Abstract
The production of 1,3-propanediol from crude glycerol and mixed anaerobic sludge was investigated in batch experiments and continuous reactors. Using a 23 complete factorial design, the effects of the concentration of glycerol (22–30 g L−1), KH2PO4 (1.50–2.00 g L−1), and vitamin B12 (7–8 mg L−1) were examined in batch reactors. As an evaluated response, the highest 1,3-PD yields occurred for high concentrations of vitamin B12 and low levels of KH2PO4, reaching 0.57 g g−1 glycerol consumed. The variable glycerol concentration was not significant in the studied range. In addition, the condition that provided the best 1,3-PD yield was applied to an anaerobic fluidized bed reactor fed with crude glycerol (26.0 g L−1), which was monitored as the hydraulic retention time (HRT) decreased from 36 to 12 h. The greatest 1,3-PD yield, of 0.31 g g−1 glycerol, was obtained with an HRT of 28 h.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The demand for alternative energy sources is growing across the world. Biomass, which can be used to produce renewable biofuels, is one of the most promising sources. Brazil is somewhat of a pioneer in the use of biofuels as clean energy sources. The Brazilian government resolved to replace gasoline with fuel alcohol in 1973, through the National Alcohol Program (“Pro-Álcool”), and by the mid-80s, around 95% of the automobiles produced in Brazil had been modified for the combustion of ethanol. This paved the way for “Flex” vehicles. These flexible-fuel vehicles, which were introduced in 2003, now represent more than 80% of the cars sold in the country [1].
Another biomass-generated biofuel is biodiesel, which is produced through the transesterification of vegetable oils or animal fats. The reaction is carried out using alcohols such as ethanol or methanol and is usually catalyzed by NaOH or KOH. Glycerol is generated as a byproduct of this process, with a yield of approximately 1–1.4 kg kg−1 of biodiesel produced [2]. The global production of biodiesel has increased considerably in the past decades—from 500 × 108 in 2004 to 7.5 × 109 gallons year−1 in 2013—and it is expected that it will continue to grow [2]. In Brazil, data from the National Agency for Petroleum, Natural Gas and Biofuels (ANP) indicate that the annual production of biodiesel in 2017 was 4.29 × 106 m3, while that of glycerol was 3.74 × 105 m3 [3].
This increased biodiesel production means that excess amounts of glycerol are generated. Furthermore, the biodiesel production process introduces impurities, such as salts, methanol, and fatty acids. Consequently, glycerol derived from this process has such a low market value that it has largely become a waste product. Moreover, its high chemical oxygen demand (COD) necessitates its adequate treatment or disposal [4]. New uses for glycerol are needed to stabilize the price and supply, and to avoid the accumulation of a material that may have an environmentally negative impact [5].
Fermentation of glycerol can produce highly valued and widely used products, including 1,3-propanediol (1,3-PD) [6,7,8], dihydroxyacetone [9], succinic acid [10], propionic acid [11, 12], ethanol [13, 14], citric acid [15], and hydrogen [16, 17]. The process involves two parallel metabolic routes: oxidative and reductive. In the oxidative route, glycerol is converted to dihydroxyacetone, and following phosphorylating, the product undergoes glycolysis to form acetate, butyrate, propionate, ethanol, H2, and other metabolites. In the reductive route, glycerol is converted to 3-hydroxypropionaldehyde (3-HPA), which goes on to generate 1,3-PD [1]. Most notably, 1,3-PD is used in cosmetics, lubricants, and medicines. Furthermore, it is a potentially important chemical intermediate in the manufacture of certain polymers (polyesters, polyethers, polyurethanes, etc.), and in the synthesis of heterocyclic compounds [18]. This product is highly specific to glycerol fermentation and cannot be obtained by any other anaerobic conversion [19].
Among the bacteria capable of converting glycerol to 1,3-PD are some species of Lactobacillus, Pantoea agglomerans (formerly Enterobacter agglomerans), Citrobacter freundii, Klebsiella pneumoniae, Clostridium pasteurianum, and Clostridium butyricum [8, 20,21,22]. Recent studies involving pure cultures in 1,3-PD production, such as da Silva et al. [8] and Tee et al. [20], reached 1,3-PD yields of 0.37 and 0.51, from 25 to 40 g L−1 glycerol, respectively. As an alternative to pure cultures, 1,3-PD can be produced using mixed anaerobic cultures [4, 5, 23, 24]. Comparing with pure cultures, microbial consortium is able to metabolize unpurified substrates, such as crude glycerol [25]. However, without the nutritional support necessary to the development of the 1,3-PD producing bacteria, the conversion of high glycerol concentrations becomes impaired, affecting the 1,3-PD yield [25]. Using inoculum from organic farm soil, Kanjilal et al. [26] studied the 1,3-PD production from pure and crude glycerol, and observed a decrease in yields by increasing the substrate concentration from 20 to 30 g L−1. A microbial consortium DL38 from marine sludge was screened which displayed high tolerance to crude glycerol and high production of 1,3-PD. A yield of 0.52 g g−1 and a final concentration of 81.40 g L−1 were obtained in batch fermentation, according to Jiang et al. [27].
In continuous operation, microbial consortium shows a significant improvement in raw material utilization and robustness against environmental fluctuations, which has potential application in industrial production of biochemical [25]. Different configurations of bench-scale reactors have been investigated in the continuous production of 1,3-PD. Varrone et al. [28] reached yields of 0.52 and 0.46 g g−1 applying anaerobic sludge and activated sludge, respectively, in continuous stirred-tank reactor (CSTR). Gallardo et al. [24] used an expanded granular sludge blanket (EGSB) reactor inoculated with granular anaerobic sludge and reached 0.43 g g−1, from 25 g L−1 of crude glycerol. Anaerobic fluidized bed reactors (AFBRs) retain large amounts of biomass through adherence to a support material. AFBRs have good stability in continuous operations with high and low hydraulic retention times (HRTs) [29], and represent a good alternative for the fermentation of glycerol using mixed anaerobic cultures. Several authors have demonstrated the potential of AFBR in the continuous fermentation of wastewater [30,31,32,33], including glycerol. Recently, Nazareth et al. [34] showed the use of an AFBR system in the continuous production of propionic acid from crude glycerol. Despite the advantages of AFBR, there are no studies in the literature known to the authors regarding the production of 1,3-PD from glycerol in this type of reactor configuration. Nevertheless, studying the behavior of the system and identifying the optimal range of operation have great importance in maximizing 1,3-PD yield.
In this work, a 23 complete factorial design was used to determine the individual and interactive effects of three components of the nutrient medium (glycerol, KH2PO4, and vitamin B12) on the 1,3-PD yield from mixed cultures. In addition, continuous 1,3-PD production was evaluated in an AFBR operated with 26.0 g L−1 of crude glycerol at HRTs between 36 and 12 h, in the best condition determined by the experimental design.
Materials and methods
Fermentation medium and inoculum
The batch experiments and AFBR operation were performed with a single carbon source, crude glycerol, provided by BioBrotas Oleoquímica (Brotas, São Paulo, Brazil). The residue consisted of approximately 84% glycerol, with impurities of salts (~ 13%), methanol (~ 1%), water (~ 2%), and fatty acids (< 0.05%). The nutrient medium from Barbirato et al. [35] was adapted for culturing the microorganisms. It was constituted by: 3.4 g L−1 K2HPO4·3H2O; 2.0 g L−1 (NH4)2SO4; 0.2 g L−1 MgSO4·7H2O; 0.02 g L−1 CaCl2·2H2O; 0.005 g L−1 FeSO4·7H2O; 0.5 g L−1 yeast extract. In addition, it was supplemented with the trace element solution SL7 at a concentration of 2 mL L−1, as described by Biebl and Pfennig [36]. KH2PO4 (1.5–2.0 g L−1) and vitamin B12 (7.0–8.0 mg L−1) were dosed according to the experimental design.
The inoculum was obtained from sludge from an upflow anaerobic sludge blanket (UASB) reactor used for the treatment of effluent from a poultry slaughterhouse (Avícola Dacar, Tietê, São Paulo, Brazil). To inhibit the development of methanogenic archaea, the sludge was subjected to a thermal pretreatment, in accordance with Kim et al. [37].
Experimental design of the batch reactors
A 23 complete factorial design was performed to verify the significance of the variables tested in the yield of 1,3-PD, produced from crude glycerol and mixed cultures. The ranges and levels of the independent variables—glycerol (X1), KH2PO4 (X2), and vitamin B12 (X3)—are shown in Table 1.
The response surface for the significant variables was generated according to the first-order polynomial equation, described by the following equation:
where Y represents the predicted response, β0 is a constant, βi is the linear coefficient, βij is the coefficient of the interaction parameter, and xi and xj are the coded independent variables Xi and Xj (i, j = 1, 2, 3). The tested variables were coded according to the following equation:
where xi is the coded value and Xi is the real value of the independent variable, X0 is the real value of the center point, and ΔXi is the value of the difference between the points.
The response surface and the analysis of the parameters [F test and analysis of variance (ANOVA)] were performed using Statistica 10.0 software (StatSoft Inc. 2010, USA). ANOVA was performed to test the significance of the factors (glycerol, KH2PO4, and vitamin B12) in the fit of the linear model. The parameters with p values less than 5% (p < 0.05) were considered significant for the maximization of 1,3-PD yield.
The experiments were conducted in batch reactors that consisted of 2-L Duran® flasks with 1 L of useful volume, into which 10% (100 mL) of inoculum was added. Crude glycerol was used as the sole source of carbon (22–30 g L−1). The nutrient medium used was previously described, and KH2PO4 (1.50–2.00 g L−1) and vitamin B12 (7–8 mg L−1) were added as additional nutrients for the development of the 1,3-PD producing bacteria at the concentrations given in Table 1. N2 was purged inside the flasks to ensure anaerobic conditions, and the pH was adjusted to 6.72 ± 0.02 with the aid of 30% HCl and/or 6 M NaOH. The flasks were kept in a temperature-controlled incubator at 37 °C under agitation at 200 rpm for 168 h.
Continuous anaerobic fluidized bed reactor (AFBR) operation conditions
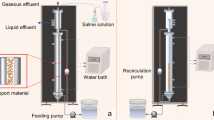
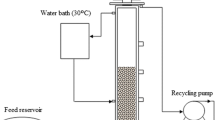
The AFBR used in this study was very similar to that used by Nazareth et al. [34]. The reactor was built in transparent acrylic, and had an internal diameter of 3.6 cm, a height of 151.5 cm, and a total volume of 1542.1 cm3. The temperature was maintained at 30 ± 1 °C with the aid of a thermostatic bath and reactor jacket. A support material was used to immobilize the biomass in the form of expanded clay particles (2.8–3.35 mm) with an apparent density of 1.06 g cm−3 and a porosity of 23% [38].
The operating conditions of the AFBR are given in Table 2. The concentration of glycerol applied to the reactor was based on the results obtained by the factorial design, as well as the adjustment of the nutritional medium in relation to the KH2PO4 and vitamin B12 concentration. To promote microbial adhesion to the support and microbial adaptation, the AFBR-containing expanded clay particles were inoculated in the batch mode by recycling the pretreated sludge (7%) with a fixed glycerol concentration (26.0 g L−1) for 15 days. N2 gas was sprayed into the fermentation medium to ensure anaerobic conditions and HCl 30% v/v was added to maintain the affluent pH adjust to 6.70 ± 0.20. For the reactor operation, the upward velocity was 1.3 times greater than the minimum fluidization velocity used. Thereafter, the reactor was transferred to the continuous mode of operation with the initial HRT of 36 h and fixed glycerol concentration of 26.0 g L−1. The next phase was begun when the steady state was reached, based on a glycerol consumption efficiency and 1,3-PD production with a variation of less than 10% for 15 days. The reactor was operated for 53 days. The biogas composition and the soluble metabolites were monitored as a function of time. Only the results obtained in the steady state were reported.
Analytical methods
The chemical oxygen demand (COD), volatile suspense solids (VSS), and pH were analyzed in accordance with standard methods [39]. The volumetric production of biogas was measured according to the method proposed by Walker et al. [40]. The biogas content was determined using a gas chromatograph (GC-2010, Shimadzu, Japan), described by Amorim et al. [38]. The glycerol concentration was determined according to the spectrophotometric method described by Bondioli and Della Bella [41].
1,3-PD and other aqueous products were measured by gas chromatography (GC-17A, Shimadzu, Japan), using the headspace method, with an automatic sampler injection sampler system. The chromatograph was equipped with a flame ionization detector (FID) and DB-WAX capillary column, 30 m × 0.25 mm × 0.25 µm, with a flow rate of 1.56 mL min−1. The injector and detector temperatures were 250 °C and 280 °C, respectively. From an initial temperature of 35 °C, the temperature ramp used was: 2 °C min−1 until 42 °C; 20 °C min−1 until 75 °C; 35 °C min−1 until 120 °C; 10 °C min−1 until 170 °C. The temperature was held at 120 °C for 1 min and at 170 °C for 2 min. The flow rates of the carrier gas (H2), auxiliary gas (N2), and flame gas (synthetic air) were kept constant at 50, 35, and 500 mL min−1, respectively.
Results and discussion
Screening of nutrient medium components in batch assays
A 23 complete factorial design was performed to verify the statistical significance of certain components of the nutrient medium, with the aim of maximizing the 1,3-PD yield. Table 3 shows that the 1,3-PD yield varied from 0.031 to 0.571 g g−1 glycerol consumed, reaching the maximum value with 30.0 g L−1 of glycerol, 1.50 g L−1 of KH2PO4, and 8.0 mg L−1 of B12. Results of the dependent variable were used to determine regression coefficients for 1,3-PD yield (Table 4), to calculate ANOVA (Table 5), and to construct three-dimensional response surface for significant results (Fig. 1).
According to Table 4, the variables KH2PO4 and vitamin B12 were considered significant for 1,3-PD yield (p < 0.05). Some studies show that high concentrations of glycerol favor 1,3-PD production [7, 42, 43]; however, as the current study covered a narrow range of high glycerol concentrations (22–30 g L−1), this variable did not show a significant effect on the response (p = 0.86 > 0.05).
Through multiple regression analysis, the first-order polynomial model was established to predict the yield of 1,3-PD. Non-significant terms (based on p value < 0.05) were neglected and the model Eq. (3) was reduced from the coded and significant variables:
where Y represents the predicted value for 1,3-PD yield, and x2 and x3 are the coded values for the independent variables KH2PO4, and B12, respectively.
The statistical significance of Eq. (3) was confirmed using F tests and ANOVA for the model (Table 5). The calculated F value was much higher than the critical value, indicating the high statistical significance of the predicted model with regard to the 1,3-PD yield. The fit of the model was also expressed as the coefficient of determination R2, where the closer the R2 value is to 1, the better the model fits the experimental data [42]. The fit had an R2 value of 0.9306, which indicated that 93.06% of the variability in the response be explained by the model. The adjusted coefficient of determination (R2 adj = 0.7920) was satisfactory to confirm the significance of the model.
The three-dimensional response surface for the 1,3-PD yield, based on the linear model described by Eq. (3) and the significant variables, is shown in Fig. 1. Higher yields of 1,3-PD correlated with higher concentrations of vitamin B12 and lower levels of KH2PO4.
Kaur et al. [44] also evaluated the statistical significance of nutrients, glycerol, (NH4)2SO4, K2HPO4, and KH2PO4 in maximizing the 1,3-PD production in batch reactors with Clostridium diolis. They verified that, in addition to glycerol, the phosphate sources (K2HPO4 and KH2PO4) were significant for the response, which shows the agreement to the results of the present study. In fact, phosphate sources are important for the maintenance of the buffer capacity of the fermentative medium, contributing to the growth and bioenergy of the bacterial cell [44]. In addition, these substances act as a source of phosphorus, which is essential in the synthesis of DNA, RNA, and phospholipids (plasma membrane constituents), and in the conversion of ADP to ATP, and vice versa, during oxidative phosphorylation [45, 46].
The effect of vitamin B12 on 1,3-PD production from glycerol has been verified previously using Lactobacillus diolivorans [22] and Halanaerobium saccharolyticum subsp. Saccharolyticum [6]. In both studies, the addition of vitamin B12 to the fermentative medium containing glycerol significantly increased 1,3-PD production. Thus, despite the limited range in the present study, the fact that the current results are in agreement with these previous studies emphasizes the significance of vitamin B12 for maximizing the 1,3-PD yield from glycerol. According to Biebl et al. [18], most of the 1,3-PD producing microorganisms are vitamin B12-dependent, since the enzyme glycerol dehydratase that is included in the first stage of 1,3-PD production requires vitamin B12 as a coenzyme for active functioning.
1,3-Propanediol (1,3-PD) production in the AFBR
The factorial design allowed some of the concentrations of the components in the nutrient medium to be adjusted to maximize 1,3-PD production in an anaerobic reactor. Therefore, the AFBR was operated continuously, and the nutritional medium applied consisted of: 26.0 g L−1 crude glycerol; 1.50 g L−1 KH2PO4; 7.8 mg L−1 B12, in addition to the nutrients described in the “Fermentation medium and inoculum”.
Figure 2 shows the variation of glycerol conversion, and the concentrations of glycerol in the affluent and glycerol effluent. The concentration of residual glycerol remained high throughout the reactor operation, which can be attributed to the high organic loading applied to the system. The highest glycerol conversion in the reactor was observed with an HRT of 36 h, and the lowest was observed with an HRT of 12 h. Gonen et al. [47] also observed reductions in glycerol consumption from 100 to 75% by decreasing the HRT from 8 to 2 h in an anaerobic packed-bed reactor (APBR), which was immobilized with Clostridium beijerinckii NRRL B-593 and fed with 45 g L−1 of glycerol.
Figure 3 shows the concentration of 1,3-PD produced in the AFBR reactor as a function of HRT, and Table 6 shows the 1,3-PD yields, substrate conversion efficiency, pH, and the byproduct concentrations formed in the different phases of operation. The AFBR produced similar concentrations of 1,3-PD with HRTs of 28 h and 20 h, reaching the maximum with a HRT of 20 h (1310.0 mg L−1). However, a lower 1,3-PD production occurred with an HRT of 12 h, as a consequence of the low conversion efficiency.
The decreased glycerol conversion and 1,3-PD production with an HRT of 12 h can be attributed to an inhibitory effect by the substrate, also leading to “feedback inhibition” by the product. According to Edwards [48], an excessive amount of substrate may unbalance the metabolism of the cell, causing overproduction of a product from one metabolic pathway, and blocking a related second pathway. On the other hand, when the concentration of this product rises, the tendency is that this metabolite acts as an allosteric inhibitor, decreasing the velocity of the pathway and its own production, causing the so-called feedback inhibition.
In this study, the high glycerol concentration applied to the reactor (26.0 g L−1) disadvantaged the substrate consumption, which remained at around 20% in the first three phases of operation. In addition to this, the 1,3-PD production was high in all the phases of reactor operation (Table 6), and there were only small amounts of metabolites from the oxidative path, such as ethanol, acetic acid, and propionic acid.
The variation of the 1,3-PD yield during the operation of the AFBR is shown in Fig. 3 as a function of the HRT. The 1,3-PD yield in the reactor reached a maximum of 0.31 ± 0.06 g g−1 glycerol consumed with an HRT of 28 h, and the HRT of 12 h gave the lowest 1,3-PD yield obtained.
Cheng et al. [49] were pioneers in reporting 1,3-PD production, using K. pneumoniae on a pilot scale. In a 5 L fermenter operated in the batch-fed mode, the authors achieved a 1,3-PD yield of 0.36 g g−1 glycerol consumed from 50 g L−1 glycerol, by varying the incubation time from 12 to 48 h. Gonen et al. [50] reported a 1,3-PD yield of 0.49 g g−1 glycerol consumed with an HRT of 8 h, in an APBR, containing K. pneumoniae immobilized on polyurethane foam, which was fed with 45 g L−1 glycerol.
Gallardo et al. [24] evaluated different pretreatments of sludge in 1,3-PD production in an expanded granular sludge blanket (EGSB) reactor which was fed with 25 g L−1 of glycerol. The HRT was varied between 24 and 3 h. The authors determined the maximum 1,3-PD yield (0.43 g g−1 glycerol consumed) in with an HRT of 12 h for the reactor with untreated sludge. However, in the reactors inoculated with fragmented sludge and thermally pretreated sludge—similar to the present study—the authors obtained 0.38 and 0.39 g g glycerol consumed in, respectively, with an HRT of 6 h.
Table 6 shows the concentrations of the main byproducts produced. Butyric acid was produced in negligible concentrations. CH4 was not detected in any phase of reactor operation, emphasizing the efficiency of the pretreatment used and indicating that the operating conditions were unfavorable for the development of methanogenic archaea. H2 was also not detected in any phase of reactor operation, indicating the complete favoring of the reductive path of glycerol fermentation.
H2 and 1,3-PD are produced by competing metabolic pathways in terms of reducing equivalents. The cells produce reduced metabolites, such as 1,3-PD, H2, acetate, butyrate, and ethanol, to maintain the balance of electrons. The reaction mechanism includes recycling of NADH and NAD+ to maintain redox balance [51]. For example, in Clostridium sp., the reducing equivalents generated in the conversion of pyruvate to acetyl-CoA are used to reduce ferredoxin, which is reoxidized through the H2 production by the enzyme hydrogenase [24]. If little or no H2 is formed, the reducing equivalents must be available for the production of other reduced compounds, such as 1,3-PD. Similarly, in the absence of 1,3-PD, the electrons are transferred to the formation of compounds such as H2, ethanol, acetate, and butyrate provided from oxidative pathway [6, 24, 51].
The theoretical maximum 1,3-PD yield in co-production with acetate is 0.70 mol mol−1 glycerol (0.59 g g−1 glycerol) when no H2 is produced. Practical maximum yields of 0.56 and 0.57 g g−1 glycerol have been previously reported by Saint-Amans et al. [52] using a pure culture of C. butyricum, and by Selembo et al. [5] from mixed wheat soil cultures, respectively. In the current work, the maximum yield obtained was 0.57 g g−1 glycerol in the batch process, and 0.31 g g−1 glycerol during continuous fermentation in the AFBR reactor. The yields obtained in this study are comparable with others reported recently in the literature (Table 7), although the 1,3-PD yield in the continuous system is considered low. This discrepancy may be a consequence of low substrate conversion during all the phases of the reactor.
The significant favoring of the reductive pathway of glycerol fermentation can be attributed to the addition of vitamin B12 in the fermentation medium, since 1,3-PD was formed in most phases of the reactor operation, and the products from the oxidative pathway—H2, acetate, ethanol—were produced in reduced concentrations. This corroborates the work by Huang et al. [54], in which it was found that adding vitamin B12 to a fermentative medium containing pure glycerol as the sole carbon source increased the 1,3-PD production with K. pneumoniae. In a similar way, Kivisto et al. [6] showed that the addition of vitamin B12 enhanced the useful reactions of glycerol, and decreased the yields of H2, CO2, and acetate, via H. saccharolyticum subsp. Saccharolyticum, in addition to inducing the production of 1,3-PD from glycerol. Recently, Vivek et al. [55] observed that the addition of Co+2 and vitamin B12 in the fermentation medium maximized the production of 1,3-PD by Lactobacillus brevis N1E9.3.3.
Conclusions
A complete factorial design showed that the variables KH2PO4 and vitamin B12 exerted a significant influence on 1,3-PD yield from crude glycerol using mixed cultures. The maximum 1,3-PD yield (0.57 g g−1 glycerol consumed) was obtained with 30 g L−1 glycerol, 1.50 g L−1 KH2PO4, and 8 mg L−1 B12 in batch tests. Based on factorial design, the AFBR was operated continuously with 26.0 g L−1 crude glycerol; 1.50 g L−1 KH2PO4; 7.8 mg L−1 B12. The continuous reactor showed a low capacity to consume the substrate concentration applied to the system. Nevertheless, satisfactory 1,3-PD yields were obtained as a result of the favoring of this reductive pathway by mixed cultures supplemented with vitamin B12.
References
da Silva GP, Mack M, Contiero J (2009) Glycerol: a promising and abundant carbon source for industry microbiology. Biotechnol Adv 27(1):30–39
Zhang X, Yan S, Tyagi RD, Surampalli RY, Valéro JR (2016) Energy balance of biofuel production from biological conversion of crude glycerol. J Environ Manag 170:169–176
National Agency of Petroleum, Natural gas and biofuels (ANP, Brazil) (2017) Brazilian statistical yearbook of oil, natural gas and biofuels, 1st edn. National Agency of Oil, Natural Gas and Biofuels, Rio de Janeiro (in Portuguese)
Sittijunda S, Reungsang A (2017) Fermentation of hydrogen, 1,3-propanediol and ethanol from glycerol as affected by organic loading rate using up-flow anaerobic sludge blanket (UASB) reactor. Int J Hydrog Energy 42:27558–27569
Selembo PA, Perez JM, Lloyd WA, Logan BE (2009) Enhanced hydrogen and 1,3-propanediol production from glycerol by fermentation using mixed cultures. Biotechnol Bioeng 104(6):1098–1106
Kivistö A, Santala V, Karp M (2011) Closing the 1,3-propanediol route enhances hydrogen production from glycerol by Halanaerobium saccharolyticum subsp. Saccharolyticum. Int J Hydrog Energy 36(12):7074–7080
Moon C, Hwan Lee C, Sang BI, Um Y (2011) Optimization of medium compositions favoring butanol and 1,3-propanediol production from glycerol by Clostridium pasteurianum. Bioresour Technol 102(22):10561–10568
da Silva GP, de Lima CJB, Contiero J (2015) Production and productivity of 1,3-propanediol from glycerol by Klebsiella pneumoniae GLC29. Catal Today 257:259–266
Bauer R, Katsikis N, Varga S, Hekmat D (2005) Study of the inhibitory effect of the product dihydroxyacetone on Gluconobacter oxydans in a semi-continuous two-stage repeated-fed-batch process. Bioprocess Biosyst Eng 28(1):37–43
Song H, Lee SY (2006) Production of succinic acid by bacterial fermentation. Enzyme Microb Technol 39(3):352–361
Himmi EH, Bories A, Boussaid A, Hassani L (2000) Propionic acid fermentation of glycerol and glucose by Propionibacterium acidipropionici and Propionibacterium freudenreichii ssp Shermanii. Appl Microbiol Biotechnol 53(4):435–440
Dishisha T, Alvarez MT, Hatti-Kaul R (2012) Batch- and continuous propionic acid production from glycerol using free and immobilized cells of Propionibacterium acidipropionici. Bioresour Technol 118:553–562
Jarvis GN, Moore ERB, Thiele JH (1997) Formate and ethanol are the major products of glycerol fermentation produced by a Klebsiella planticola strain isolated from red deer. J Appl Microbiol 83(2):166–174
Ito T, Nakashimada Y, Senba K, Matsui T, Nishio N (2005) Hydrogen and ethanol production from glycerol-containing wastes discharged after biodiesel manufacturing process. J Biosci Bioeng 100(3):260–265
Imandi SB, Bandaru VR, Somalanka SR, Garapati HR (2007) Optimization of medium constituents for the production of citric acid from byproduct glycerol using Doehlert experimental design. Enzyme Microb Technol 40(5):1367–1372
Reungsang A, Sittijunda S, O-Thong S (2013) Bio-hydrogen production from glycerol by immobilized Enterobacter aerogenes ATCC 13048 on heat-treated UASB granules as affected by organic loading rate. Int J Hydrog Energy 38(17):6970–6979
Chookaew T, O-Thong S, Prasertsan P (2014) Biohydrogen production from crude glycerol by immobilized by Klebsiella sp TR17 in a UASB reactor and bacterial quantification under non-sterile conditions. Int J Hydrog Energy 39(18):9580–9587
Biebl H, Menzel K, Zeng AP, Deckwer WD (1999) Microbial production of 1,3-propanediol. Appl Microbiol Biotechnol 52(3):289–297
Homann T, Tag CG, Biebl H, Deckwer W-D, Schink B (1990) Fermentation of glycerol to 1,3-propanediol by Klebsiella and Citrobacter strains. Appl Microbiol Biotechnol 33:121–126
Tee ZK, Jahim JM, Tan JP, Kim BH (2017) Preeminent productivity of 1,3-propanediol by Clostridium butyricum JKT37 and the role of using calcium carbonate as pH neutraliser in glycerol fermentation. Bioresour Technol 233:296–304
Khanna S, Goyal A, Moholkar VS (2013) Production of n-butanol from biodiesel derived crude glycerol using Clostridium pasteurianum immobilized on Amberlite. Fuel 112:557–561
Pflügl S, Marx H, Mattanovich D, Sauer M (2012) 1,3-Propanediol production from glycerol with Lactobacillus diolivorans. Bioresour Technol 119:133–140
Liu B, Christiansen K, Parnas R, Xu Z, Li B (2013) Optimizing the production of hydrogen and 1,3-propanediol in anaerobic fermentation of biodiesel glycerol. Int J Hydrog Energy 38(8):3196–3205
Gallardo R, Faria C, Rodrigues LR, Pereira MA, Alves MM (2014) Anaerobic granular sludge as a biocatalyst for 1,3-propanediol production from glycerol in continuous bioreactors. Bioresour Technol 155:28–33
Sun Y-Q, Shen J-T, Yan L, Zhou J-J, Jiang L-L, Chen Y, Yuanb J-L, Feng E, Xiu Z-L (2018) Advances in bioconversion of glycerol to 1,3-propanediol: prospects and challenges. Process Biochem. https://doi.org/10.1016/j.procbio.2018.05.009
Kanjilal B, Noshadi I, Bautista EJ, Srivastava R, Parnas RS (2015) Batch, design optimization, and DNA sequencing study for continuous 1,3-propanediol production from waste glycerol by a soil-based inoculum. Appl Microbiol Biotechnol 99(5):2105–2117
Jiang LL, Liu HF, Mu Y, Sun YQ, Xiu ZL (2017) High tolerance to glycerol and high production of 1,3-propanediol in batch fermentations by microbial consortium from marine sludge. Eng Life Sci 17(6):635–644
Varrone C, Floriotis G, Heggeset TMB, Le SB, Markussen S, Skiadas IV, Gavala HN (2017) Continuous fermentation and kinetic experiments for the conversion of crude glycerol derived from second-generation biodiesel into 1,3 propanediol and butyric acid. Biochem Eng J 128:149–161
Fuentes M, Mussati MC, Scenna NJ, Aguirre PA (2009) Global modeling and simulation of a three-phase fluidized bed bioreactor. Comput Chem Eng 33(1):359–370
Rosa PRF, Santos SC, Sakamoto IK, Varesche MBA, Silva EL (2014) Hydrogen production from cheese whey with ethanol-type fermentation: effect of hydraulic retention time on the microbial community composition. Bioresour Technol 161:10–19
Santos SC, Rosa PRF, Sakamoto IK, Varesche MBA, Silva EL (2014) Hydrogen production from diluted and raw sugarcane vinasse under thermophilic anaerobic conditions. Int J Hydrog Energy 39(18):9599–9610
Ottaviano LM, Ramos LR, Botta LS, Varesche MBA, Silva EL (2017) Continuous thermophilic hydrogen production from cheese whey powder solution in an anaerobic fluidized bed reactor: effect of hydraulic retention time and initial substrate concentration. Int J Hydrog Energy 42(8):4848–4860
Ramos LR, Silva EL (2016) Continuous hydrogen production from agricultural wastewaters at thermophilic and hyperthermophilic temperatures. Appl Biochem Biotechnol 182:846–869
Nazareth TC, de Oliveira Paranhos AG, Ramos LR, Silva EL (2018) Valorization of the crude glycerol for propionic acid production using an anaerobic fluidized bed reactor with grounded tires as support material. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-018-2754-y
Barbirato F, Grivet JP, Soucaille P, Bories A (1996) 3-Hydroxypropionaldehyde, an inhibitory metabolite of glycerol fermentation to 1,3-propanediol by enterobacterial species. Appl Environ Microb 62(4):3–7
Biebl H, Pfennig N (1981) Isolation of members of the family Rhodosprillaceae. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The prokaryotes. Springer, Berlin, pp 267–273
Kim S, Han S, Shin H (2006) Effect of substrate concentration on hydrogen production and 16S rDNA-based analysis of the microbial community in a continuous fermenter. Process Biochem 41(1):199–207
Amorim ELC, Barros AR, Damianovic MHRZ, Silva EL (2009) Anaerobic fluidized bed reactor with expanded clay as support for hydrogen production through dark fermentation of glucose. Int J Hydrog Energy 34(2):783–790
American Public Health Association (2012) Standard methods for the examination for water and wastewater, 22nd edn. American Water Works Association, Water Environmental Federation, Washington
Walker M, Zhang Y, Heaven S, Banks C (2009) Potential errors in the quantitative evaluation of biogas production in anaerobic digestion process. Bioresour Technol 100(24):116–123
Bondioli P, Della Bella L (2005) An alternative spectrophotometric method for the determination of free glycerol in biodiesel. Eur J Lipid Sci Technol 107(3):153–157
Oh B-R, Seo J-W, Choi MH, Kim CH (2008) Optimization of culture conditions for 1,3-propanediol production from crude glycerol by Klebsiella pneumoniae using response surface methodology. Biotechnol Bioprocess Eng 13(6):666–670
Hong E, Yoon S, Kim J, Kim E, Kim D, Rhie S, Ryu Y-W (2013) Isolation of microorganisms able to produce 1,3-propanediol and optimization of medium constituents for Klebsiella pneumoniae. Bioprocess Biosyst Eng AJ4(6):835–843 36(
Kaur G, Srivastava AK, Chand S (2012) Determination of kinetic parameters of 1,3-propanediol fermentation by Clostridium diolis using statistically optimized medium. Bioprocess Biosyst Eng 35(7):1147–1156
Wang J, Wan W (2009) Factors influencing fermentative hydrogen production: a review. Int J Hydrog Energy 34:799–811
Angelidaki I, Sanders W (2004) Assessment of the anaerobic biodegradability of macropollutants. Rev Environ Sci Biotechnol 3:117–129
Gonen C, Gungormusler M, Azbar N (2013) Continuous production of 1,3-propanediol using waste glycerol with Clostridium beijerinckii NRRL B-593 immobilized on glass beads and glass rushing rings. Chem Biochem Eng 27(2):227–234
Edwards VH (1970) The influence of high substrate concentrations on microbial kinetics. Biotechnol Bioeng 12(5):679–712
Cheng KK, Zhang J-A, Liu D-H, Sun Y, Liu H-J, Yang M-D, Xu J-M (2007) Pilot-scale production of 1,3-propanediol using Klebsiella pneumoniae. Process Biochem 42(4):740–744
Gonen C, Gungormusler M, Azbar N (2012) Comparative evaluation of pumice stone as an alternative immobilization material for 1,3-propanodiol production from waste glycerol by immobilized Klebsiella pneumonia. Appl Biochem Biotechnol 168(8):2136–2147
Saint-Amans S, Girbal L, Andrade J, Ahrens K, Soucaille P (2001) Regulation of carbon and electron flow in Clostridium butyricum VPI 3266 grown on glucose–glycerol mixtures. J Bacteriol 183(5):1748–1754
Saint-Amans S, Perlot P, Goma G, Soucaille P (1994) High production of 1,3-propanediol from glycerol by Clostridium butyricum VPI 3266 in a simply controlled fed-batch system. Biotechnol Lett 16(8):831–836
Khanna S, Goyal A, Moholkar VS (2013) Effect of fermentation parameters on bio-alcohols production from glycerol using immobilized Clostridium pasteurianum: an optimization study. Prep Biochem Biotechnol 43(8):828–847
Huang H, Gong CS, Tsao GT (2002) Production of 1,3-propanediol by Klebsiella pneumoniae. Appl Biochem Biotechnol 98:687–698
Vivek N, Pandey A, Binod P (2016) Biological valorization of pure and crude glycerol into 1,3-propanediol using a novel isolate Lactobacillus brevis N1E9.3.3. Bioresour Technol 213:222–230
Acknowledgements
The authors gratefully acknowledge the financial support of the National Council for Scientific and Technological Development (CNPq), Coordination for the Improvement of Higher Education Personnel (CAPES), and São Paulo Research Foundation (FAPESP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Edson Luiz Silva: Scopus ID: 24167115300.
Rights and permissions
About this article
Cite this article
Paranhos, A.G.d.O., Silva, E.L. Optimized 1,3-propanediol production from crude glycerol using mixed cultures in batch and continuous reactors. Bioprocess Biosyst Eng 41, 1807–1816 (2018). https://doi.org/10.1007/s00449-018-2003-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-2003-3