Abstract
Solid-state cultivation (SSC) is the microbial growth on solid supports, producing a nutrient-rich solution by cell enzymes that may be further used as a generic microbial medium. “Second-generation” ethanol is obtained by fermentation from mainly the acid hydrolysates of lignocellulosic wastes, generating several microbial growth inhibitors. Thus, this research aimed at evaluating the feasibility of ethanol fermentation from sugarcane bagasse hydrolysate after SSC with vinasse as the impregnating solution by a consortium of A. niger and T. reesei as opposed to the conventional method of acid hydrolysis. Fermentation of the hydrolysate from SSC leading to the yield of 0.40 g g−1, i.e., about 78% of maximum stoichiometric indicating that the nonconventional process allowed the use of two by-products from sugarcane processing in addition to ethanol production from glucose release.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ethanol is obtained by the processes of fermentation–distillation in Brazil from sugarcane molasses, juices, or a mixture of both. Alternatively, it can also be obtained from lignocellulosic wastes composed mainly of cellulose and hemicellulose in the so-called “second-generation ethanol–2G” [1]. Thus, to meet the increasing demand of 2G ethanol, sugarcane bagasse, a low-cost by-product of sugarcane processing, has been extensively investigated for over a decade [2,3,4,5]. The high degree of structural complexity due to the presence of lignin and hemicellulose is considered the main limiting factor for the efficient conversion of sugarcane into fermentable sugars; this results in lower ethanol yield and a higher selling price of ethanol.
The main components of lignocellulosic wastes are cellulose and hemicelluloses that consist of hard and fibrous structures formed by aromatic alcohols known as lignin [6]. In contrast, different types of pretreatment of lignocellulosic biomass (chemical, physical, biological or a combination of all) can be used to make the cellulose and hemicellulose chains more accessible to hydrolytic agents, whether in acid or enzymatic hydrolyses [7].
Different approaches in dilute acid pretreatments have been employed prior the enzymatic hydrolysis [8, 9]. This type of pretreatment promotes the hydrolysis of hemicellulose and partial decomposition of lignin, increasing the digestibility of biomass and favoring subsequent hydrolysis of this material [10, 11].
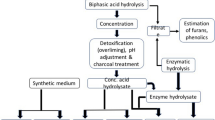
The acid hydrolysis process utilizes concentrated or dilute acids, and the main products of these hydrolytic chemical reactions are hexoses (glucose, galactose, and mannose), pentoses (xylose and arabinose), lignin, and acetic acid, in varying proportions depending on the raw material [7, 12]. However, acid hydrolysis leads to the formation of fermentation-inhibiting components such as 5-hydroxymethylfurfural (HMF) and furfural. These compounds, if not removed, can lead to microbial inhibition when the hydrolysates are used as culture media in bioconversion processes [13,14,15]. Alternatively, enzymatic hydrolysis presents advantages associated with superior yields under moderate temperatures. However, operational aspects related to high process times and the high cost of enzymes has led to uncertainties regarding the economic viability [7, 16].
The development of an integrated simultaneous saccharification–fermentation process of bagasse for ethanol production has been of great interest [16]. On the other hand, studies on microbial growth, particularly co-cultures, would make it possible to obtain higher amounts of easily fermentable hydrolysates from lignocellulosic material and also minimize the action of inhibitors in the subsequent process. As an example, while Trichoderma reesei produces three cellulases and presents a low activity of β-glucosidase, Aspergillus niger displays a higher β-glucosidase activity and a lower production of two cellulases [17]. Therefore, solid-state cultivation (SSC) may be defined as microbial growth on solid supports in the absence of free water, i.e., similar to the occurrence of fungi in nature [18, 19]. This is a potential process for the in situ production of cellulolytic enzymes from several strains of A. niger and T. reesei [4]. Moreover, mixed cultures of microorganisms could complement the metabolic capacities in SSC, increasing the bioconversion capacity of solid substrates [20, 21]. In SSC, solid supports are usually impregnated with nutrient solution. Thus, vinasse, the main wastewater from the sugarcane process, has been used for moistening bagasse and providing nutrient supply for SSC [21].
Thus, SSC can generate sufficient enzyme titer; additionally, the undigested substrate may be further hydrolyzed to produce a nutrient-rich solution. This solution can be used as a generic culture medium in the form of a “microbial hydrolysate” with potential use to produce several bioproducts. This sequential process is innovative and has been the focus of few studies in the literature [22,23,24].
This research aimed at evaluating the feasibility of ethanol fermentation from bagasse hydrolysate after SSC by the consortium of A. niger and T. reesei in comparison with the acid hydrolysate process.
Material and methods
Inoculum
Trichoderma reesei CMAA 1168 and Aspergillus niger CCT 4355 strains used for SSC were maintained on a medium consisting of 200 g L−1 sucrose, 2.5 g L−1 ammonium nitrate, 1.0 g L−1 potassium phosphate, 0.25 g L−1 magnesium sulfate, and 0.04 g L−1 copper sulfate (w/v) pH 4.0 at 4 °C [20]. Prior to each experiment, the inocula were grown in flasks for at least 7 days, in an incubator at 30 °C without agitation in the medium mentioned above. The industrial strain PE-2 of the Saccharomyces cerevisiae yeast was used for the ethanol fermentation experiments and was maintained on the YPD medium (10 g L−1 yeast extract, 20 g L–1 peptone, 20 g L−1 dextrose, and 20 g L−1 agar).
Sugarcane bagasse and vinasse
Sugarcane bagasse and vinasse used in the experiments were collected in a sugarcane processing industry located in the city of Araras, São Paulo, Brazil. After collection, the samples were dried in an oven at 50 oC for approximately 48 h, reaching a final moisture of 10%. After this stage, the particles were crushed in a knife mill and then classified in a set of TYLER sieves FOBRAS® (São Paulo, Brazil) to select material with a particle size (mean diameter) between 0.59 and 0.84 mm. Sterilization of the material used in the tests was performed in an autoclave at 127 °C, 1.5 kgf cm−2 for 20 min. Bagasse was characterized by total organic carbon (TOC) in the SHIMADZU® (Tokyo, Japan) SSM-5000A solid sample combustion unit, vinasse was characterized in terms of pH by potentiometry, glucose by the method of glucose oxidase–peroxidase enzyme LABORLAB® (Guarulhos, Brazil), carbon (TOC), and total nitrogen (TN) were characterized by SHIMADZU® (Tokyo, Japan) TOC-LCPN total organic carbon analyzer.
Bagasse hydrolysates
Solid-state cultivation for microbial hydrolysates
Solid-state cultivation (SSC) was set up with a 50:50 suspension (inoculum volume ratio) of A. niger and T. reesei (according to Inoculum) corresponding approximately to 50% in volume of the moisture liquid impregnating the solid particles and vinasse as the nutrient solution [21, 25, 26]. In addition to TOC and TN, sugarcane vinasse was characterized in terms of zinc, copper, iron, and manganese by atomic absorption spectroscopy in AAS Perkin Elmer®—PINAACLE T900 (Waltham, Massachusetts, EUA) [27]. A packed-bed column bioreactor of 30 mm diameter was filled up to 60 mm bed height with the selected particles of sugarcane bagasse without any further pretreatment besides the sterilization in autoclave as solid support. SSC conditions were 30 °C and air flow-rate of 0.2 L min–1 of water-saturation as shown in Fig. 1 [26]. The solid medium presented initial moisture of 80% (vinasse as impregnating solution and inoculum suspension with 50% of A. niger and 50% of T. reesei). All SSC experiments were set up in triplicate.
The fungal extract was obtained with 1:15 deionized water (solid–water) by rotating in an orbital shaker at 100 rpm and 28 °C for 90 min according to conditions adapted from [28, 29] and optimized according to [26]. The fungal extract obtained for each condition was filtered to 0.45 µm pores diameter to remove bagasse and spores. The pH was determined by potentiometry, glucose by the LABORLAB® kit; and carbon and total nitrogen by SHIMADZU® TOC-LCPN. For ethanol fermentation by yeasts, the filtration of fungal extract (filter unit of 0.45 µm) allows cell-free extract according to the SSC conditions optimized previously [22, 25, 26, 30, 31].
For SSC, the yield “bulk” from nutrient solution and bagasse “solid medium” were calculated as a function of the TOC present in the impregnating solution and the TOC in the sugarcane bagasse particles, respectively.
Acid hydrolysates
The sugarcane bagasse particles were pretreated with 1% (volume) sulfuric acid at 127 °C and 1.5 kgf cm−2 for 60 min. After autoclaving, the particles were vacuum-filtered to separate the liquid and the bagasse particles. In sequence of the pretreatment, sulfuric acid was added in the proportion of 100 mg of sulfuric acid per g of bagasse at a ratio of 1:10 solid/liquid, 127 °C and 1.5 kgf cm−2 for 50 min in the autoclave [32]. The material was then vacuum-filtered with a qualitative filter paper to separate the liquid. The pH was adjusted to 5.5 using NaOH and then centrifuged an RCF 1844 × G at 15 min by SOLAB® (Piracicaba, Brazil).
Ethanol fermentation from hydrolysates
The inoculum was centrifuged and transferred to microbial (SSC) or acid hydrolysate for testing. For acid hydrolysate, the inoculum was subjected to adaptation containing 50% of the hydrolysate and 50% of sterile distilled water for 24 h. In each vial containing 30 mL of the sterile hydrolysate, 10 mL of the cell suspension (about 1 g of yeast wet mass) was incubated at 30 °C and rotated at 150 rpm. Biomass was evaluated by measuring the optical density at 600 nm, glucose by the LABORLAB® kit, ethanol, acetic acid, furfural and 5-hydroxymetylfurfural by Gas-Phase Chromatography. Ethanol fermentation was set up in triplicate and evaluated in terms of the kinetic profiles of ethanol production, yeast growth, and glucose consumption, allowing the estimation of yield and maximum productivity in ethanol, and specific production rates from both hydrolysates.
Analyses
Ethanol, acetic acid, furfural and 5-hydroxymethylfural (5-HMF) were determined by gas chromatography on a GC-2010 Plus SHIMADZU® instrument, Stabilwax®-DA 30 m, 0.25 mm ID, 0.25 μm column and FID detector.
Results and discussion
Solid-state cultivation by Aspergillus niger and Trichoderma reesei for glucose release from sugarcane bagasse
Studies conducted with SSC revealed that T. reesei and A. niger are potential fungi for cellulase production and growth from sugarcane bagasse as the solid support [21]. SSC processes can be effectively used to produce cellulases from lignocellulosic material. Endoglucanases and β-glucosidases can be produced from SSC with this type of microbial association [33, 34]. Significant increases in the activities of total cellulases, endoglucanases, and xylanases were related to the addition of simple sources of nitrogen, yeast extract, peptone, and potassium phosphate in A. niger cultures [35]. Mineral salts and trace elements such as iron sulfate, manganese sulfate, zinc sulfate, and cobalt chloride were also effective for T. reesei cellulase production [33]. Thus, the solid support sugarcane bagasse was impregnated with vinasse, the wastewater from ethanol fermentation-distillation processes. It has several mineral salts that contributed to the production of cellulases, as well as low concentration of alcohol that influenced the fungal growth. It should be noted that the vinasse used to moisten the solid particles of bagasse had a favorable C/N ratio of around 20 (TOC 10.360 mg L–1, total nitrogen 502.7 mg L–1, zinc 0.69 mg L–1, copper 0.035 mg L–1, iron 14.5 mg L–1, and manganese 3.11 mg L–1). According to Khonngram and Salakkam [4], fungal growth should be enhanced to increase the hydrolysis efficiency and improve the activities of the cellulolytic enzymes. Thus, it is fundamental to adjust the media components, particularly to obtain a suitable C/N ratio. Therefore, usually solid supports such as bagasse with carbon sources are impregnated with nutrient solutions containing nitrogen source and ions as wastewaters (vinasse or dry spent yeast) [4, 21, 22].
According to Table 1, the glucose content after 24 h of SSC was approximately 0.8 g L–1, i.e., this was the amount of glucose released in the extract after fungal cultivation. Whereas the initial glucose concentration in vinasse was 0.14 g L–1, the total (final) amount of glucose released was due to the hydrolysis of the structural polysaccharides of bagasse by the process of SSC. However, after the sterilization process to fermentation, the glucose content of the extract obtained from SSC decreased from 0.8 to 0.35 g L−1. This factor could be attributed to the synergistic effect of temperature and acidic pH in the medium containing a larger amount of vinasse; it presented colloidal organic matter that may have been associated with glucose, making this sugar unavailable. A relatively lower glucose concentration (0.8 g L–1) was obtained in the current study in comparison with other studies on enzymatic hydrolysis [2, 35, 36]. However, it should be clarified that the sugarcane bagasse used here did not undergo a pretreatment process, a step normally used in enzymatic hydrolysis and in this sense, pretreatment can be an alternative to decrease the cellulose crystallinity and increase the sugar yield [37]. This is the glucose content obtained from the extract in the 1:15 ratio (solid–water). Thus, considering that cellulose around 45% [37] of the dry mass of the particles, there was the release of 0.8 g L−1 of glucose from an original amount 5.3 g of cellulose per liter. Moreover, it should also be considered that part of this glucose was used for fungal growth.
Rodrigues et al. [38] obtained a glucose concentration of around 1 g L–1 in the acid hydrolysate from sugarcane bagasse. On the other hand, Martini et al. [3] reported values close to 3 g L–1, but with high concentrations of inhibitors such as furfural and 5-hydroxymethylfurural. Rocha et al. [37] found similar values but with pretreated sugarcane bagasse. The glucose yield from the mass of sugarcane bagasse particles was in the same magnitude (between 0.04 and 0.08 g g–1 in terms of reducing sugars) as the values obtained by Khonngam and Salakkam [4] in their study on A. niger. According to these authors, the highest first-order reaction rate for the release of the reducing sugar by hydrolysis of sugarcane bagasse with dry spent yeast was 0.15 h–1; it resulted in a generation time of about 5 h in less than 24 h of SSC (Table 1). In addition, there was no optimization of glucose release by SSC, suggesting the potential of higher glucose content in different conditions of solid particle size, fungal inoculum ratio, and temperature, as examples.
The TOC content of the impregnating solution with vinasse (10,360 mg L–1) resulted in hydrolytic enzyme activity due to the limited use of carbon in the liquid phase; very less amount was available in the form of simple sugars. Thus, the structural polysaccharides of the sugarcane bagasse also led to slightly higher glucose content (0.8 g L–1). The results from Table 1 indicate the organic carbon consumption of sugarcane bagasse, suggesting polysaccharide degradation and sequential glucose release in the fungal extract for use in ethanol fermentation. Yields of carbon-glucose indicated carbon consumption from both the solid medium (0.2 mg C glucose to mg C bagasse) as well as from vinasse as the nutrient solution (0.077 mg C glucose to mg C of bulk phase). Thus, SSC allowed the release of glucose and obtained a fungal extract that could be reused as a culture medium for fermentation, i.e., a “microbial hydrolysate”. In terms of mass balance of carbon, the difference between TOC sugarcane bagasse particles in the 24 h of SSC was an amount of 0.024 g of carbon consumed and the release of 0.012 g of carbon released in terms of glucose, both per gram of bagasse. Therefore, results from this mass balance indicates that a half of the carbon from sugarcane bagasse has been converted into biomass or fungal metabolites, while the other half being released into the extract as glucose, in addition to part of the carbon from the nutrient solution (vinasse).
Ethanol fermentation of hydrolysates from solid-state cultivation and acid treatment of sugarcane bagasse
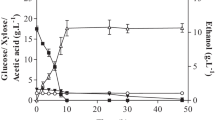
Fermentative assays with S. cerevisiae PE-2 yeast were set up for 72 h from the microbial hydrolysates obtained by SSC for 24 h with sugarcane bagasse particles impregnated with vinasse. Biomass and pH (data not shown) values remained practically constant during the tests. Della-Bianca et al. [39] reported that the PE-2 strain proved efficient for product formation in the pH range of 3–5. In addition, the pH of 4.5 ensured better ethanol yield and higher cell integrity during the fermentation process with the PE-2 strains [40]. The profiles indicated that ethanol production was directly linked to glucose depletion in the first 6 h, thus limiting yeast growth.
Table 2 presents ethanol-glucose yields of 0.40 g g–1, about 78% of maximum stoichiometry. This yield was similar (0.42 g g–1) to the one reported by Salakkam et al. [23]. However, these authors utilized soybean residue supplemented with sugarcane molasses at the concentration of about 110 g L–1 of total sugars to produce ethanol. Blomqvist et al. [41], in fermentations with diluted hydrolysate, reported a yield of 0.2 g g–1 for Dekkera bruxellensis and Saccharomyces cerevisiae yeasts. Several studies using Pichia stipitis, one of the most important pentose-fermenting yeasts from hydrolysates, reported yields of 0.35–0.48 g g–1 at 36 h of cultivation [42,43,44,45,46,47,48]. In contrast, we observed depletion of glucose after only six hours of cultivation, leading to a maximum yield of ethanol within this time. It is important to note that concentrations of acetic acid, 5-HMF, and furfural inhibitors were not detected in this microbial hydrolysate (data not shown), which may suggest the highest yield. Thus, these results are promising, since there was no optimization of SSC or complementation of the fungal extract with other sugars.
Even though the Saccharomyces yeast was admittedly unable to consume pentose’s, similar or even higher yields were obtained from both extraction conditions when compared with the production of 2G ethanol from acid hydrolysates [1, 49]. Thus, it was suggested that there was little influence of inhibitors, the results were promising, and could be further optimized with sugarcane bagasse pretreatment steps.
Table 3 shows the kinetic parameters for ethanol fermentation from acid hydrolysates. Glucose depletion occurred after 12 h of cultivation, i.e., it was slower than that for the fermentation condition from “microbial hydrolysates”, suggesting a lower conversion rate. Moreover, the yield of glucose in ethanol was around 0.08 g g–1, which means approximately 16% of efficiency, suggesting some limitation or inhibition. The results of inhibitors present in the acid hydrolysate may indicate that the synergic effect of acetic acid, 5-HMF, and furfural contributed as a potential inhibitor of ethanol fermentation. This was in addition to lower productivity as a probable response to the effect of other components in the medium.
Results suggest that, unlike the “second-generation ethanol” conventionally obtained via the acid hydrolysis of lignocellulosic materials, there was no considerable inhibition of yeast growth in the present method. Potential inhibitors of ethanol fermentation were formed and released from hemicellulose, cellulose, and lignin by the thermochemical route of hydrolysis. Examples include furfural, 5-hydroxymethylfurfural (HMF), levulinic acid, acetic acid, and formic acid among others [49]. In this context, phenols are considered inhibitors and deactivators of cellulolytic enzymes and glycosidases. Tannic acid was a major inhibitor and deactivator for all enzyme activities tested in the literature, with monomeric phenolic compounds having a less pronounced effect [50]. In this research, the inhibitory effects of polymeric and monomeric phenols were evaluated in different microorganisms commonly used to produce commercial enzymes aimed at converting cellulose into ethanol. These authors indicate that alternative strategies should be tested including enzymatic hydrolysis in shorter periods to minimize the effect of time, removing phenolics before the enzyme hydrolysis by separation methods, including washing the solids, or using microbial, enzymatic, and chemicals to inactivate phenolics. It is known that phenolics compounds derived from pretreated sugarcane bagasse (liquid hot water) pretreatment deactivate cellulolytic and hemicellulolytic enzymes [51]. In this sense, proposals for detoxification by bio-abatement have been set up to remove enzymatic inhibitors from biomass liquors. Bio-abatement by Coniochaeta ligniaria led to a 1.2- to1.5-fold increase for cellulose conversion in comparison of non-biotreatment [52]. Thus, it was concluded that these inhibitors, if present in the fermentation from SSC hydrolysates, occur in non-inhibitory concentrations.
In terms of specific production rates (µP), the results indicated higher values for fermentation in the medium containing microbial hydrolysate. Even with the lowest biomass production (0.35 g L–1, Table 2), there was high productivity (23.45 mg per liter per hour), indicating anaerobic conditions and supply of carbon, nitrogen, and nutrients that made ethanol fermentation possible. This SSC hydrolysate could be used as a culture medium under these conditions. Therefore, the fermentation results from “microbial hydrolysates” could have been even better and the next steps need to evaluate the composition of the crude extract in addition to that of glucose. In addition, the selection of the SSC inoculum proportion (A. niger and T. reesei) itself was critical for greater glucose release and nutrient balance in the unconventional medium that would be used for ethanol fermentation.
Conclusions
The results indicated that the proposal of a sequential SSC-ethanol fermentation allowed the use of two relevant industrial by-products of this region of Brazil (sugarcane bagasse and vinasse as the impregnating solution) and glucose was released. SSC with the fungal consortium released glucose, and high yields were obtained in the ethanol fermentation from these sugars in the hydrolysate. This indicated the technical viability of its use for 2G ethanol production due to the release of sugars without the presence of inhibitors in comparison to acid hydrolysates.
References
Codato CB, Martini C, Ceccato-Antonini SR, Bastos RG (2018) Ethanol production from Dekkera bruxellensis in synthetic media with pentose. Braz J Chem Eng 35:11–17. https://doi.org/10.1590/0104-6632.20180351s20160475
Barcelos CA, Maeda RN, Betancur GJV, Pereira JRN (2013) The essentialness of delignification on enzymatic hydrolysis of sugarcane bagasse cellulignin for second generation ethanol production. Waste Biomass Valorization 4:341–346. https://doi.org/10.1007/s12649-012-9137-3
Martini C, Tauk-Tornisielo SM, Codato CB, Basto RG, Ceccato-Antonini SR (2016) A strain of Meyerozyma guilliermondii isolated from sugarcane juice able to grow and ferment pentoses in synthetic and bagasse hydrolysates media. World J Microbiol Biotechnol 32:1–9. https://doi.org/10.1007/s11274-016-2036-1
Khonngam T, Salakkam A (2019) Bioconversion of sugarcane bagasse and dry spent yeast to ethanol through a sequential process consisting of solid-state fermentation, hydrolysis, and submerged fermentation. Biochem Eng J 150:107284. https://doi.org/10.1016/j.bej.2019.107284
Prajapati BP, Jana UK, Suryawanshi RK, Kango N (2020) Sugarcane bagasse saccharification using Aspergillus tubingensis enzymatic cocktail for 2G bio-ethanol production. Renew Energy 152:653–663. https://doi.org/10.1016/j.renene.2020.01.063
Langan P, Petridis LO’, Neill HM, Pingali SV, Foston M, Yoshiharu N, Schulz R, Lindner B, Hanson BL, Harton S, Heller WT, Urban V, Evans BR, Gnanakaran S, Ragauskas AJ, Smith JC, Davison B (2014) Common processes drive the thermochemical pretreatment of lignocellulosic biomass. Green Chem 16:63–68. https://doi.org/10.1039/C3GC41962B
Sun Y, Cheng J (2005) Dilute acid pretreatment of rye straw and bermudagrass for ethanol production. Bioresource Technol 96:1599–1606. https://doi.org/10.1016/j.biortech.2004.12.022
Taherzadeh MJ, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci 9:1621–1651
Kumar R, Mago G, Balan V, Wyman CE (2009) Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies. Bioresource Technol 100:3948–3962. https://doi.org/10.1016/j.biortech.2009.01.075
Yan X, Wang Z, Zhang K, Si M, Liu M, Chai L, Liu X, Shi Y (2017) Bacteria-enhanced dilute acid pretreatment of lignocellulosic biomass. Bioresource Technol 245:419–425. https://doi.org/10.1016/j.biortech.2017.08.037
Solarte-Toro J, Romero-Gacrcía JM, Martínez-Patiño JC, Ruiz-Ramos E, Castro-Galiano E, Cardona-Alzate CA (2019) Acid pretreatment of lignocellulosic biomass for energy vectors production: a review focused on operational conditions and techno-economic assessment for bioethanol production . Renewable Sustainable Energy Rev 107:587–601. https://doi.org/10.1016/j.rser.2019.02.024
Soares PA, Vaz-Rossel CE (2007) Conversão de celulose pela tecnologia organosolv, vol 3. NAIPPE-USP, São Paulo
Olsson L, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates II: inhibitors and mechanisms of inhibition. Bioresource Technol 74:25–33. https://doi.org/10.1016/S0960-8524(99)00161-3
Hahn-Hägerdal B, Karhumaa K, Fonseca C, Spencer-Martins I, Gorwa-Grauslund M (2007) Towards industrial pentose-fermenting yeast strains. Appl Microbiol Biotechnol 74:937–953. https://doi.org/10.1007/s00253-006-0827-2
Fonseca BG, Moutta RO, Ferraz FO, Vieira ER, Nogueira AS, Baratella BF, Rodrigues LC, Hou-Rui Z, da Silva SS (2011) Biological detoxification of different hemicellulosic hydrolysates using Issatchenkia occidentalis CCTCC M 206097. J Ind Microbiol Biotechnol 38:199–207. https://doi.org/10.1007/s10295-010-0845-z
Khajeeram S, Unrean P (2017) Techno economic assessment of high solid simultaneous saccharification and fermentation and economic impacts of yeast consortium and on-site enzyme production technologies. Energy 122:194–203. https://doi.org/10.1016/j.energy.2017.01.090
Gutierrez-Correa M, Tengerdy RP (1997) Production of cellulase on sugar cane bagasse by fungal mixed culture solid substrate fermentation. Biotechnol Lett 19:665–667. https://doi.org/10.1023/A:1018342916095
Pandey A (2003) Solid-state fermentation. Biochem Eng J 13:81–84. https://doi.org/10.1016/S1369-703X(02)00121-3
Thomas L, Larroche C, Pandey A (2013) Current developments in solid-state fermentation. Biochem Eng J 81:146–161. https://doi.org/10.1016/j.bej.2013.10.013
Gutiérrez-Correa M, Villena GK (2017) Batch and repeated batch cellulase production by mixed cultures of Trichoderma reesei and Aspergillus niger or Aspergillus phoenicis. J Microbiol Biotechnol Res 2(6):929–935
Campanhol BS, Silveira GC, Castro MC, Ceccato-Antonini SR, Bastos RG (2019) Effect of the nutrient solution in the microbial production of citric acid from sugarcane bagasse and vinasse. Biocatal Agric Biotechnol 19:101147. https://doi.org/10.1016/j.bcab.2019.101147
Bastos RG, França HCR, Campanhol BS, Castro MC, Silveira GC (2017). Sequential process of citric acid production in sugarcane bagasse by microbial consortium and ethanol fermentation from fungal extract. In: 2017 ASABE Annual International Meeting, Spokane, WA. Proceedings ASABE 2017 1700161. https://doi.org/10.13031/aim.201700161
Salakkam A, Kingpho Y, Najunhom S, Aiamsonthi K, Kaewlao S, Reungsang A (2017) Bioconversion of soybean residue for use as alternative nutrient source for ethanol fermentation. Biochem Eng J 125:65–72. https://doi.org/10.1016/j.bej.2017.05.020
Kumar D, Jain VK, Shanker G, Srivastava A (2003) Citric acid production by solid state fermentation using sugarcane bagasse. Process Biochem 38:1731–1738. https://doi.org/10.1016/S0032-9592(02)00252-2
Oliveira AF, Matos VC, Bastos RG (2012) Cultivation of Aspergillus niger on sugarcane bagasse with vinasse. Biosci J 28:889–894
Bastos RG, Morais DV, Volpi MPC (2015) Influence of solid moisture and bed height on cultivation of Aspergillus niger from sugarcane bagasse with vinasse. Braz J Chem Eng 32:377–384. https://doi.org/10.1590/0104-6632.20150322s00003423
Federation WE, APH Association (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA): Washington, DC, p 1220
Motta FL, Santana MHA (2014) Solid-state fermentation for humic acids production by a Trichoderma reesei strain using an oil palm empty fruit bunch as the substrate. Appl Biochem Biotechnol 172:2205–2217. https://doi.org/10.1007/s12010-013-0668-2
Khosravi-Darani K, Zoghi A (2008) Comparison of pretreatment strategies of sugarcane bagasse experimental design for citric acid production. Bioresource Technol 99:6986–6993. https://doi.org/10.1016/j.biortech.2008.01.024
Bastos RG, Motta FL, Santana MHA (2016) Oxygen transfer in the solid-state cultivation of D. monoceras on polyurethane foam as an inert support. Braz J Chem Eng 33:793–799. https://doi.org/10.1590/0104-6632.20160334s20150262
Ferrari FA, Motta FL, Bastos RG, Santana MHA (2013) The solid state cultivation of Streptococcus zooepidemicus in polyurethane foam as a strategy for the production of hyaluronic acid. Appl Biochem Biotechnol 170:1491–1502. https://doi.org/10.1007/s12010-013-0293-0
Silva JPA, Carneiro LM, Conceição IR (2014) Assessment of advanced oxidative processes based on heterogeneous catalysis as a detoxification method of rice straw hemicellulose hydrolysate and their effect on ethanol production by Pichia stipites. Biomass Convers Biorefinery 4:225–236. https://doi.org/10.1007/s13399-013-0104-4
Mekala NK, Singhania RR, Sukumaran RK, Pandey A (2008) Cellulase production under solidstate fermentation by Trichoderma reesei RUT C30 Statistical optimization of process parameters. Appl Biochem Biotechnol 151:122–131. https://doi.org/10.1007/s12010-008-8156-9
Maeda RN, Serpa VI, Rocha VAL, Mesquita RAA, Anna LMMS, Castro AM, Driemeier CE, Pereira-Junior N, Polikarpov I (2011) Enzymatic hydrolysis of pretreated sugar cane bagasse using Penicillium funiculosum and Trichoderma harzianum cellulases. Process Biochem 46:1196–1201. https://doi.org/10.1016/j.procbio.2011.01.022
Rodríguez-Zúñiga UF, Neto VB, Couri S, Crestana S, Farinas CS (2014) Use of spectroscopic and imaging techniques to evaluate pretreatment sugarcane bagasse as a substrate for cellulase production under solid-state fermentation. Appl Biochem Biotechnol 172(5):2348–2362
Sørensen A, Andersen JJ, Ahring BK, Teller PJ, Lübeck M (2014) Screening of carbon sources for beta-glucosidase production by Aspergillus saccharolyticus. Int Biodeterior Biodegrad 93:78–83. https://doi.org/10.1016/j.ibiod.2014.05.011
Rocha NRDAF, Martin C, Soares IB, Maior AMS, Baudel HM, Abreu CAM (2011) Dilute mixed-acid pretreatment of sugarcane bagasse for ethanol production. Biomass Bioenerg 35(1):663–670. https://doi.org/10.1016/j.biombioe.2010.10.018
Rodrigues RCLB, Rocha GJM, Rodrigues-Junior D, Filho HJI, Felipe MDGA (2010) Scale-up of diluted sulfuric acid hydrolysis for producing sugarcane bagasse hemicellulosic hydrolisate (SBHH). Bioresource Technol 101:1247–1253. https://doi.org/10.1016/j.biortech.2009.09.034
Della-Bianca BE, Hulster E, Pronk JT, van Maris AJA, Gombert AK (2014) Physiology of the fuel ethanol strain Saccharomyces cerevisiae PE-2 at low pH indicates a context-dependent performance relevant for industrial applications. FEMS Yeast Res 14(8):1196–1205. https://doi.org/10.1111/1567-1364.12217
Dorta C, de Oliva-Neto P, Abreu-Neto MS, Nicolau-Junior N, Nagashima AI (2006) Synergism among lactic acid, sulfite, pH and ethanol in alcoholic fermentation of Saccharomyces cerevisiae (PE-2 and M-26). World J Microbiol Biotechnol 22:177–182. https://doi.org/10.1007/s11274-005-9016-1
Blomqvist J, South E, Tiukova L, Momeni MH, Hansson H, Ståhlberg J, Horn SJ, Schnürer J, Passoth V (2011) Fermentation of lignocellulosic hydrolysate by the alternative industrial ethanol yeast Dekkera bruxellensis. Lett Appl Microbiol 53(1):73–78. https://doi.org/10.1111/j.1472-765X.2011.03067.x
Hahn-Hagerdal B, Linden T, Senac T, Skoog K (1991) Ethanolic fermentation of pentoses in lignocellulose hydrolysate. Appl Biochem Biotechnol 28:28–29. https://doi.org/10.1007/bf02922595
Amartey S, Jeffries T (1996) An improvement in Pichia stipitis fermentation of acid-hydrolysed hemicellulose achieved by overliming (calcium hydroxide treatment) and strain adaptation. World J Microbiol Biotechnol 12:281–283. https://doi.org/10.1007/bf00360928
Awafo VA, Chahal DS, Simpson BK (1998) Optimization of ethanol production by Saccharomyces cerevisiae (ATCC 60868) and Pichia stipitis y-7124: a response surface model for simultaneous hydrolysis and fermentation of wheat straw. J Food Biochem 22:489–509. https://doi.org/10.1111/j.1745-4514.1998.tb00258.x
Nigam JN (2001) Development of xylose-fermenting yeast Pichia stipitis for ethanol production through adaptation on hardwood hemicellulose acid prehydrolysate. J Appl Microbiol 90:208–215. https://doi.org/10.1046/j.1365-2672.2001.01234.x
Agbogbo FK, Cowaed-Kelly G, Torry-Smith M, Wenger K, Jeffries TW (2007) The effect of initial cell concentration on xylose fermentation by Pichia stipites. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-007-9086-7
Kuhar S, Nair LM, Kuhad RC (2008) Pretreatment of lignocellulosic material with fungi capable of higher lignin degradation and lower carbohydrate degradation improves substrate acid hydrolysis and the eventual conversion to ethanol. Can J Microbiol 54:305–313. https://doi.org/10.1139/w08-003
Ceccato-Antonini SR, Codato CB, Martini C, Bastos RG, Tauk-Tornisielo SM (2017) Yeast for pentose fermentation: isolation, screening, performance, manipulation and prospects. In: Buckeridge MSD, Souza AP (eds) Advances of basic science for second generation bioethanol from sugarcane. Springer, Switzerland, pp 133–157
Canilha L, Carvalho W, Felipe MGA, Silva JBA, Giulietti M (2010) Ethanol production from sugarcane bagasse hydrolysate using Pichia stipitis. Appl Biochem Biotechnol 161:84–92. https://doi.org/10.1007/s12010-009-8792-8
Ximenes E, Kim Y, Mosier N, Dien B, Ladisch MR (2011) Deactivation of cellulases by phenols. Enzym Microb Technol 48:54–60. https://doi.org/10.1016/j.enzmictec.2010.09.006
Michelin M, Ximenes E, Polizeli MLTM, Ladisch MR (2016) Effect of phenolic compounds from pretreated sugarcane bagasse on cellulolytic and hemicellulolytic activities. Bioresour Technol 199:275–278. https://doi.org/10.1016/j.biortech.2015.08.120
Cao G, Ximenes E, Nichols NN, Zhang L, Ladisch MR (2013) Biological abatement of cellulase inhibitors. Bioresour Technol 146:604–610. https://doi.org/10.1016/j.biortech.2013.07.112
Acknowledgements
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Brazil (Finance Code 88882.378479/2019-01 and 001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brito Codato, C., Gaspar Bastos, R. & Ceccato-Antonini, S.R. Sequential process of solid-state cultivation with fungal consortium and ethanol fermentation by Saccharomyces cerevisiae from sugarcane bagasse. Bioprocess Biosyst Eng 44, 1–8 (2021). https://doi.org/10.1007/s00449-021-02588-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02588-6