Abstract
1,3-propanediol (1,3-PD) is a chemical compound of immense importance primarily used as a raw material for fiber and textile industry. It can be produced by the fermentation of glycerol available abundantly as a by-product from the biodiesel plant. The present study was aimed at determination of key kinetic parameters of 1,3-PD fermentation by Clostridium diolis. Initial experiments on microbial growth inhibition were followed by optimization of nutrient medium recipe by statistical means. Batch kinetic data from studies in bioreactor using optimum concentration of variables obtained from statistical medium design was used for estimation of kinetic parameters of 1,3-PD production. Direct use of raw glycerol from biodiesel plant without any pre-treatment for 1,3-PD production using this strain investigated for the first time in this work gave results comparable to commercial glycerol. The parameter values obtained in this study would be used to develop a mathematical model for 1,3-PD to be used as a guide for designing various reactor operating strategies for further improving 1,3-PD production. An outline of protocol for model development has been discussed in the present work.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The escalating global energy demands, environmental concerns and a predilection for use of cleaner, inexhaustible and environment friendly resources over the exhaustible and dwindling petrochemical resources call for green production of industrially important metabolites. In the recent past, there has been an increasing interest in the production of biodiesel as an alternative fuel. It is a non-toxic fuel which is derived from vegetable or animal fats by the process of trans-esterification [1]. A principal by-product of the biodiesel plant is glycerol or glycerin, which accounts for 10% (w/w) of the biodiesel produced. The biodiesel plant producing a green fuel on one hand leaves behind on the other hand enormous quantities of ‘waste’ glycerol thereby raising serious concerns for disposal of this waste in order to increase the sustainability of the plant. Besides this huge amounts of glycerol are generated as by-product from soap industries as well [2]. Therefore, biotechnological processes need to be developed to valorize glycerol into a higher valued product like 1,3-propanediol (1,3-PD). 1,3-PD is an organic compound with noticeable properties particularly for polycondensation reactions to synthesize polyesters, polyethers and polyurethanes. It has gained commercial attention as an important monomer to synthesis a new polyester polytrimethylene terephthalate (PTT) which has excellent properties for use by fiber and textile industries [2, 3]. 1,3-PD also finds enormous other applications e.g. solvent, adhesives, lubricant, antifreeze, cosmetics, detergents, medicines etc [4]. The classic production route to this valuable monomer is the chemical process which entails the use of high pressure and temperature, expensive catalysts and accumulation of toxic by-products [5, 6]. Biological alternative to chemical synthesis for 1,3-PD is possible by the use of microorganisms belonging to the genera Klebsiella, Citrobacter, Enterobacter and Clostridium which can use glycerol for bioconversion to 1,3-PD [7–12].
Glycerol assimilation in nature occurs via a coupled ‘oxidative’ and ‘reductive’ metabolic pathway [13, 14]. The oxidative branch is predominantly involved in the production of energy (ATP) and reducing equivalents (NADH + H+) with simultaneous formation of the by-products whereas the reductive branch yields 1,3-PD while regenerating NADH + H+ for continuation of metabolic activities [15]. Though the biotechnological method presents a cleaner, environmentally favorable route to 1,3-PD there are certain bottlenecks in this process. The concentration and productivities achievable by these methods are quite low which could be mainly attributed to the fact that biological processes are carried out at mild temperatures and pressures as opposed to the chemical methods [16]. Besides this, the biotechnological process of 1,3-PD suffers from severe inhibition by both substrate and product. Optimization of fermentation recipe and selection of right bioreactor operating strategies are the fundamental bioprocess engineering tools for improving the production of the desired metabolite. Selection of right medium recipe and environmental conditions is considered to play a critical role in enhancing microbial growth and metabolite production.
The present study was aimed at determination of macrokinetic parameters of 1,3-PD fermentation by an obligate anaerobe Clostridium diolis which would be mainly used for 1,3-PD model development. The investigation was initiated by study of growth inhibition of C. diolis using varying concentrations of substrate (glycerol) to ascertain the nature of relationship between specific growth rate and glycerol concentration and to establish the glycerol concentration which might inhibit growth of the microorganism. This was followed by statistical optimization of medium recipe for growth and 1,3-PD formation by C. diolis using Plackett–Burman Design and Response Surface Methodology (RSM). This is the first report on nutrient medium optimization for 1,3-PD production by statistical means using a non-pathogenic and strict anaerobe. The optimal concentrations of key process variables obtained from statistical media design were then used in bioreactor (3.7 L) with pH-control conditions to estimate the key kinetic parameters of 1,3-PD fermentation by C. diolis. The values of parameters thus obtained would be used to propose a mathematical model for 1,3-PD production in our future investigations. An outline of the steps involved in model development has been discussed in the present work. The developed medium recipe was also used with raw glycerol derived from Jatropha biodiesel plant to investigate the efficiency of its bioconversion to 1,3-PD. Production of 1,3-PD directly from biodiesel-derived raw glycerol without any pretreatment has been reported for the first time in this work.
Materials and methods
Microorganism
Clostridium diolis DSM 15410 (previously Clostridium butyricum DSM 5431) procured from German Collection of Microorganisms (DSMZ, Germany) was used in this study. The microorganism was maintained on Reinforced Clostridium Medium (RCM) and stored at 4 °C.
Medium and culture conditions
The precultures were developed in 120 ml serum bottles which contained 50 ml nitrogen gassed sterile medium and were sealed with rubber septa and aluminum caps. This modification of culture conditions facilitated the transfer/withdrawal of active inoculum under anaerobic conditions. The bottles were incubated at 33 °C and 150 rpm in an orbital incubator shaker for 24 h. The medium used for setting the microorganism in exponential phase contained (per litre) pure glycerol 20 g; K2HPO4 3.4 g; KH2PO4 1.3 g; (NH4)2SO4 2 g; MgSO4·7H2O 0.2 g; CaCl2·2H2O 0.02 g; FeSO4·7H2O 5 mg; Yeast extract 2 g; Trace element solution (TES) 2 ml [9]. The TES per liter consisted of: 70 mg ZnCl2; 0.1 g MnCl2·4H2O; 60 mg H3BO3; 0.2 g CoCl2·2H2O; 20 mg CuCl2·2H2O; 25 mg NiCl2·6H2O; 0.9 ml HCl (37%). FeSO4·7H2O and TES were filtered sterilized using 0.22 μm pore size syringe filter (Millipore, Ireland) while the rest of the medium components were autoclaved. Experiments to study growth inhibition were conducted using the same medium composition as described above but with varying glycerol concentration. Similarly, the control shake flask experiment with un-optimized medium had the above mentioned composition with a glycerol concentration of 50 g/L.
Preliminary experiments on growth inhibition
For studying the effect of limiting nutrient (glycerol) on growth of C. diolis preliminary experiments were carried out in 500 ml specially modified Erlenmeyer flasks (mouth size reduced to 10 mm) containing 100 ml autoclaved medium and different concentrations of glycerol (98% pure, Merck, India) ranging from 20 to 100 g/L. Infusion of nitrogen gas was performed in flasks to ensure anaerobic conditions. The pH of the medium was adjusted to 7.0 before autoclaving using 2 N NaOH/HCl. An exponentially growing culture (24 h) from serum bottles was taken as inoculum for shake flask cultivation. Inoculated flasks were kept in an orbital incubator shaker with the set point for temperature at 33 °C. Growth was obtained at 33 °C and 150 rpm. The experiments were carried out in triplicate. Samples were taken at regular intervals of 2 h. Growth of C. diolis on different concentrations of glycerol was monitored by estimation of optical density (OD) at 650 nm and specific growth rate was determined from the slope of the least square regression lines of the logarithm of OD versus time data in the exponential growth phase of the culture.
Optimization of nutrient medium recipe by Plackett–Burman design and RSM
Plackett–Burman Design representing the first stage of medium optimization was predominantly used as a screening tool to identify those process variables which have really a significant effect on the responses under study. An experimental design of 12 experiments for four factors namely glycerol, (NH4)2SO4, K2HPO4 and KH2PO4 was formulated by the Plackett–Burman Design (Design Expert 5.0, software developed by StatEase Inc, MN, USA). All the process variables were studied at two levels of concentrations, high (+1) and low (−1). The responses under study were (1) Biomass (g/L) and (2) 1,3-PD (g/L). These factors (glycerol, (NH4)2SO4, K2HPO4 and KH2PO4) were chosen on the basis of these being the carbon (glycerol), nitrogen ((NH4)2SO4) and phosphate (K2HPO4 and KH2PO4) sources for the microorganism which can possibly effect the biomass and 1,3-PD production. The range of glycerol concentration taken in this design was decided on the basis of results obtained from trial experiments conducted to study the effect of glycerol on microbial growth. The concentration range for the rest of the parameters was taken from extensive literature data on 1,3-PD fermentation on the basis of the fact that the statistical optimization methods used in this study account for intriguing interactions between high and low concentration values of the input variables. This nature of statistical methods obviates any chances of erroneous results which may arise due to variation in medium constituent quality, experimental set up etc. All the experiments were conducted in 500 ml modified Erlenmeyer flasks containing 100 ml autoclaved medium and sparged with nitrogen for maintaining anerobiosis which was established by the change of color of resazurin dye. The medium pH was adjusted to 7.0 with 2 N NaOH/HCl. It was inoculated with 5% (v/v) exponentially growing culture (24 h) from serum bottles. The inoculated flasks were kept at 33 °C and agitated at 150 rpm in orbital incubator shaker for 25 h. All the experiments were performed in duplicate. Samples were taken at the end of 25 h and analyzed for the two responses. These responses (biomass and 1,3-PD) were then subjected to compatible analysis of Plackett–Burman protocol to yield t coefficient values for all the four process variables. The variables which were found to be statistically significant as reflected by their high positive t values (glycerol, K2HPO4 and KH2PO4) with respect to the two responses were considered for RSM to obtain a statistical model accounting for both individual and interactive effects of these key process variables and eventually obtain optimal concentrations of process parameters. The optimal values of these three independent process variables were determined by a 2n factorial Central Composite Design (CCD) developed using Design Expert (version 5.0.9) software (Stat-Ease Corp., USA). A set of 20 experiments for three factors was designed by the software whereas the remaining one factor with a low t value was set at a constant non-limiting concentration. The experimental conditions were same as described above. The responses obtained for different experimental recipe in RSM were used for regression and graphical analysis with the help of software to find the optimal values for glycerol, K2HPO4 and KH2PO4. A special feature of Design Expert known as Point Prediction was also used to confirm the model predictions. The responses predicted by the model were also validated by study of batch kinetics in shake flask.
Shake flask study using statistically optimized medium
For batch cultivation studies in shake flask 100 ml medium was taken in 500 ml modified Erlenmeyer flasks and inoculated with 5 ml exponentially growing culture (24 h) from the serum bottles. The ordinary Erlenmeyer flasks (500 ml) have a mouth size (diameter) of 30 mm. The mouth size has been modified to 10 mm for our experiments using serum bottle neck. The modified flasks are then sealed with rubber and aluminum caps with the help of a crimper and nitrogen gas infused in the flasks to maintain anaerobic conditions. The latter is established by the pink color of resazurin dye. This makes it possible to carry out anaerobic fermentation outside the anaerobic tent. Statistically obtained medium composition (“Results and discussion”) was used in this study. The medium pH was adjusted to 7.0 with 2 N NaOH/HCl. The cultivation conditions were 33 °C and 150 rpm. Samples were withdrawn at regular intervals of 3 h for biomass and 1,3-PD estimation.
Estimation of kinetic parameters of 1,3-PD fermentation by C. diolis
The kinetic parameters of glycerol conversion to 1,3-PD by C. diolis were determined by cultivation of microorganism in a 3.7 L bioreactor (Bioengineering AG CH Switzerland) (working volume 1.5 L) using optimized nutrient medium recipe. Bioreactor was inoculated with 10% inoculum (v/v) grown in 500 ml shake flasks. Nitrogen gas was sparged at 0.5 vvm in the bioreactor to maintain anaerobic conditions. pH was controlled at 7.0 by automatic addition of 2 N NaOH/HCl. Agitation was done by a flat blade turbine impeller at 150 rpm and temperature was maintained at 33 °C by chilled water unit (Julabo, Germany). Biomass and 1,3-PD were analyzed at regular intervals of 3 h. These values of parameters form initial guesses for model parameter optimization which would be attempted for proposing batch 1,3-PD model.
Growth and 1,3-PD production on biodiesel derived raw glycerol
Experiments were conducted in 500 ml modified Erlenmeyer flasks containing 100 ml autoclaved nitrogen gassed medium. However, raw glycerol (containing around 40% glycerol) obtained as a by-product from Jatropha-based biodiesel production (Centre for Rural Development and Technology, IIT, Delhi) was used for growth and 1,3-PD production in this experiment. Rest of the conditions were same as described above for shake flask cultivation studies. Samples were analyzed after 24 h for biomass and 1,3-PD.
Analytical methods
Biomass was measured turbidometrically at 650 nm (OD 650) and correlated with dry cell weight (DCW) directly by a previously established standard curve between OD 650 and DCW. Glycerol and 1,3-PD were quantitated by High Performance Liquid Chromatography (Agilent 1200 Series) using a Refractive-Index Detector (RI) and Bio-Rad Aminex HPX-87H column. Analysis was carried out at 30 °C temperature using 0.5 mM H2SO4 as mobile phase at a flow rate of 0.5 ml/min [17].
Results and discussion
Preliminary experiments on growth inhibition
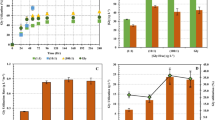
The preliminary studies on the effect of initial glycerol concentration on clostridial growth were carried out using different concentrations of carbon source glycerol while keeping the rest of the medium constituents at a constant level. The experiments distinctly demonstrated inhibition of growth beyond a particular concentration of glycerol. A decrease in the specific growth rate (μ) of C. diolis was evident when initial glycerol concentration higher than 40 g/L was taken in the growth medium. This decrease was observed as soon as a glycerol concentration of 50 g/L was used and μ dipped to 0.65 h−1 from 0.76 h−1 obtained on 40 g/L glycerol. However, a sharp fall in maximum specific growth rate to 0.24 h−1 occurred when the medium contained 70 g/L glycerol. It continued to decrease further till it reached zero at a glycerol concentration of 100 g/L. Also a longer lag phase (10–11 h vs. 5 h at lower concentrations) was observed in cultures grown on still higher concentrations (80–90 g/L) of glycerol. S m value indicating the critical glycerol concentration which caused complete inhibition of growth was found to be 98.3 g/L in this study. Figure 1 shows the effect of increasing initial glycerol concentration on μ of C. diolis. This relation between μ and substrate concentration facilitates in selection of appropriate substrate limitation and/or inhibition terms for proposing the model.
Optimization of nutrient medium recipe by Plackett–Burman Design and RSM
The complexity caused by the multivariable nature of biological processes makes it rather difficult to zero in on ‘the’ significant process variables which really effect the response(s) and optimize them using conventional trial and error approach. Thus the use of intelligent, fast and reliable screening tool like Plackett–Burman is advantageous in optimizing such complex processes [18]. Four factors namely glycerol, (NH4)2SO4, K2HPO4 and KH2PO4 were submitted to Plackett–Burman protocol which led to a design of 12 experiments. Table 1 shows the range of variables taken for the study while Table 2 describes the experimental recipe and the responses obtained. Table 3 shows the t value coefficients obtained for independent variables under study thereby highlighting those variables which had a significant effect on biomass and 1,3-PD production. It is apparent from the table that all factors but one ((NH4)2SO4) significantly affected the process as indicated by their significantly high positive t values. A positive influence of glycerol was expected for it being the substrate for microbial growth as well as bioconversion to 1,3-PD. The phosphate (mineral) sources (K2HPO4 and KH2PO4) were also found to be positive effectors which could be explained on the basis of their requirement for maintenance of buffering capacity in the medium which is essential for growth of the cells and production of 1,3-PD and also due to their contribution towards bioenergetics of the cell. Table 2 clearly shows that maximum 1,3-PD concentration was obtained when these two factors were at maximum (experiment 4 and 5).
Response surface methodology helps in evaluating the concentration of process variables while highlighting the effect of variations in these variables on the responses under study. It also facilitates the development of a model equation which fits the experimental data and helps in comprehensive study of the system and arriving at the optimal response of the process [19]. This is not possible in the conventional optimization approach. By the use of Plackett–Burman Design, three key process variables namely glycerol, K2HPO4 and KH2PO4 with positive influence on responses were identified and utilized in CCD. Table 4 shows the experimental design of 20 experiments formulated by CCD and the responses obtained in the study. The following quadratic regression equations were obtained for the two responses by the analysis of experiments:
-
1.
Biomass: 0.88 + 0.011A + 0.027B + 0.11C − 0.034A2 + 0.23B2 + 0.092C2 − 0.20AB − 0.20AC + 0.17BC
-
2.
1,3-propanediol: 1.83 + 0.28A + 0.096B + 0.17C + 0.59A2 + 0.14B2 − 0.57C2 + 0.50AB + 1.12AC − 0.16BC
where A = Glycerol, B = K2HPO4, C = KH2PO4.
The accuracy of the model evaluated by determination coefficient R 2 was found to be 0.9213 and 0.9135 for biomass and 1,3-PD, respectively. A low coefficient of variation 13.14 and 11.29% for response (1) and response (2), respectively, indicated a high degree of precision. The significance of the model was further confirmed by the ‘Model F-value’ of 13.45 and 15.32 for biomass and 1,3-PD, respectively. The 2D contour plots (Figs. 2a, b, 3a, b) serving as graphical representations of the solution of equations for biomass and 1,3-PD were thoroughly analyzed to find the optimum levels of the three process variables. These showed the changes in the response(s) with respect to variation in two variables while the third variable is held at a constant (zero) level. Contour plots in Fig. 2a, b represent the relation of two variables with respect to biomass. While Fig. 2a shows the contour lines of glycerol and K2HPO4 (with KH2PO4 held at a constant value of 2.25) that have the same response value for biomass, Fig. 2b indicates the contours of constant response (biomass) with the axis systems being glycerol and KH2PO4 (with K2HPO4 held at a constant value of 2.25). As seen in the graphs, maximum predicted response i.e. peak biomass concentration is achieved when all three factors are at a high level. Similarly Fig. 3 shows the effect of change in concentrations of two variables i.e. glycerol and K2HPO4 (Fig. 3a) and glycerol and KH2PO4 (Fig. 3b) on 1,3-PD production. A significantly high 1,3-PD concentration could be predicted by taking high concentrations of the three factors in the medium recipe. Besides the information provided by contour plots on the optimal levels of process variables, a unique feature of Design Expert software-Point Prediction was used to reconfirm these values. This feature facilitated in varying the concentrations of all three factors and consequently reinforcing the results predicted by contour plots. Design Expert presented the maximum numerical solution with the predicted biomass and 1,3-PD concentration of 1.4 and 3.2 g/L, respectively, at the following values of process variables: glycerol 54.15 g/L, K2HPO4 3.21 g/L and KH2PO4 2.75 g/L.
Study of batch kinetics in shake flask
The validation of the predicted medium composition was done by study of batch kinetics in shake flask. The kinetics obtained by growth of C. diolis on 54.15 g/L glycerol as a carbon source is shown in Fig. 4. A maximum biomass of 1.5 g/L and 3.5 g/L 1,3-PD was obtained upon growth in the shake flask which was in close agreement with the values predicted by the model (shown above). A glycerol concentration of 20.4 g/L was left unconsumed in the shake flask. Control experiment with un-optimized medium (results not shown) gave a lesser biomass and 1,3-PD concentration of 1.1 and 2.2 g/L, respectively.
Estimation of kinetic parameters of 1,3-PD fermentation
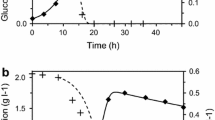
Figure 5 depicts the time course of cultivation of C. diolis for the production of 1,3-PD in a 3.7 L bioreactor. After an initial lag of around 6 h the culture started to grow exponentially. This was soon followed by an increase in 1,3-PD concentration at 8 h. Though a decline in biomass growth was observed at around 30 h, 1,3-PD production continued to increase till the end of 36 h. It featured a maximum biomass concentration of 3.4 g/L and 1,3-PD concentration of 26 g/L at the end of 36 h. A very small amount (3.1 g/L) of unconsumed glycerol was left behind in the fermentation broth at the end of fermentation. Higher product concentrations are generally obtained in the bioreactor compared to the shake flask due to better control of environmental conditions such as pH, temperature etc. inside the bioreactor. An important factor which is believed to significantly influence the microbial growth and hence metabolic activities is culture pH. While the fermentation in bioreactor is carried out at a constant pH (maintained throughout by addition of acid/base) it is rather difficult to maintain constant pH conditions in the shake flask. It is only expected that non-maintenance of constant pH during fermentation (in shake flask) would eventually lead to a significant pH reduction in 1,3-PD fermentation. This would give rise to highly dynamic conditions in the shake flask particularly with respect to pH thereby severely affecting the activities of the key enzymes. It has been reported in the literature that with the decrease in pH the detrimental nature of organic acids of 1,3-PD fermentation particularly butyric acid becomes more pronounced [20]. This toxicity may eventually lead to a cessation of microbial activity or even consequent termination of fermentation in shake flask. The above mechanism could have been the main reason for lower observed yield of 1,3-PD in shake flask fermentation in the present investigation. This may also be the reason for appearance of large amounts of unconsumed substrate (20.4 g/L) in the fermentation broth in shake flask towards the end of the fermentation. On the other hand, constant pH conditions appeared to have favored activities of enzymes involved in 1,3-PD production thereby resulting in much higher 1,3-PD yield in the bioreactor and relatively lesser substrate (3.1 g/L) left in the bioreactor. Besides pH, anaerobiosis strategy used for 1,3-PD production also has an important effect on the latter [21]. Continual sparging with nitrogen is reported to result in complete utilization of glycerol (substrate) with high 1,3-PD yields in experiments with less broth volume. On the other hand, termination of nitrogen supply after inoculation (for self-generated anaerobiosis environment) caused significant amounts of unconsumed glycerol to be left behind in the fermentation broth and therefore significantly reduced 1,3-PD conversion yield by this approach. The latter strategy had been used in the present study for shake flask fermentation which consequently featured lower 1,3-PD yields as compared to bioreactor in which nitrogen was continuously sparged. Thus, a combined effect of pH and anaerobiosis strategy is postulated to be the causative agent for lower 1,3-PD accumulation in shake flask cultivation and higher 1,3-PD yield in bioreactor than shake flask in the present investigation. A μmax of 0.65 h−1 and K s value of 12.8 g/L was observed in batch cultivation using statistically optimized medium which meant faster growth and substrate consumption by the microorganism and therefore could explain the higher biomass and 1,3-PD concentration achieved in optimized medium. A cell yield (Y X/S) of 0.07 g/g glycerol, 1,3-PD yield (Y P/S) of 0.50 g 1,3-PD/g glycerol consumed with a 1,3-PD productivity (Q 1,3-PD) of 0.72 g/L/h was obtained. Table 5 shows the results of different fermentation procedures followed in this study. Table 6 gives the list of key kinetic parameters of 1,3-PD fermentation obtained in the study.
The batch kinetic data obtained in the present study can be correlated with the rates and specific rates of growth and product formation which form the basis for proposal of the model for 1,3-PD process. The model consists of differential material balance equations which describe the substrate consumption, biomass and product formation rates as described below:
where Y, m, K 1, K 2 represent the yield of biomass and 1,3-PD with respect to substrate, maintenance coefficient, growth associated and non-growth associated product constant, respectively. The kinetic parameter values thus obtained from batch studies would be further optimized by minimizing the difference between the original experimental data and model simulation by non-linear regression using original logarithm of Rosenbrock [22] and other computer programs and methodology as described by Volesky and Votruba [23]. The model equations would be then simulated on computer using optimal values of model parameters. Once the batch model has been developed and validated, it could be extrapolated to other reactor operating strategies such as fed-batch, continuous etc. for further improvement in 1,3-PD concentration and/or productivity.
A lot of research work has been done on the biotechnological production of 1,3-PD. There have been few recent reports on statistical optimization of 1,3-PD production by Klebsiella pneumoniae [24, 25] and even recombinant E. coli [26]. But to the best of our knowledge there have been no reports on statistical optimization of growth and 1,3-PD formation by a strict anaerobe like C. diolis. This work is particularly important considering the high 1,3-PD titres and yield offered by Clostridium sp. and its non-pathogenic nature as compared to K. pneumoniae. Biebl et al. [27] investigated the use of C. butyricum DSM 5431 for the production of 1,3-PD. A concentration of 29.5 g/L 1,3-PD was reported using 52 g/L glycerol giving a 1,3-PD yield of 0.56 mol/mol. Attempts with other strains of C. butyricum (CNCM 1211 and VPI 3266) were made by Barbirato et al. [9] and González-Pajuelo et al. [1], respectively. CNCM 1211 gave a higher 1,3-PD yield with a high initial glycerol concentration of 70 g/L (0.64 mol/mol) as compared to low yield (0.62 mol/mol) on low glycerol concentration of 20 g/L. Batch experiments without pH regulation using VPI 3266 and commercial glycerol by González-Pajuelo et al. [1] featured a consumption of 41% glycerol yielding 0.58 mol 1,3-PD/ mol glycerol. In this work, a 1.3-fold increase in biomass and a 1.6-fold increase in 1,3-PD concentration could be achieved in the shake flask (as compared to the un-optimized medium) as a result of optimization. A higher biomass growth (3.4 g/L), 1,3-PD concentration (26 g/L) and improved 1,3-PD yield of 0.50 g/g (0.65 mol/mol) was obtained in the bioreactor. The yield obtained in this study is the highest achieved so far using C. diolis DSM 15410. Moreover, the merit of the present work is the determination of key kinetic parameters of 1,3-PD fermentation which would form the basis for model development in our future investigation as discussed above.
Growth and 1,3-PD production on biodiesel derived raw glycerol
Use of raw glycerol obtained as a by-product from biodiesel production for growth and 1,3-PD production by C. diolis was investigated in this work. There was some microbial growth inhibition upon growth on raw glycerol which could be due to the presence of certain impurities such as methanol, un-reacted fatty acids, salts (used as catalysts during biodiesel production) etc. in it. However, a biomass concentration of 1.3 g/L with production of 3.1 g/L 1,3-PD could be obtained which is comparable with the results obtained above using commercial glycerol. The direct use of raw glycerol from biodiesel trans-esterification process without any pre-treatment for the production of 1,3-PD adds to the simplicity and price relaxation of the process.
Conclusion
Bioconversion of glycerol to 1,3-PD represents an interesting renewable and eco-friendly method of producing a commercially important metabolite (1,3-PD) from a waste product. Inhibition of microbial growth by high initial substrate concentration meant fed-batch cultivation could be a possible means for obtaining significantly high 1,3-PD concentration and productivity. But for this to be implemented, the initial glycerol concentration to be taken in the bioreactor has to be just appropriate to support good microbial growth and 1,3-PD production from the very beginning as well as be non-limiting and non-inhibitory to the microorganism. Also taking the optimal concentration of other key nutrients which influence the bioprocess is important to ensure best responses with respect to biomass and product (1,3-PD).
Statistical optimization of the nutrient medium recipe in this study could increase the biomass and 1,3-PD concentration in the shake flask by 1.3- and 1.6-fold, respectively. A maximum biomass concentration of 3.4 g/L and 1,3-PD concentration of 26 g/L could be obtained in the bioreactor giving the highest 1,3-PD yield achieved so far of 0.50 g/g for this strain. The batch kinetics data thus obtained would be particularly useful for developing a mathematical model which would serve as a guide for designing various reactor operating strategies like fed-batch, continuous etc. for further improving 1,3-PD concentration and/or productivity.
References
González-Pajuelo M, Andrade J, Vasconcelos I (2004) Production of 1,3-propanediol by Clostridium butyricum VPI 3266 using a synthetic medium and raw glycerol. J Ind Microbiol Biotechnol 31:442–446
Maervoet VET, Mey MD, Beauprez J, Maeseneire SD, Soetaert WK (2011) Enhancing the microbial conversion of glycerol to 1,3-propanediol using metabolic engineering. Org Process Res Dev 15:189–202
Yu C, Cao Y, Zou H, Xian M (2011) Metabolic engineering of Escherichia coli for biotechnological production of high-value organic acids and alcohols. Appl Microbiol Biotechnol 89:573–583
Zeng AP, Biebl H (2002) Bulk chemicals from biotechnology: the case of 1,3-propanediol production and the new trends. Adv Biochem Eng Biotechnol 74:239–259
Arntz D, Haas T, Müller A, Wiegand N (1991) Chem-Ung-Tech 63:733
Chaminand J, Djakovitch LA, Gallezot P, Marion P, Pinel C, Rosier C (2004) Glycerol hydrogenolysis on heterogenous catalysts. Green Chem 6:359–361
Abbad-Andaloussi S, Guedon E, Spiesser E, Petitdemange H (1996) Glycerol dehydratase activity: the limiting step for 1,3-propanediol production by Clostridium butyricum. Lett Appl Microbiol 22:311–314
Barbirato F, Camarasa C, Claret Grivet JP, Bories A (1995) Glycerol fermentation of 1,3-propanediol producing microorganism: Enterobacter agglomerans. Appl Microbiol Biotechnol 43:786–793
Barbirato F, Himmi EH, Conte T, Bories A (1998) 1,3-Propanediol production by fermentation: an interesting way to valorize glycerin from the ester and ethanol industries. Ind Crop Prod 7:281–289
Boenigk R, Bowien S, Gottschalk G (1993) Fermentation of glycerol to 1,3-propanediol in continuous cultures of Citrobacter freundii. Appl Microbiol Biotechnol 38:453–457
Huang H, Gong CS, Tsao GT (2002) Production of 1,3-propanediol by Klebsiella pneumoniae. Appl Biochem Biotechnol 98–100:687–698
Yang G, Tian J, Li J (2007) Fermentation of 1,3-propanediol by a lactate deficient mutant of Klebsiella oxytoca under microaerobic conditions. Appl Microbiol Biotechnol 73:1017–1024
Papanikolaou S, Fick M, Aggelis G (2004) The effect of raw glycerol concentration on the production of 1,3-propanediol by Clostridium butyricum. J Chem Technol Biotechnol 79:1189–1196
Zhu MM, Lawman PD, Cameron DC (2002) Improving 1.3-propanediol production from glycerol in a metabolically engineered Escherichia coli by reducing accumulation of sn-glycerol-3-phosphate. Biotechnol Prog 18:694–699
Daniel R, Boenigk R, Gottschalk G (1995) Purification of 1.3-propanediol dehydrogenase from Citrobacter freundii and cloning, sequencing, and overexpression of the corresponding gene in E. coli. J Bacteriol 177:2151–2156
Celinska E (2010) Debottlenecking the 1,3-propanediol pathway by metabolic engineering. Biotechnol Adv 28:519–530
Kaur G, Sharma R, Srivastava AK, Chand S (2012) On-line characterization of metabolic state in batch cultivation of Clostridium diolis for 1,3-propanediol production using NADH + H+ fluorescence. Appl Biochem Biotechnol 166:138–145
Plackett RL, Burman JP (1944) The design of optimum multifactorial experiments. Biometrika 33:305–325
Khuri AI, Cornell JA (1987) Response surfaces: design and analysis. Marcel Dekker, New York
Zeng AP, Ross A, Biebl H, Tag C, Gunzel B, Deckwer W.-D. (1994) Multiple product inhibition and growth modeling of Clostridium butyricum and Klebsiella pneumoniae in glycerol fermentation. Biotechnol Bioeng 44:902–911
Chatzifragkou A, Aggelis G, Komaitis M, Zeng AP, Papanikolaou S (2011) Impact of anaerobiosis strategy and bioreactor geometry on the biochemical response of Clostridium butyricum VPI 1718 during 1,3-propanediol fermentation. Bioresour Technol 102:10625–10632
Rosenbrock HH (1960) An automatic method of finding the greatest or the least value of a function. J Comput 3:175–184
Volesky B, Votruba J (1992) Modelling and optimization of fermentation processes. Elsevier, Amsterdam
Zheng Z-m, Q-i Hu, Hao J, Xu F, Guo N-n, Sun Y, Liu D-h (2008) Statistical optimization of culture conditions for 1,3-propanediol by Klebsiella pnuemoniae AC 15 via central composite design. Bioresour Technol 99:1052–1056
Oh B-R, Seo J-W, Choi MH, Kim CH (2008) Optimization of culture conditions for 1,3-propanediol production from crude glycerol by Klebsiella pnuemoniae using response surface methodology. Biotechnol Bioprocess Eng 13:666–670
Zhang X, Li Y, Zhuge B, Tang X, Shen W, Rao Z, Fang H, Zhuge J (2006) Optimization of 1,3-propanediol production by novel recombinant Escherichia coli using response surface methodology. J Chem Technol Biotechnol 81:1075–1078
Biebl H, Marten S, Hippe H, Deckwer W-D (1992) Glycerol conversion to 1,3-propanediol by newly isolated clostridia. Appl Microbiol Biotechnol 36:592–597
Acknowledgments
The Senior Research Fellowship (SRF) award by Indian Council of Medical Research (ICMR), Govt. of India, New Delhi for the execution of the project is gratefully acknowledged by one of the authors (Ms. Guneet Kaur).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, G., Srivastava, A.K. & Chand, S. Determination of kinetic parameters of 1,3-propanediol fermentation by Clostridium diolis using statistically optimized medium. Bioprocess Biosyst Eng 35, 1147–1156 (2012). https://doi.org/10.1007/s00449-012-0700-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-012-0700-x