Abstract
In the present investigation, biocorrosion inhibition efficiency of Syzygium aromaticum (clove) aqueous extract on carbon steel in presence of four corrosion causing bacterial strains (Bacillus subtilis, Streptomyces parvus, Pseudomonas stutzeri, and Acinetobacter baumannii) was explored. Weight loss, potentiodynamic polarization, and AC impedance studies were carried out with and without bacterial strains and clove extract. The results obtained from weight loss and AC impedance studies indicate that these corrosion causing bacterial strains accelerated the biocorrosion reaction and biofilm playing a key role in this process. However, the addition of clove extract into the corrosive medium decreased the corrosion current and increased the solution and charge transfer resistance. The significant inhibition efficiency of about 87% was archived in the mixed consortia system with clove extract. The bioactive compounds were playing an important role in the antibacterial activity of the clove extract. It was revealed that clove extract has both biocidal and corrosion inhibition properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Corrosion is the destruction of the metallic structure by chemical and electrochemical reactions which is a major issue in almost all industrial sectors. Cost of the corrosion by direct and indirect causes is about 5% of the gross national product (GNP) of almost all the countries worldwide [1]. The United States alone spends about $500 billion for the management of corrosion issues [2]. Among the corrosion cost, microbiologically influenced corrosion (MIC) contributed about 20–50% directly or indirectly [3, 4]. Acceleration or speed up of the corrosion phenomenon by the action of a different kind of microorganisms such as bacteria, fungi, and archaea is defined as MIC, which leads to system failure and metal deterioration [5,6,7,8,9]. Biofilms playing a key role in the biocorrosion process in the pipeline structures [10,11,12]. Biofilms are highly hydrated, a mixture of both aerobic and anaerobic microbial colonies with the self-produced matrix of extracellular polymeric substances (EPS), which include both organic and inorganic (together with metals) substances [13,14,15,16,17]. Compared to the planktonic microbiota, biofilm consists of wide community ranges with massive cell concentrations up to 106 to 1011 [18, 19].
The impact and seriousness of the MIC have been frequently encountered in many industrial sectors such as in oil and gas industries, nuclear power plants [20], process plants, fuel reprocessing units, geothermal plants, power plants, sewage drains [21], storage tanks, pipelines [22], oilfields and their relevant areas like pumps, valves, and vessels [23], oil recovery systems, cooling water towers, fire sprinkler systems [24], pulp and paper industry [25], railway tracks [26] and radioactive disposal facilities [27]. So many accidents and incidents were recorded due to the direct and indirect causes of MIC in history. In 1977, a weld failure due to the MIC caused by sulfate-reducing bacteria (SRB) in hydro-testing water leads to the refrigerated propane tank explosion, which turns to a loss of about $179 million. One of the well-known major issues due to the MIC is the Prudhoe Bay oil spill in the Alaskan North slope, which makes a huge impact on the marine habituates, since 212,000 gallons of crude oil was discharged into the seawater due to the resolving of the deep-water leakage problem was taken several months [28]. Notable other accident due to the MIC is natural gas pipeline leakage followed by an explosion due to the internal corrosion in Carlsbad, New Mexico, United States.
Carbon steel is widely used in many industrial applications including oil and gas refineries, power plants, petrochemical plants, ships, and distillers due to its strong mechanical strength and low cost, and easy availability [29, 30]. The major problem associated with carbon steel is a higher susceptibility to corrosive environments especially in the higher alkaline and acid conditions [31, 32]. Different methods/approaches are tried to minimize the corrosion problems like using protective coatings and inorganic/organic inhibitors. These approaches are given significant protection to the carbon steel but the major problems are their higher cost, toxicity to the environment as well as to the living organisms. The alternative options to replace the chemical inhibitors are green inhibitors.
Plant-based green inhibitors are low cost, easily available, non-toxic, eco-friendly, and biodegradable. Some of the plant's derivatives are tested as the green inhibitors for the protection of carbon steel and other metallic structures from the corrosion problems such as Melia azedarach L. extract [33], ginger roots extract [34], Ficus tikoua leaves extract [35], Areca plant extracts [36], Salvia officinalis plant extract [37], curcumin, parsley, and cassia bark extracts [38], lemongrass extract [39], Phellodendron chinense Schneid bark extract [40], Cistus ladanifer [41], etc. Very surprisingly, very few green inhibitors only investigated for carbon steel which is susceptible to microbial corrosion such as ginger extract [42], Azadirachta indica leaves extract [6, 43, 44], Allium sativum extract [45], Daphne gnidium L. [46].
The spices are supposed to have many valuable properties and are used in the food for their aroma and nature. Among the spices, clove (Syzygium aromaticum) buds are widely considered for their antimicrobial and antioxidant properties. The clove tree is usually grown well in the coastal region with maximum altitudes of 200 m above sea level. Once the tree has grown up it will start to produce buds, before the flowering, flower buds will be harvested for commercial uses [47]. From the literature, it is believed that clove is rich with many bioactive compounds like acetyl eugenol, vanillin, betacaryophyllene, tannins, crategolic acid, methyl salicylate, gallotannic acid; the flavonoids like kaempferol, eugenin, eugenitin, and rhamnetin; triterpenoids like stigmasterol, oleanolic acid. Among these compounds, eugenol is the key bioactive compound present in the clove buds [48].
To date, so many green inhibitors are tried to minimize the chemical corrosion as well as microbial corrosion for carbon steel, and still, it is very challenging to find an inhibitor with the highest capability and potential alternative to the chemical inhibitors. With this current shortfall, we selected clove as an alternative green inhibitor to minimize the microbial growth and control the biocorrosion problems in the hypersaline condition. The primary interest in chosen clove bud extract as green inhibitors is their antimicrobial properties to the broad microbial groups and also it is enriched with many bioactive compounds. Hence in this study, we applied the clove buds extract to the carbon steel API 5LX, which is suspected of the biocorrosion caused by the four corrosive bacterial strains. In this study, weight loss experiments, electrochemical impedance spectroscopy, Tafel polarization, and surface analysis with scanning electron microscopy (SEM) and X-ray diffraction (XRD) are carried out to confirm the anticorrosion mechanism of the clove extract on carbon steel in hypersaline conditions.
Materials and methods
Bacterial strains and culture conditions
From our previous works [19, 49], we have isolated and identified many bacterial strains from the oil reservoir samples such as crude oil and produced water samples. Among the isolated bacterial strains, four strains were confirmed as potential corrosion causing bacterial strains such as Bacillus subtilis A1 (National Center for Biotechnology Information Accession No. KP895564), Streptomyces parvus B7 (KP895570), Pseudomonas stutzeri NA3 (KU708859), and Acinetobacter baumannii MN3 (KU708860). These strains were retrieved from the glycerol stock (-20 °C) and sub-cultured into the Luria–Bertani (LB) agar plates (g/L: 10.0 sodium chloride, 5.0 yeast extract, 10.0 tryptone, and 15.0 agar (Himedia, Mumbai, India)) and incubated for 24 h at 37 °C. From these plates, a contamination-free pure single colony and sub-cultured using the LB broth medium in the shaking condition.
Preparation of corrosion inhibitor
The clove (Syzygium aromaticum) buds were purchased from the local market and air-dried at 40 °C for 2 days and mortar and pestle were used to ground into the fine powder. 10 g of this powder was added with 100 mL of the deionized water and kept at room temperature for 24 h. After a day of immersion in the deionized water, clove (Syzygium aromaticum) buds extract (SAE) was filtered using Whatman filter paper and the filtrate was kept in a vacuum oven at 40 °C and the obtained residual extract was stored in the refrigerator for the further uses. The functional groups present in clove buds extract was analyzed using the Fourier transform infrared spectrum (FT-IR). The extracted sample was ground well with potassium bromide in a ratio of 1:99 and the sample was analyzed in the range of 400–4000 cm−1.
Antibacterial activity of the clove buds extract
The antibacterial activity and minimal inhibitory concentration of clove bud extract were confirmed as previously mentioned in Parthipan et al. [6]. Briefly, Mueller Hinton Agar (MH) (Himedia-India) plates were used for this purpose and six various concentrations of clove bud extract were tested such as 20, 50, 100, 150, 200, and 250 ppm. Selected four corrosion causing bacterial strains such as B. subtilis A1, S. parvus B7, P. stutzeri NA3, and A. baumannii MN3 were used for this antibacterial study. Pre-cultured bacterial strains were spread over the agar plates and a glass borer was used to make wells and 50 µL of each selected concentration of the clove buds extract were added in each well of four bacterial plates separately. Gentamycin (10 μg) was used as a positive bactericidal compound, which has inhibition activity against a wide range of bacterial strains. Zone of the inhibition was calculated after the 24 h of incubation at 37 °C and all the experiment was carried out in triplicates.
Biocorrosion studies
The biocorrosion experiments were carried out as described in our previous work [6]. Carbon steel API 5LX with weight percentage (wt%) of: C 0.070, Mn 1.05, Si 0.195, Ni 0.02, Cu 0.05, Cr 0.03, Al 0.029 and balanced with Fe. Carbon steel with two dimensions such as 25 × 25 × 4 mm and 10 × 10 × 2 mm were prepared for the weight loss and electrochemical studies, respectively. These metal coupons were initially smoothened using a different grade of silicon carbide papers from 180 to 1500 and 0.3 μM alumina powder was used for the final polishing. Produced water (salinity: 36 g/L) collected from the crude oil reservoir was used as the corrosive medium for the weight loss and electrochemical studies.
Weight loss studies
The coupons for the weight loss and electrochemical studies were surface sterilized with trichloroethylene and ultraviolet (UV) light before immersion into the corrosive medium. For weight loss experiments, initial weight was measured. A weight loss study was carried out as mentioned in Parthipan et al. [6]. Briefly, 300 mL of produced water alone taken into a wide-open 500 mL Erlenmeyer flask and triplicate coupons (both weight loss and electrochemical study coupons) were immersed and noted as a system I. System II–XII are similar to that of a system I, system II was added with 150 ppm of SAE inhibitor. Systems III, V, VII, and IX were added with 104 colony-forming unit per milliliter (CFU/mL) of each bacterial strain separately and system IV, VI, VIII, and X were inoculated with each bacterial strain and 150 ppm of SAE inhibitor, respectively. System XI was inoculated with mixed consortia (B. subtilis A1, S. parvus B7, P. stutzeri NA3, and A. baumannii MN3) of four strains and system XII was similar to system XI in addition to that, the inhibitor was added. These corrosion systems were kept stagnantly without any disturbance for the 20 days at room temperature. During the weight loss studies, coupons were retrieved at 2 days interval for sessile cell count. At the end of the weight loss experiments, rust products formed over the metal surface was carefully scraped and dried for the X-ray diffraction (XRD) analysis using the Bruker D8, samples are scanned with CuKα radiation (Ni filter). The coupons were then pickled with Clark solution as described in Parthipan et al. [6]. Coupons from the abiotic control system and mixed consortia system with and without inhibitor were subjected for the scanning electron microscopy (SEM) observation using Hitachi, S-3400N model. Besides, pits formed over the surface of the mixed consortia system was observed using SEM as described in Parthipan et al. [49]. After the picking process, the coupons were dried, further final weight of each coupon was measured and their weight loss was calculated from their initial weight. The corrosion rate was calculated from the weight loss information [50]. The inhibition efficiency (IE%) of SAE on the different corrosion systems was calculated based on the weight loss in each system as mentioned in our previous report [19]. All the studies were performed in triplicates.
Electrochemical analysis
The potentiodynamic polarization and AC impedance studies were carried out using the CH Instruments Inc., USA (Model: CHI-608E). At the end of the 20th day, the carbon steel retrieved from each corrosion system was used as the working electrode, saturated calomel electrode (SCE) used as reference electrode and platinum wire was used as a counter electrode. Corrosive medium recovered from the weight loss studies was utilized as the electrolyte solution for these studies. AC impedance was recorded at the frequency of 0.1–105 Hz with a scan rate of 10 mV/min. The potentiodynamic polarization measurements were carried out by polarizing towards +200 mV anodically and -200 mV cathodically with the scan rate of 0.002 V/s concerning corrosion potential (Ecorr). All the experiments were repeated three times. Electrochemical data obtained from this study were analyzed using the Zsim Demo 3.20d software.
Results and discussion
FT-IR analysis of clove extract
The FT-IR analysis of the clove extract was done as shown in Fig. 1, accordance with the wavenumbers, their functional groups were confirmed. This will provide us the structural properties of clove extract. The highly intensive band at 3427 cm−1 represents the OH group, alkyl CH stretch (sp3) was noted at 2932 cm−1. A sharp peak at 1723 cm−1 represented the frequency pattern of ester group C–O, a peak at 1617 cm−1 belong to the aliphatic alkenes. A sharp peak at 1512 cm−1 represents the aromatic group, a peak at 1451 cm−1 indicates the presence of methylen (CH2), sharp peaks at 1358 and 1205 cm−1 belongs to methyl groups (CH3). Another sharp peak at 1043 cm−1 represents C–O. Moderate bands at 916 and 760 cm−1 indicate the presence of CH2 and C=C, respectively [51]. These specific functional groups are well-matched with the standard eugenol functional groups; hence it is confirmed the presence of eugenol in the clove extract [52].
Antibacterial activity of SAE on corrosive bacterial strains
The aqueous extract of clove buds was tested against four corrosion causing bacterial strains. As mentioned in Table 1, very less inhibition activity was observed at the lower concentrations like 20, 50, and 100 ppm. Inhibition activity was increased with the increasing concentration of the extract. Significantly, 10–12 ± 1 mm of the inhibition was obtained at 150 ppm. Maximum inhibition of 20 ± 1 mm was observed at the tested concentration of 250 ppm. These outcomes revealed that clove buds contain a broad range of bioactive compounds, which shows a very good bactericidal activity on both Gram-positive and Gram-negative bacterial strains [47, 53]. Based on the zone of inhibition, 150 ppm of SAE was selected as the inhibitor concentration to apply to the corrosion systems.
Biocorrosion studies
Weight loss and inhibition efficiency
Weight loss study was carried out with and without bacterial strains and clove inhibitor. For each system, triplicates carbon steel coupons were utilized to confirm the accurate weight loss during the 20 days of immersion period. The weight loss obtained from different corrosion systems were presented in Table 2. The produced water alone used in system I was considered as the abiotic control system which was recorded with 16 ± 1 mg. This weight loss confirmed the presents of the higher content of chloride ions in the produced water, which causes chemical corrosion over the surface of carbon steel. At the same time, while adding the clove extract into the produced water (system II) weight loss was decreased considerably with 3.3 ± 0.2 mg. Corrosion causing bacterial strains leads to very severe corrosion over the carbon steel surface by recording a higher amount of weight loss comparatively with an abiotic control system with the range of 32.8 ± 1 (system IX, with strain MN3) to 40.2 ± 2 mg (system-V, with strain B7). Higher weight loss was noticed in the mixed consortia system with 48.2 ± 2 mg. Similar to system II, the addition of clove extract into the bacterial systems (IV, VI, VIII, X, and XII) decreased the weight loss enormously with a range of 4.4 to 6.4 ± 0.5 mg. From the weight loss studies, it revealed that clove extract added into different biocorrosion systems inhibited bacterial growth and development. Besides, clove extracts playing a dual role by suppressing bacterial growth as well as by forming a protective layer over the surface of carbon steel, which leads to very less chemical damage in the rich chloride environment [54].
The corrosion rate in the presence and absence of the bacterial strains and clove inhibitor was calculated from weight loss information and presented in Table 2. The corrosion rate in the abiotic control system is 0.060 mm/y, which was reduced to the 0.012 mm/y while included with the clove extract. Corrosion rates were very high ranging from 0.114 to 0.150 mm/y in presence of each bacterial strain. At the same time, the inhibitor added bacterial system found less corrosion rate with the range of 0.016 to 0.018 mm/y. Among these corrosion systems, a very higher corrosion rate was recorded in the mixed consortia system with 0.179 mm/y due to the vigorous bacterial corrosion. Outcomes from this observation strongly support that bacterial strains actively participated in the biocorrosion process and leads to the metal deterioration, interestingly the clove extract showed very good corrosion inhibition activity in the presence of mixed consortia by inhibiting both the Gram-positive and Gram-negative biofilms forming bacterial strains.
Based on the weight loss information, corrosion inhibition efficiency (IE) of the clove extract on different corrosion systems was calculated and presented in Table 2. Strain S. parvus was highly sensitive to the clove extract with an IE of 89% followed by the B. subtilis A1 (88%), P. stutzeri NA3 (88%), and A. baumannii MN3 (85%). Least IE was noticed in the abiotic control system with 79%. Still, clove extract performed a considerable level of corrosion inhibition. Clove extract showed good inhibition activity while adding into the mixed consortia system with an IE of 87%. The inhibition efficiency obtained from this study was much higher than the clove extract tested on the acidic and alkaline corrosive medium on different metals [54, 55].
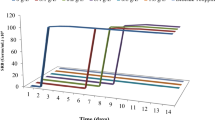
During the biocorrosion experiments, the total viable count of each corrosion system was calculated at 2 days interval to confirm their viability towards the end of the corrosion experiments and presented in Fig. 2. There is no bacterial colony was observed in the abiotic control which ensures that the corrosion system is free from the contaminations. In presence of the bacterial strains, growth was counted up to 105 to 106 (CFU/cm2). The stationary growth phase for each bacterial strain was observed between 8 to 10th day of incubation and further colonies were started to decrease with an increasing incubation period. A very few colonies 101 (CFU/cm2) was observed on the bacterial system while the addition of the clove extract by the second day of incubation, which was subsequently disappeared, and no visible colonies were observed with a further incubation period. These biofilms forming bacterial strains were formed as very dense biofilm over the metal surface of carbon steel and create vigorous pitting type of corrosion, at the same time clove extract inhibited bacterial growth and also decreased the corrosion rate as well.
Total viable count of bacterial colonies from different biocorrosion systems. The viable colonies are monitored every 2 days interval during the weight loss studies. Note. AC abiotic control; A1 B. subtilis; B7 S. parvus; NA3 P. stutzeri; MN3 A. baumannii; SAE Syzygium aromaticum extract; MC mixed consortia
Electrochemical studies
Potentiodynamic polarization analysis
Potentiodynamic polarization curves were recorded after the 20 days of the corrosion studies and presented in Fig. 3. From polarization curves, the corrosion current density (Icorr), corrosion potential (Ecorr), anodic Tafel slopes (βa) and cathodic Tafel slopes (βc) were calculated and were given in Table 3. A very high corrosion current was observed in the bacterial system which indicates that bacterial strains promoted the corrosion reactions. From Fig. 3, it was very clear that Ecorr of the bacterial systems was moved towards the negative direction. At the same time, this shift was changed towards the positive side when included with the clove inhibitor. From Table 3, the Icorr value of the mixed consortia is 3.19 ± 0.17 × 10–4 V vs SCE. But it was reduced to 1.11 ± 0.12 × 10–4 V vs SCE in addition of the clove extract into the corrosive medium. This shifting trend was noticed from many green inhibitors in recent studies [45, 56]. Interestingly, both the anodic and the cathodic current densities were decreased in the inhibitor included systems compared to the respective uninhibited systems, which indicates that added SAE inhibitor solution adsorbed over the carbon steel surface and inhibited both the anodic and the cathodic corrosion reactions. Also, SAE inhibitor acting as a mixed type of inhibitor for the biocorrosion control.
The potentiodynamic polarization curves of different corrosion systems with and without bacterial strains and clove extract. Respective growth medium (produced water) is used as an electrolyte solution for this measurement. These polarization measurements are carried out by polarizing towards +200 mV anodically and -200 mV cathodically with the scan rate of 0.002 V/s concerning corrosion potential. Note. AC abiotic control; A1 B. subtilis; B7 S. parvus; NA3 P. stutzeri; MN3 A. baumannii; SAE Syzygium aromaticum extract; MC mixed consortia
AC impedance analysis
AC impedance was analysed at the end of the biocorrosion experiments and the equivalent circuit (Fig. 4) was used to fit the impedance data and calculated different parameters such as Rs: solution resistance, Qf: capacitance of biofilm, Rb: biofilm resistance, Qdl, capacitance of electric double layer and Rct, charge transfer resistance were presented in Table 4 (Fig. 5). During the corrosion reactions, the accumulation of solution species at the metal–solution interface consequences the development of an electric double layer with capacitance and resistance structures to transfer of charges. So, this double layer could affect the rate of electron transfer and mechanism between anodic and cathodic sites over the surface of the metal. In this study, Table 4 provides details of dielectric properties of metal–solution interface, the effect of the inhibitor on electric double layer.
AC impedance curve of different corrosion systems with and without bacterial strains and clove extract. Impedance is recorded at the frequency range of 0.1–105 Hz with a scan rate of 10 mV/min. Note. AC abiotic control; A1 B. subtilis; B7 S. parvus; NA3 P. stutzeri; MN3 A. baumannii; SAE Syzygium aromaticum extract, MC mixed consortia
It was clearly illustrated that the semi-circle curves in the inhibitor system were larger than that of in bacterial strain alone system, which suggesting that very little corrosion with the clove extract added system [57, 58]. Solution resistance (Rs) in the abiotic control system was observed at 6.253 ± 0.24 Ω cm2, which was very lesser compared to the bacterial systems (i.e. 9.102 ± 0.29 Ω cm2 in the mixed consortia system). Rs was increased while the corrosive medium was included with the clove extract in both biotic and abiotic systems. Similarly, Rct in the biotic systems was very less, which indicates that bacterial strains formed the biofilm over the metal surface and decreased the metal resistance [59]. At the movement, when clove extract was added into the corrosive medium, the Rct was increased into 3–4 folds than their respective biotic and abiotic systems. Interesting information was obtained from the biofilm resistance (Rb) that, Rb is very high in the biotic system and which was decreased when the clove extract suppressed the biofilm development; this trend was according to the recent works by Zhai et al. [60] and Jia et al. [57]. These observations strongly support that bioactive compound playing a key role in biofilm control as well as corrosion protection from the chloride attack.
Surface analysis
At the end of the weight loss study, rust product formed over the surface of the carbon steel during the corrosion reaction was collected and analyzed using the XRD and presented in Fig. 6. In the abiotic system, the major rust products were ferrous hydroxide (Fe(OH)2), manganese (II) hydroxide (Mn(OH)2), and ferrous chloride (Fe(Cl)2). In turn, ferric oxide (Fe2O3) and manganese oxide (Mn3O4) were found as the major rust products on the biotic systems. The observation of these rust products in the individual as well as in the mixed consortia indicates that all these bacterial strains can oxidize the inorganic metal components present in the carbon steel API 5LX [6, 45, 61]. The intensity of these peaks almost disappears or reduced in the clove extract added system, which indicates that clove extract formed as a protective film over the carbon steel surface and protect them from further corrosion attack as does other green inhibitors [42, 45].
The surface of the abiotic coupons and mixed consortia coupons was analysed using the SEM and presented in Fig. 7. The bare metal clearly shows the smooth surface of the coupons (Fig. 7a). Abiotic control coupon in presence of produced water was vigorously corroded as shown in Fig. 7b, but SAE added abiotic coupon shows no such corrosion (Fig. 7c). Similarly, mixed consortia examined using SEM shows sessile biofilm formation over the carbon steel surface (Fig. 7d, e). At the same time, SAE extract added into the mixed consortia system clearly shown that added inhibitor utterly suppressed the biofilm formation over the carbon steel surface (Fig. 7f) [49]. Massively, a very vigorous pitting type of corrosion was observed in the surface of the mixed consortia coupons (Fig. 8a, b). These surface analysis studies confirmed that SAE inhibitor suppressed corrosion in both biotic and abiotic systems as well mixed consortia caused pitting type of corrosion in the carbon steel surface.
Mechanism of biocorrosion inhibition by clove extract
The highly corrosive nature of the produced water was initiated the attacking type of corrosion over the carbon steel surface in the abiotic and biotic systems. In the biotic systems, in addition to chloride ion, bacterial biofilm playing a key role in corrosion acceleration, it can be found from the weight loss and corrosion rate of the biotic and abiotic systems. Biofilm formation in each system was monitored by the sessile cell counts as well as from the electrochemical analysis. In each biotic system color of the medium was turning into the yellowish-orange (rust) color which indicates that bacterial strains added to the corrosive medium started to colonize over the surface of the carbon steel and started to corrode them. This biofilm formation was made an impact on the metal/solution interface, in specific, the negative shift in Ecorr values and also increase the corrosion rate of carbon steel [56, 62]. The development of biofilm over the carbon steel can enhance these corrosion processes by accepting electrons from the metal matrix, which lead to the formation of a pathway of electron transfer from the anodic site (beneath biofilms) to the cathodic site (over the surface metal), where electrons were accepted by oxygen, and consequently, corrosion reactions were accelerated [63].
However, biotic systems included with the clove extract showed a positive shift in the Ecorr and Icorr (Fig. 3, Table 3). The Rct was also increased, which was confirmed from the AC impedance studies (Fig. 5, Table 4). SEM analysis of the biotic and abiotic systems (Figs. 7, 8) indicates that green inhibitors protected the carbon steel from corrosion problems. The biocorrosion protection mechanism by the clove extract can be explained based on the outcome of these studies. It is well known that clove extract contains vast bioactive molecules and among all, eugenol was present in the higher quantity (9300 to 14,600 mg per 100 g clove) and which has very good antibacterial activity against a broad range of microbial strains [47]. Before included in the corrosion system, the antibacterial activity of the clove extract was confirmed against these corrosive bacterial strains (Table 1). These interesting observations proposed that adsorption and development of bioactive molecules present in the clove extract protected carbon steel from the bacterial attack and minimized the biocorrosion reactions.
Conclusions
Corrosion inhibition capability of clove extract has been studied for the first time on carbon steel API 5LX in hypersaline corrosive medium with and without corrosion causing bacterial strains (B. subtilis A1, S. parvus B7, P. stutzeri NA3, and A. baumannii MN3). Antibacterial activity of the clove extract was confirmed against these corrosive bacterial strains and 150 ppm was selected as minimum inhibitory concentration. Clove extract reduced the weight loss and corrosion rate tremendously. The inhibition efficiency of the clove extract in presence of the mixed consortia was 87%. The potentiodynamic polarization parameter showed that corrosion current in the inhibitor system was shifted towards the positive direction which means very less corrosion. This statement was strongly supported by AC impedance analysis in which charge transfer resistance in the mixed consortia was very less (237.9 ± 7.6 Ω cm2) and at the same time, the addition of clove extract, that was increased to 863.4 ± 18.5 Ω cm2. Very less corrosion resistance was observed from the bacterial systems, which confirmed that these corrosion causing bacterial strains formed a dense biofilm over the carbon steel and accelerating the pitting type of corrosion. XRD analysis revealed that bacterial strains convert Fe2+ to Fe2O3, which confirms that they can oxidize the inorganic metal species present on the surface of carbon steel API 5LX, which was suppressed by clove inhibitor. The bioactive and antibacterial compounds present in the clove extract such as eugenol forming a protective layer over the metal surface and control the biofilm as well as corrosion reaction.
References
Javaherdashti R (2011) Impact of sulphate-reducing bacteria on the performance of engineering materials. Appl Microbiol Biotechnol 91:1507–1517
Kannan P, Su SS, Mannan MS, Castaneda H, Vaddiraju S (2018) A review of characterization and quantification tools for microbiologically influenced corrosion in the oil and gas industry: current and future trends. Ind Eng Chem Res 57:13895–13922
Wan H, Song D, Zhang D, Du C, Xu D, Liu Z, Ding D, Li X (2018) Corrosion effect of Bacillus cereus on X80 pipeline steel in a Beijing soil environment. Bioelectrochemistry 121:18–26
Jia R, Yang D, Xu D, Gu T (2018) Carbon steel biocorrosion at 80 °C by a thermophilic sulfate reducing archaeon biofilm provides evidence for its utilization of elemental iron as electron donor through extracellular electron transfer. Corros Sci 145:47–54
Rajasekar A, Babu TG, Pandian SK, Maruthamuthu S, Palaniswamy N, Rajendran A (2007) Biodegradation and corrosion behavior of manganese oxidizer Bacillus cereus ACE4 in diesel transporting pipeline. Corros Sci 49:2694–2710
Parthipan P, Narenkumar J, Elumalai P, Preethi PS, Nanthini AUR, Agrawal A, Rajasekar A (2017) Neem extract as a green inhibitor for microbiologically influenced corrosion of carbon steel API 5LX in a hypersaline environments. J Mol Liq 240:121–127
Kotu SP, Mannan MS, Jayaraman A (2019) Emerging molecular techniques for studying microbial community composition and function in microbiologically influenced corrosion. Int Biodeterior Biodegrad 144:104722
Elumalai P, AlSalhi MS, Mehariya S, Karthikeyan OP, Devanesan S, Parthipan P, Rajasekar A (2020) Bacterial community analysis of biofilm on API 5LX carbon steel in an oil reservoir environment. Bioprocess Biosyst Eng. https://doi.org/10.1007/s00449-020-02447-w
Tsarenko IV, Makarevich AV, Orekhov DA (1998) Microbicidal properties of polymer films modified by five-membered polynitrogen heterocycles. Bioprocess Eng 19(1998):469–473
Li E, Wu J, Zhang D, Sun Y, Chen J (2018) D-phenylalanine inhibits the corrosion of Q235 carbon steel caused by Desulfovibrio sp. Int Biodeterior Biodegrad 127:178–184
Liu H, Xu D, Dao AQ, Zhang G, Lv Y, Liu H (2015) Study of corrosion behavior and mechanism of carbon steel in the presence of Chlorella vulgaris. Corros Sci 101:84–93
Zhou E, Li H, Yang C, Wang J, Xu D, Zhang D, Gu T (2018) Accelerated corrosion of 2304 duplex stainless steel by marine Pseudomonas aeruginosa biofilm. Int Biodeterior Biodegrad 127:1–9
Zacheus OM, Lehtola MJ, Korhonen LK, Martikainen PJ (2001) Soft deposits, the key site for microbial growth in drinking water distribution networks. Water Res 35:1757–1765
Parthipan P, Elumalai P, Karthikeyan OP, Ting YP, Rajasekar A (2017) A review on biodegradation of hydrocarbon and their influence on corrosion of carbon steel with special reference to petroleum industry. J Environ Biotech Res 6:12–33
Liduino VS, Cravo-Laureau C, Noel C, Carbon A, Duran R, Lutterbach MT, Servulo EFC (2019) Comparison of flow regimes on biocorrosion of steel pipe weldments: community composition and diversity of biofilms. Int Biodeterior Biodegrad 143:104717
Liduino VS, Filho JCP, Cravo-Laureau C, Lutterbach MT, SErvulo EFC, (2019) Comparison of flow regimes on biocorrosion of steel pipe weldments: fluid characterization and pitting analysis. Int Biodeterior Biodegrad 144:104750
Ramalingam V, Dhinesh P, Sundaramahalingam S, Rajaram R (2019) Green fabrication of iron oxide nanoparticles using grey mangrove Avicennia marina for antibiofilm activity and in vitro toxicity. Surf Interf 15:70–77
Fish KE, Osborn AM, Boxall J (2016) Characterising and understanding the impact of microbial biofilms and the extracellular polymeric substance (EPS) matrix in drinking water distribution systems. Environ Sci Water Res Technol 2:614
Parthipan P, Babu TG, Anandkumar B, Rajasekar A (2017) Biocorrosion and its impact on carbon steel API 5LX by Bacillus subtilis A1 and Bacillus cereus A4 isolated from Indian crude oil reservoir. J Bio Tribo Corros 3:32
Xu D, Gu T (2015) The war against problematic biofilms in the oil and gas industry. J Microb Biochem Technol 7:124
Tator KB (2003) In hydrogen sulfide and microbiologically induced corrosion of concrete steel and ductile iron in waste water facilities. Corrosion. NACE International, Texas
Rajasekar A, Anandkumar B, Maruthamuthu S, Ting YP, Rahman PK (2010) Characterization of corrosive bacterial consortia isolated from petroleum product transporting pipelines. Appl Microbiol Biotechnol 85:1175–1188
Cetin D, Aksu ML (2009) Corrosion behavior of low-alloy steel in the presence of Desulfotomaculum sp. Corros Sci 51:1584–1588
Bsharat TK (1998) Detection, treatment, and prevention of microbiologically influenced corrosion in water-based fire protection systems. National Fire Sprinkler Association
Blanco M, Negro C, Gaspar I, Tijero J (1996) Slime problems in the paper and board industry. Appl Microbiol Biotechnol 46:203–208
Maruthamuthu S, Nagendran T, Anandkumar B, Karthikeyan M, Palaniswamy N, Narayanan G (2011) Microbiologically influenced corrosion on rail. Curr Sci 100:870–880
Flitton MKA, Yoder TS (2012) In twelve year study of underground corrosion of activated metals. Corrosion. NACE International, Texas
Jacobson GA (2007) Corrosion at Prudhoe Bay: a lesson on the line. Mater Perform 46(8):26–35
Ismail AS, Farag AA (2020) Experimental, theoretical and simulation studies of extracted crab waste protein as a green polymer inhibitor for carbon steel corrosion in 2 M H3PO4. Surf Interf 19:100483
Kaskah SE, Pfeiffer M, Klock H, Bergen H, Ehrenhaft G, Ferreira P, Gollnick J, Fischer CB (2017) Surface protection of low carbon steel with N-acyl sarcosine derivatives as green corrosion inhibitors. Surf Interf 9:70–78
Aktas DF, Sorrell KR, Duncan KE, Wawrik B, Callaghan AV, Suflita JM (2017) Anaerobic hydrocarbon biodegradation and biocorrosion of carbon steel in marine environments: the impact of different ultra low sulfur diesels and bioaugmentation. Int Biodeterior Biodegrad 118:45–56
Suarez EM, Lepkova L, Kinsella B, Machuca LL (2019) Aggressive corrosion of steel by a thermophilic microbial consortium in the presence and absence of sand. Int Biodeterior Biodegrad 137:137–146
Ali AI, Mahrous YS (2017) Corrosion inhibition of c-steel in acidic media from fruiting bodies of Melia azedarach L. extract and a synergistic Ni2+ additive. RSC Adv 7:23687
Gadow HS, Motawea MM (2017) Investigation of the corrosion inhibition of carbon steel in hydrochloric acid solution by using ginger roots extract. RSC Adv 7:24576
Wang O, Tan B, Bao H, Xie Y, Mou Y, Li P, Chen D, Shi Y, Li X, Yang W (2019) Evaluation of Ficus tikoua leaves extract as an eco-friendly corrosion inhibitor for carbon steel in HCl media. Bioelectrochemistry 128:49–55
Raghavendra N (2018) Areca plant extracts as a green corrosion inhibitor of carbon steel metal in 3 M hydrochloric acid: gasometric, colorimetry and atomic absorption spectroscopy views. J Mol Eng Mater 6:1850004
Bourazmi H, Tabyaoui M, Hattabi LE, Aoufir YE, Taleb M (2018) Methanolic extract of Salvia officinalis plant as a green inhibitor for the corrosion of carbon steel in 1 M HCl. J Mater Environ Sci 9:928–938
Abdallah M, Altass HM, Jahdaly BAA, Salem MM (2018) Some natural aqueous extracts of plants as green inhibitor for carbon steel corrosion in 0.5 M sulfuric acid. Green Chem Lett Rev 11:189–196
Deyab MA, Osman MM, Elkholy AE, Heakal FET (2017) Green approach towards corrosion inhibition of carbon steel in produced oilfield water using lemongrass extract. RSC Adv 7:45241
He T, Emori W, Zhang R, Okafor PC, Yang M, Cheng C (2019) Detailed characterization of Phellodendron chinense Schneid and its application in the corrosion inhibition of carbon steel in acidic media. Bioelectrochemistry 130:107332
Lekbach Y, Xu D, Abed SE, Dong Y, Liu D, Khan MS, Koraichi SI, Yang K (2018) Mitigation of microbiologically influenced corrosion of 304L stainless steel in the presence of Pseudomonas aeruginosa by Cistus ladanifer leaves extract. Int Biodeterior Biodegrad 133:159–169
Narenkumar J, Parthipan P, Nanthini AUR, Benelli G, Murugan K, Rajasekar A (2017) Ginger extract as green biocide to control microbial corrosion of mild steel. 3 Biotech 7:133
Bhola SM, Alabbas FM, Bhola R, Spear JR, Mishra B, Olson DL, Kakpovbia AE (2014) Neem extract as an inhibitor for biocorrosion influenced by sulfate reducing bacteria: a preliminary investigation. Eng Fail Anal 36:92–103
Swaroop BS, Victoria SN, Manivannan R (2016) Azadirachta indica leaves extract as inhibitor for microbial corrosion of copper by Arthrobacter sulfureus in neutral pH conditions—a remedy to blue green water problem. J Taiwan Inst Chem Eng 64:269–278
Parthipan P, Elumalai P, Narenkumar J, Machuca LL, Murugan K, Karthikeyan OP, Rajasekar A (2018) Allium sativum (garlic extract) as a green corrosion inhibitor with biocidal properties for the control of MIC in carbon steel and stainless steel in oilfield environments. Int Biodeterior Biodegrad 132:66–73
Djouahra-Fahem D, Angar Y, Gana LM, Khoukhi F, Kebbouche-Gana S (2019) A comprehensive study on crude methanolic extract of Daphne gnidium L. as effective corrosion inhibitors of mild steel induced by SRB consortium. J Bio Tribo Corros 5:18
Cortes-Rojas DF, Fernandes de Souza CR, Oliveira WP (2014) Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed 4:90–96
Yang YC, Lee SH (2003) Ovicidal and adulticidal effects of Eugenia caryophyllata bud and leaf oil compounds on Pediculus capitis. J Agri Food Chem 51:4884–4888
Parthipan P, Elumalai P, Ting YP, Rahman PKSM, Rajasekar A (2018) Characterization of hydrocarbon degrading bacteria isolated from Indian crude oil reservoir and their influence on biocorrosion of carbon steel API 5LX. Int Biodeterior Biodegrad 129:67–80
Rajasekar A, Xiao W, Sethuraman M, Parthipan P, Elumalai P (2017) Airborne microorganisms associated with corrosion of structural engineering materials. Environ Sci Pollut Res 24:8120–8136
Mohammed KAK, Abdulkadhim HM, Noori SI (2016) Chemical composition and anti-bacterial effects of clove (Syzygium aromaticum) flowers. Int J Curr Microbiol App Sci 5:483–489
Chowdhry BZ, Ryall JP, Dines TJ, Mendham AP (2015) Infrared and raman spectroscopy of eugenol, isoeugenol, and methyl eugenol: conformational analysis and vibrational assignments from density functional theory calculations of the anharmonic fundamentals. J Phys Chem A 119:11280–11292
Hemalatha R, Nivetha P, Mohanapriya C, Sharmila G, Muthukumaran C, Gopinath M (2016) Phytochemical composition, GC-MS analysis, in vitro antioxidant and antibacterial potential of clove flower bud (Eugenia caryophyllus) methanolic extract. J Food Sci Technol 53:1189–1198
Saxena A, Sharma A, Saxena D, Jain P (2012) Corrosion inhibition and adsorption behavior of clove oil on iron in acidic medium. E J Chem 9:2044–2051
Azzouyahar E, Abu-Obaid A, Hajji ME, Bazzi L, Belkhaouda M, Lamiri A, Salghi R, Jodeh S, Essahli M (2016) Plants extract as green corrosion inhibitors: the case of eugenol from clove. Der Pharm Chem 8:467–475
Lekbach Y, Li Z, Xu D, Abed SE, Dong Y, Liu D, Gu T, Koraichi SI, Yang K, Wang F (2019) Salvia officinalis extract mitigates the microbiologically influenced corrosion of 304L stainless steel by Pseudomonas aeruginosa biofilm. Bioelectrochemistry 128:193–203
Jia R, Yang D, Rahman HBA, Gu T (2017) Laboratory testing of enhanced biocide mitigation of an oilfield biofilm and its microbiologically influenced corrosion of carbon steel in the presence of oilfield chemicals. Int Biodeterior Biodegrad 125:116–124
Narenkumar J, Parthipan P, Madhavan J, Murugan K, Marpu SB, Suresh AK, Rajasekar A (2018) Bioengineered silver nanoparticles as potent anti-corrosive inhibitor for mild steel in cooling towers. Environ Sci Pollut Res 25:5412–5420
Parthipan P, Sabarinathan D, Angaiah S, Rajasekar A (2018) Glycolipid biosurfactant as an eco-friendly microbial inhibitor for the corrosion of carbon steel in vulnerable corrosive bacterial strains. J Mol Liq 261:473–479
Zhai X, Myamina M, Duan J, Hou B (2013) Microbial corrosion resistance of galvanized coatings with 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one as a biocidal ingredient in electrolytes. Corro Sci 72:99–107
Rajasekar A, Babu TG, Pandian STK, Maruthamuthu S, Palaniswamy N, Rajendran A (2007) Role of Serratia marcescens ACE2 on diesel degradation and its influence on corrosion. J Ind Microbiol Biotechnol 34:589–598
Li H, Yang C, Zhou E, Yang C, Feng H, Jiang Z, Xu D, Gu T, Yang K (2017) Microbiologically influenced corrosion behavior of S32654 super austenitic stainless steel in the presence of marine Pseudomonas aeruginosa biofilm. J Mater Sci Technol 33:1596–1603
Li Y, Xu D, Chen C, Li X, Jia R, Zhang D, Sand W, Wang F, Gu T (2018) Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: a review. J Mater Sci Technol 34:1713–1718
Acknowledgments
Dr. P. Parthipan, gratefully acknowledges the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), for providing research fellowship under National Postdoctoral Fellowship (PDF/2017/001134). Also, authors are grateful to the researchers supporting project number (RSP-2020/68), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parthipan, P., AlSalhi, M.S., Devanesan, S. et al. Evaluation of Syzygium aromaticum aqueous extract as an eco-friendly inhibitor for microbiologically influenced corrosion of carbon steel in oil reservoir environment. Bioprocess Biosyst Eng 44, 1441–1452 (2021). https://doi.org/10.1007/s00449-021-02524-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02524-8