae and cyanobacteria. Aquat Toxicol 80:338–345. https://doi.org/10.1016/j.aquatox.2006.09.011
Li ZR, Amist N, Bai LY (2019) Allelopathy in sustainable weeds management. Allelopath J 48:109–138
Pardo-Muras M, Puig CG, López-Nogueira A et al (2018) On the bioherbicide potential of Ulex europaeus and Cytisus scoparius: profiles of volatile organic compounds and their phytotoxic effects. PLoS ONE. https://doi.org/10.1371/journal.pone.0205997
Norsworthy JK, Meehan JT (2005) Use of isothiocyanates for suppression of palmer amaranth (Amaranthus palmeri ), pitted morningglory (Ipomoea lacunosa), and yellow nutsedge (Cyperus esculentus). Weed Sci 53:884–890. https://doi.org/10.1614/WS-05-056R.1
Khanh TD, Chung IM, Tawata S, Xuan TD (2006) Weed suppression by Passiflora edulis and its potential allelochemicals. Weed Res 46:296–303. https://doi.org/10.1111/j.1365-3180.2006.00512.x
Ulloa-Benítez Á, Medina-Romero YM, Sánchez-Fernández RE et al (2016) Phytotoxic and antimicrobial activity of volatile and semi-volatile organic compounds from the endophyte Hypoxylon anthochroum strain Blaci isolated from Bursera lancifolia (Burseraceae). J Appl Microbiol 121:380–400. https://doi.org/10.1111/jam.13174
Triolet M, Guillemin J, Andre O, Steinberg C (2020) Fungal-based bioherbicides for weed control: a myth or a reality? Weed Res 60:60–77. https://doi.org/10.1111/wre.12389
Michalak I, Chojnacka K (2015) Algae as production systems of bioactive compounds. Eng Life Sci 15:160–176. https://doi.org/10.1002/elsc.201400191
Tubeileh AM, Souikane RT (2020) Effect of olive vegetation water and compost extracts on seed germination of four weed species. Curr Plant Biol 22:1–6. https://doi.org/10.1016/j.cpb.2020.100150
Mutale-joan C, Redouane B, Najib E et al (2020) Screening of microalgae liquid extracts for their bio stimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Sci Rep 10:2820. https://doi.org/10.1038/s41598-020-59840-4
Mzibra A, Aasfar A, Benhima R et al (2021) Biostimulants derived from moroccan seaweeds: seed germination metabolomics and Growth promotion of tomato plant. J Plant Growth Regul 40:353–370. https://doi.org/10.1007/s00344-020-10104-5
Acknowledgements
The authors thank the Brazilian Funding Agencies: Brazilian National Council for Scientific and Technological Development (CNPq), Coordination of the Superior Level Staff Improvement (CAPES), the support of the Bioprocess and Biotechnology for Food Research Center (Biofood), which is funded through the Research Support Foundation of Rio Grande do Sul (FAPERGS) (FAPERGS-22/2551-0000397-4), Federal University of Fronteira Sul (UFFS) and Federal University of Santa Catarina (UFSC) for the financial support.
Funding
CAPES, CNPq and FAPERGS.
Author information
Authors and Affiliations
Contributions
AFC, GF, and HT conceived and designed the study. AFC analyzed the data. AFC and CB drafted the manuscript. CD, AU, TS, CB, NK, WM, and LL helped in the experimental part and carefully revised the manuscript. SLAJ, MAT, and OB helped isolate strains from the intestines of caterpillars. SLAJ, AJM, MAT, OB, SRP, GF, HT critically reviewed and supervised the development of the paper. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
All authors agreed with this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Camargo, A.F., Dalastra, C., Ulrich, A. et al. The bioherbicidal potential of isolated fungi cultivated in microalgal biomass. Bioprocess Biosyst Eng 46, 665–679 (2023). https://doi.org/10.1007/s00449-023-02852-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02852-x