Abstract
Plant species vary in their growth response to arbuscular mycorrhizal (AM) fungi, with responses ranging from negative to positive. Differences in response to AM fungi may affect competition between plant species, influencing their ability to coexist. We hypothesized that positively responding species, whose growth is stimulated by AM fungi, will experience stronger intraspecific competition and weaker interspecific competition in soil containing AM fungi, while neutrally or negatively responding species should experience weaker intraspecific and stronger interspecific competition. We grew Plantago lanceolata, which responds positively to AM fungi, and Bromus inermis, which responds negatively to AM fungi, in an additive response surface competition experiment that varied the total density and relative frequency of each species. Plants were grown in sterilized background soil that had been inoculated with whole soil biota, which includes AM fungi, or a microbial wash, that contained other soil microbes but no AM fungi, or in sterilized soil that contained no biota. The positively responding P. lanceolata was more strongly limited by intraspecific than interspecific competition when AM fungi were present. By contrast, the presence of AM fungi decreased the strength of intraspecific competition experienced by the negatively responding B. inermis. Because AM fungi are almost always present in soil, strong intraspecific competition in positively responding species would prevent them from outcompeting species that respond neutrally or negatively to AM fungi. The potential for increased intraspecific competition to offset growth benefits of AM fungi could, therefore, be a stabilizing mechanism that promotes coexistence among plant species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The interaction between plants and soil microbes is increasingly being appreciated as a key mechanism influencing the coexistence of plant species (Bever et al. 2012). Species coexistence can be maintained by stabilizing mechanisms, which occur when intraspecific competition exceeds interspecific competition (Chesson 2000; Adler et al. 2007, 2010). Increased intraspecific competition counteracts the effects of relative fitness differences to limit the population growth of dominant species (Chesson 2000; Adler et al. 2007; Levine and HilleRisLambers 2009; Adler et al. 2010; HilleRisLambers et al. 2012). Numerous studies suggest that negative feedbacks between plants and pathogenic soil microbes can cause self-limitation of dominant species and, therefore, promote coexistence (Bever 1994; van der Putten and Peters 1997; Alexander and Holt 1998; Klironomos 2002; Bever et al. 2012; Heinze et al. 2015; Chung and Rudgers 2016). However, few studies have directly investigated whether self-limitation can also be caused by mutualistic soil microbes (Veresoglou et al. 2018; Siefert et al. 2019).
A common and typically mutualistic interaction between plants and soil microbes involves arbuscular mycorrhizal (AM) fungi, obligate symbionts that colonize the roots of > 75% of plant species (Brundrett 2009). These fungi facilitate plant access to limiting nutrients such as phosphorus in exchange for sugars from photosynthesis. AM fungi may also provide pathogen protection to their plant hosts, either by competing directly with pathogenic microbes for space and resources within plant roots or indirectly by improving plant tolerance of pathogens through increasing nutrient acquisition (Wehner et al. 2010). Because of these nutrient acquisition and pathogen protection services, most plant species accumulate more biomass when inoculated by AM fungi relative to non-inoculated controls, increasing competitive ability (Hoeksema et al. 2010). However, though the relationship between plants and AM fungi is mutualistic on average (Hoeksema et al. 2010), the net benefit plants obtain from AM fungi varies among plant species, ranging along a continuum from positive to neutral and even negative growth responses (Klironomos 2002; Janos 2007; Bever et al. 2012; Jiang et al. 2017).
Variation in plant response to AM fungal inoculation may influence coexistence through effects on both inter- and intraspecific competition. For example, positively responding plant species may be more likely to outcompete species that respond neutrally or negatively to AM fungi (Fitter 1977; Hetrick and Wilson 1989; West 1996; Scheublin et al. 2007; Derelle et al. 2015), possibly due to their increased ability to uptake soil nutrients via AM fungal hyphae (George et al. 1995; Bever et al. 2010). However, the benefit of AM fungal inoculation can decrease with increasing density, possibly due to increased overlap among individuals’ nutrient depletion zones (Hartnett et al. 1993; Koide and Dickie 2002). This suggests that positively responding species may also experience strong intraspecific competition in the presence of AM fungi and increased nutrient depletion zone overlap, and therefore may be more likely to self-limit (Hartnett et al. 1993; Moora and Zobel 1996; Watkinson and Freckleton 1997). The greater likelihood of positively responding species experiencing negative density dependence (in the form of stronger intraspecific relative to interspecific competition) than neutrally or negatively responding species could act as a stabilizing mechanism that promotes coexistence in plant communities. However, few studies have explicitly investigated how differences in plant response to AM fungi influence the relative strength of intra- and interspecific competition (Hartnett et al. 1993; Moora and Zobel 1996), and so the contribution of variation in mycorrhizal growth response to stabilizing mechanisms that promote species coexistence remains a critical knowledge gap (Hartnett et al. 1993; Moora and Zobel 1996; Veresoglou et al. 2018).
In this study, we explored whether differences in plant response to AM fungi could contribute to stabilizing mechanisms of coexistence by investigating the strength of both intraspecific and interspecific competition in two co-occurring plant species that differ in their response to AM fungal colonization. To test this hypothesis, we grew plants in soil containing whole soil biota (i.e., with AM fungi and other microbes), soil containing only non-AM fungal biota, and in sterilized soil. When grown together in soils with AM fungi, we predicted that positively responding species would experience stronger intraspecific competition and weaker interspecific competition than in soil lacking AM fungi. By contrast, neutrally or negatively responding species would experience greater interspecific competition and less intraspecific competition in soils containing AM fungi. We further hypothesized that if positively responding species normally rely on AM fungi for pathogen protection, they may be more vulnerable to soil pathogens in the absence of their fungal mutualists. Therefore, a species that responds positively to AM fungi would experience stronger interspecific competition and weaker intraspecific competition in soil that contained non-AM biota than in sterilized soil. By contrast, we predicted that neutrally or negatively responding species would experience weaker interspecific competition but greater intraspecific competition in soil containing only non-AM biota compared to sterilized soil.
Materials and methods
We studied competition between Bromus inermis, a species that has neutral to negative responses to AM fungi (Sherrard and Maherali 2012), and Plantago lanceolata, a species that responds positively to AM fungi (Stanescu and Maherali 2017), in a greenhouse environment. These species naturally co-occur across much of North America and Eurasia in fields and anthropogenically disturbed environments (Cavers et al. 1980; Otfinowski et al. 2007). To create a common background environment to which soil biota treatments could be added, we grew plants in pots containing a sterilized 2:1 mixture of field soil and silica sand. The field soil was collected from a nutrient poor old field at the Long Term Mycorrhizal Research Site in Guelph, Ontario, where both study species co-occur. The unsterilized field soil contained 2.1-mg phosphorus, 3.3-mg NO3 and 2.9-mg NH4 per kg of soil (Sherrard and Maherali 2012). The silica sand contained undetectable levels of phosphorus, 0.28-mg NO3 and 1.71-mg NH4 per kg of sand (Rekret and Maherali unpublished). To remove soil biota, the soil mix was sterilized by autoclaving it at 120 °C for two 90-min cycles. The greenhouse conditions consisted of 14-h days, with a 24 °C day temperature and 16–18 °C night temperature. B. inermis and P. lanceolata seeds were obtained from local seed suppliers (OSC Seeds, Waterloo, Canada, and Richters Herbs, Goodwood, Canada, respectively).
Experimental design and treatments
To examine how competitive interactions between the two plant species were affected by AM fungi and other soil microbes, three different soil treatments were created: whole soil, microbial wash or sterilized soil. The soil inoculum was obtained by collecting soil cores to a depth of 30 cm at regular intervals along four transects spanning the old field community at the Long Term Mycorrhizal Research Site and then pooling all cores. The ‘whole soil’ treatment consisted of 25 g of live soil inoculum (containing all microbes found in the soil, including AM fungi). The ‘microbial wash’ treatment consisted of 25 g of sterilized soil inoculum (inoculum soil that had been autoclaved at 120 °C for two 90-min cycles) and 100 mL of live microbial wash (containing live soil microbes but excluding AM fungi). The microbial wash was produced by suspending inoculum soil in water in a 1:4 volume ratio for 24 h with occasional mixing, and then passing this solution through a 25-µm sieve. This allowed smaller soil microbes to pass through and remain in the wash but excluded the larger AM fungal spores (Ames et al. 1987; Koide and Li 1989; Bever 1994). Previous studies have shown this wash contains many non-AM fungal soil microbes, and so comparing plant performance between the whole soil and microbial wash treatments is effective for isolating the effect of AM fungi on plant growth and competitive ability (Ames et al. 1987; Koide and Li 1989; Bever 1994). The ‘sterilized soil’ treatment consisted of 25 g of sterilized soil inoculum (no live soil microbes) that had been autoclaved at 120 °C for two 90-min cycles. Comparing the microbial wash treatment with this sterilized soil treatment, therefore, isolated the effect of other soil microbes on plant growth and competitive ability.
To examine how differences in plant growth response to AM fungi influence interspecific and intraspecific competition across the three soil treatments, we applied a fully additive response surface competition design (Fig. S1 in Supplementary material 1) where both total plant density and relative frequency of each species were manipulated (Law and Watkinson 1987; Freckleton and Watkinson 2000). This type of study design is considered the best approach to distinguish between the relative effects of intraspecific and interspecific competition because the density of both conspecific and heterospecific competitors is manipulated within a single experimental design (Freckleton and Watkinson 2000; Inouye 2001). Each of the three soil treatments was applied to all twelve of the following B. inermis:P. lanceolata density combinations: 0:2, 2:0, 1:1, 4:0, 0:4, 3:1, 1:3, 2:2, 4:2, 2:4, 3:3 and 4:4. Each soil treatment/density combination was replicated four times for a total of 144 pots.
To apply the treatments, each 948-mL pot, 15 cm in diameter and 15-cm deep, was sterilized with bleach and then filled with 600 mL of the sterilized 2:1 field soil:sand mixture. Another 300 mL of this mixture was mixed with 25 g of either the live or sterilized inoculum and added to the top of the pot. Seeds of the appropriate species were placed in the pot and covered lightly with the soil. Since germination was expected to be less than 100%, for density treatments consisting of one or two individuals of a species, ten seeds of that species were planted in the pot. For densities of three or four individuals, 20 seeds were planted in the pot. This method of seed addition also allowed the spatial arrangement of individuals within pots to be more random than would be possible if germinated seedlings were planted to initiate the experiment (e.g., Veresoglou et al. 2018). For the non-AM microbial wash treatment, 85 mL of microbial wash was added to each pot, whereas for the sterilized and whole soil treatments, 85 mL of distilled water was added to each pot. Pots were then randomly assigned to locations on a greenhouse bench in a checkerboard pattern to minimize aboveground competition for light.
A drip irrigation system was used to provide 50 mL of water per day to each pot for the first 16 days of growth (approximately 8.3 mL of water was provided to each pot every 4 h for a total of 50 mL in a 24 h period), and as plants grew larger, this was increased to 60 mL of water per day to each pot for the next 37 days of growth (10 mL every 4 h), and 70 mL of water to each pot for the remainder of the experiment (~ 11.7 mL every 4 h). The pots were also misted once daily for the first 10 days after planting to encourage germination. Ten days after planting, plants were thinned to achieve the desired density treatment for each pot, taking care to ensure that the remaining individuals were well spaced within the pot to reduce aboveground competition for light. For two pots that did not contain enough seedlings to achieve the desired density, seedlings of the appropriate species were transplanted into these pots from different pots within the same soil treatment. Any newly germinating seedlings were weeded from the pots for the next 15 days to maintain the density treatments. No weeding was done after this to avoid accidentally removing tillers produced by B. inermis individuals. To prevent extreme nutrient limitation to the plants and fungi, each pot received 50 mL of 17-5-17 NPK Plant-Prod® Solutions fertilizer (Master Plant-Prod Inc., Brampton, Canada) diluted in water to ¼ strength 29 days and 59 days after planting.
Harvest and data collection
After 84 days of growth, the aboveground plant biomass for each species in each pot was harvested. Belowground biomass was not measured because the root systems of both plant species could not be separated when grown in the same pot. However, allometric relationships between above- and belowground biomass are strong and positive in temperate grassland species (e.g., Husáková et al. 2018), and so we rely on aboveground biomass to estimate the magnitude of intraspecific and interspecific interactions. Aboveground biomass has previously been used to estimate competitive interactions in other studies exploring the relationship between plant response to AM fungi and competitive interactions (Hartnett et al. 1993; Moora and Zobel 1996; Watkinson and Freckleton 1997).
Following harvest, plant material was dried at 60 °C for 48 h and weighed to calculate a mean biomass per individual for each species in each pot, which served as an indicator of plant performance. To verify that colonization occurred in the whole soil treatment but did not occur in the other treatments, one pot from each soil treatment and density combination was randomly selected for root sampling. A subsample of roots in this pot was harvested, washed and stored in 50% ethanol until the roots could be cleared and stained. Roots could not be separated by species, so the samples from pots containing both B. inermis and P. lanceolata included roots of both species. Roots were cleared in 10% KOH and stained with a solution of Black Sheaffer ink (Sheaffer Pen and Art Supply Co./A.T. Cross Company, Providence, RI) and vinegar (Vierheilig et al. 1998). Fungal colonization of these roots was quantified using the gridline intersect method (McGonigle et al. 1990). We recorded the presence of AM hyphae, vesicles, arbuscules and non-AM fungal structures for each intersect. A total of 50 intersects distributed along fifteen root segments, each ~ 1 cm in length, were examined for each pot sampled.
Statistical analysis
The effect of the soil treatment and density combination on plant biomass after 12 weeks of growth was evaluated using an ANOVA with Type III sums of squares, which is recommended when the intent is to assess the significance of each factor after controlling for the effects of the other factors in the model (Zar 1998). Biomass data were log-transformed to meet homogeneity of variance assumptions. A Tukey HSD post hoc test was used to determine which pairs of soil treatments were significantly different. Since transplanting could negatively affect plant growth, we analyzed data using all observations and then excluding the two pots that contained transplanted individuals. Results of the analysis run without the pots containing transplants are presented here, though the outcome was nearly identical in both cases. We also assessed whether root colonization by AM fungal structures (arbuscules, vesicles and hyphae) and non-AM fungi was influenced by soil treatments using a one-way ANOVA, followed by a Tukey HSD post hoc test after finding a significant treatment main effect.
Since the fully additive response surface design incorporates several densities of each species as well as multiple total densities, multiple regression can be used to quantify interspecific and intraspecific competition (Freckleton and Watkinson 2000; Inouye 2001). Thus, to estimate intraspecific and interspecific competition coefficients, the mean individual aboveground biomass of each species for the variety of density combinations was incorporated into a multiple regression model: 1/Wx = bx0+ biNx+ bjNy (Spitters 1983; Van et al. 1999), where Wx represents the mean aboveground dry biomass per individual for species x, Nx is the density of species x and Ny is the density of species y. In this regression model, the reciprocal of aboveground biomass (1/Wx) is used as the dependent variable because it allows a more straightforward interpretation of the competition coefficients. Specifically, a more positive coefficient represents a stronger competitive effect, or ability of density to suppress the per individual biomass of the focal species. As a result, a more positive coefficient can be interpreted as being indicative of stronger competition (Spitters 1983; Van et al. 1999; Thompson et al. 2015). To permit competition coefficients to be compared across species and soil treatments, the reciprocal biomass (1/Wx) of each species was standardized within treatments using a z-transformation before being incorporated into the regression model. The intercept bx0 estimates the reciprocal biomass of individual plants grown alone and the slope coefficients bi and bj estimate the strength of intraspecific and interspecific competition for a given focal species. Separate regressions were done for each of the two species in each of the three soil treatments to produce a total of six response surfaces.
The 95% confidence intervals of the competition coefficients (bi and bj) were used to determine whether intraspecific and interspecific competition had a significant effect on aboveground biomass for each species in each treatment. The effect was considered significant if the confidence intervals did not overlap zero. We also used the 95% confidence intervals to determine if there was a significant difference between the strength of intraspecific and interspecific competition for each species in each soil treatment and to compare the strength of intraspecific and interspecific competition across treatments. The difference was considered significant if the confidence intervals of the two coefficients did not overlap. Confidence intervals were used to evaluate differences between competition coefficients because they are straightforward to apply and are considered to be conservative post hoc tests (Cumming and Finch 2005). All data used in statistical analyses are provided in Supplementary material 2 (biomass data) and Supplementary material 3 (fungal colonization of roots).
Results
Effects of soil biota and plant density combinations on biomass
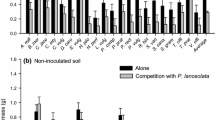
Soil biota influenced average individual aboveground biomass of both B. inermis and P. lanceolata (F2,88 = 203.369, P < 0.001 for B. inermis and F2,88 = 43.583, P < 0.001 for P. lanceolata, Table 1). B. inermis individuals grown in the sterilized soil were 30% larger than those in microbial wash soil and 140% larger than those in whole soil (Fig. 1a). P. lanceolata individuals grown in the whole soil treatment were 70% and 130% larger than those in the sterilized soil and microbial wash treatments, respectively, but P. lanceolata individual biomass did not differ between the sterilized soil and microbial wash treatments based on Tukey’s post hoc comparisons (Fig. 1b). The combination of B. inermis and P. lanceolata densities also significantly influenced average individual aboveground biomass for both B. inermis and P. lanceolata (F9,88 = 51.220, P < 0.001 for B. inermis and F9,88 = 14.396, P < 0.001 for P. lanceolata, Table 1). There was a significant interaction between soil treatment and density combination for both B. inermis and P. lanceolata, suggesting that the effect of each density combination on biomass varied among soil treatments (F18,88 = 3.038, P < 0.001 for B. inermis and F18,88 = 2.886, P < 0.001 for P. lanceolata, Table 1). This interaction was explored further by examining the competition coefficients estimated by the multiple regression models.
Individual aboveground biomass (mean ± SE) of aBromus inermis and bPlantago lanceolata averaged across all densities in each soil treatment after 84 days of growth (n = 40 for the sterilized soil treatment and n = 39 for the microbial wash and whole soil treatments). Values represent arithmetic means, and different letters represent statistically significant differences (P < 0.05) in biomass between soil treatments based on Tukey HSD post hoc comparisons
Competition coefficients
Soil treatment affected the relative strength of intraspecific and interspecific competition experienced by B. inermis. The magnitudes of intraspecific and interspecific competition experienced by B. inermis were significantly > 0 in all three soil treatments (i.e., the 95% confidence intervals around the competition coefficients did not overlap zero), meaning that both intraspecific and interspecific competition reduced B. inermis aboveground biomass in all three soil treatments (Table 2; Fig. 2a, c, e). In the whole soil treatment, there was no significant difference between intraspecific and interspecific competition. However, intraspecific competition was 210% greater than interspecific competition in the microbial wash treatment, and 460% greater than interspecific competition in the sterilized soil treatment, indicating that intraspecific competition suppressed B. inermis aboveground biomass to a greater degree than interspecific competition in these treatments. Intraspecific competition was weaker in whole soil compared to the sterilized soil and microbial wash treatments; whereas, interspecific competition was similar among all soil treatments (Table 2; Fig. 2a, c, e).
Three-dimensional regression planes representing the effect of conspecific and heterospecific plant density on the standardized reciprocal of individual plant biomass (1/W) for a, c, eB. inermis and b, d, fP. lanceolata in a, b sterilized soil, c, d microbial wash and e, f whole soil treatments. A more positive slope indicates a stronger response of plants to conspecific or heterospecific density, and thus stronger competition. n = 40 for the sterilized soil treatment and n = 39 for the microbial wash and whole soil treatments
Soil treatment also affected the relative strength of intraspecific and interspecific competition experienced by P. lanceolata. The magnitude of intraspecific competition was significantly > 0 only in the whole soil treatment; whereas, the magnitude of interspecific competition was significantly positive in all three treatments (Table 2; Fig. 2b, d, f). This indicates that intraspecific competition caused a reduction in P. lanceolata individual biomass only in the whole soil treatment, whereas interspecific competition reduced P. lanceolata biomass in all three soil treatments. In the whole soil treatment, the magnitude of intraspecific competition was 110% higher than interspecific competition, indicating that intraspecific competition suppressed P. lanceolata biomass to a greater degree than interspecific competition. By contrast, the magnitudes of intraspecific and interspecific competition were not significantly different from each other in the sterilized soil and microbial wash treatments, indicating that they had similar effects on P. lanceolata biomass. Intraspecific competition was higher (i.e., had a stronger negative effect on P. lanceolata biomass) in whole soil relative to other soil treatments; whereas, interspecific competition did not vary among soil treatments (Table 2; Fig. 2b, d, f).
Fungal colonization
In the whole soil treatment, all plants sampled were colonized by AM fungal hyphae, vesicles and arbuscules, with 18% of root intersects containing arbuscules, 7% containing vesicles and 68% containing AM hyphae, on average (Fig. S2a–c in Supplementary material 1). Though we were unable to separate roots by species in pots containing both species, AM fungi were present in all pots in the whole soil treatment that were sampled for colonization, including those containing only a single plant species. The sterilized soil and microbial wash treatments were largely free of colonization by AM fungi, though a very small number of samples did have a low level of fungal colonization. Specifically, AM hyphae were observed in 1 out of the 12 plants sampled from the sterilized soil treatment and 2 out of the 12 plants sampled from the microbial wash treatment, with one of those samples containing arbuscules as well. (Fig. S2a–c). Overall, the whole soil treatment contained significantly and substantially greater proportions of all AM fungal structures compared to the other two soil treatments (P < 0.001 in all cases, Table S1 in Supplementary material 1), indicating that comparing the whole soil and microbial wash treatments was an effective way of isolating the effects of AM fungi on plant responses to intra- and interspecific competition. All soil treatments contained a small amount of non-AM fungal hyphae (Fig. S2d) and there were no statistically significant differences in the proportion of roots colonized by non-AM fungal hyphae among soil treatments (F2,33 = 0.696, P = 0.506, Table S1).
Discussion
Our results provide insight into the potential mechanisms by which interactions with mycorrhizal fungi promote the coexistence of plant species. Specifically, we show that a positively responding species is more strongly limited by intraspecific than interspecific competition in the presence of AM fungi; whereas, a negatively responding species experienced reduced intraspecific competition in the presence of AM fungi. Because AM fungi are almost always present in soil (Stürmer et al. 2018), the greater likelihood of self-limitation in species that respond positively to AM fungi should prevent them from competitively excluding neutrally or negatively responding plant species (Klironomos 2002; Stanescu and Maherali 2017). Our findings conflict with predictions from theoretical models that ignore self-limitation (Urcelay and Diaz 2003) but are nonetheless consistent with previous reports of stronger intraspecific than interspecific competition in other positively responding grasses (Hartnett et al. 1993; Watkinson and Freckleton 1997) and forbs (Moora and Zobel 1996). Recent work has shown that plant species mixtures differing in growth response to AM fungi are more likely to coexist than mixtures of species that have similar growth responses to AM fungi, potentially due to a positive correlation between divergence in mycorrhizal growth response and divergence in niche requirements (Veresoglou et al. 2018). If plant species that respond positively to AM fungi are more likely to experience self-limitation than those with neutral or negative responses, as shown here, then a stabilizing mechanism could also promote the coexistence of species that differ in mycorrhizal growth response.
Although species with weak or negative responses to AM fungi are generally thought to be at a competitive disadvantage, reduced intraspecific competition for these species in the presence of AM fungi could explain their persistence in plant communities. For example, inoculation with AM fungi appeared to release the negatively responding species, B. inermis, from strong self-limitation, an inference based on the observation that intraspecific competition in this species was lower in soil containing AM fungi compared to the non-AM fungal treatments; whereas, interspecific competition remained the same across soil treatments (Table 2; Fig. 2). The reduction in intraspecific competition was likely due to suppression of B. inermis individual biomass by AM fungi. This result is consistent with those of Hartnett et al. (1993), who found that the weakly but positively responding species Elymus canadensis experienced less intraspecific competition in the presence of AM fungi than its more positively responding competitor. The presence or absence of AM fungi did not affect the strength of intraspecific competition for E. canadensis (Hartnett et al. 1993); whereas, here we report that the presence of AM fungi significantly decreased intraspecific competition for a negatively responding species. Thus, weaker intraspecific competition in the presence vs absence of AM fungi may only occur if a species has a negative mycorrhizal growth response.
Our findings suggest that differences in mycorrhizal growth response primarily influence intraspecific competition rather than interspecific competition. Though interspecific competition occurred in all soil treatments, there were no significant differences between interspecific competition coefficients among soil treatments for either species. This result contrasts with those of previous studies who report that in soil containing AM fungi, interspecific competition was higher for more neutral or negatively responding species than for positively responding species (Fitter 1977; Hetrick and Wilson 1989; Hartnett et al. 1993; Moora and Zobel 1996). However, these previous studies did not employ experimental designs that separate the effects of interspecific and intraspecific competition (Hartnett et al. 1993) or measured competition in pairs rather than using a range of densities (Fitter 1977; Hetrick and Wilson 1989; Moora and Zobel 1996), as was done in the present study. Thus, it is possible that experiments that do not manipulate both intra- and interspecific competition may overestimate the effect of differences in mycorrhizal growth response on interspecific competition.
Soil microbes other than AM fungi did not appear to have a strong effect on plant performance or competition. For example, B. inermis had a much stronger negative response to AM fungi than to other soil microbes (Fig. 1a) and P. lanceolata growth did not differ between the microbial wash and sterilized soil treatments (Fig. 1b). Furthermore, there were no differences for either species in the strength of intraspecific or interspecific competition between the sterilized soil and microbial wash treatments. This lack of a pathogenic effect differed from the results of other studies which have found that non-AM fungal soil microbes often negatively affect plant growth (van der Putten and Peters 1997; Alexander and Holt 1998; Klironomos 2002; Bever et al. 2012; Hodge and Fitter 2013; Heinze et al. 2015; Laliberté et al. 2015; Liang et al. 2015; Chung and Rudgers 2016). However, many studies investigating plant pathogens in soil show that they build up over time as the density of a particular plant species increases, via a negative feedback mechanism (Alexander and Holt 1998; Klironomos 2002; Bever et al. 2012; Heinze et al. 2015; Chung and Rudgers 2016). Our experiment was relatively short term and involved novel combinations of plant genotypes and soil communities; so, there was little opportunity for species-specific pathogens to accumulate to a level that would cause observable detrimental effects. Alternatively, it is also possible that the microbial wash treatment contained both beneficial and pathogenic soil microbes, as has been observed previously in investigations of microbe-mediated competition (Chung and Rudgers 2016). In this case, the positive and negative effects of different soil microbes may have counteracted one another, resulting in the lack of an observable effect.
Comparing the effects of whole soil and microbial wash inoculum treatments was effective at isolating the effects of AM fungi on plant growth and competition. Although this approach has been criticized because there may be other biotic differences between these two inocula treatments (Ames et al. 1987), it has been deemed to be effective at isolating AM fungal effects in prior studies (Ames et al. 1987; Koide and Li 1989; Bever 1994). Consistent with this prior work, we observed large differences in root colonization by AM fungi between the whole soil and microbial wash treatments, which support the assumption that the primary difference between the two treatments was the presence of AM fungi (Fig. S2a–c). Specifically, roots in the whole soil treatment were abundantly colonized by AM fungal structures, whereas AM fungi were consistently absent from roots in the microbial wash treatment. Furthermore, there was no difference in the abundance of non-AM fungal structures in plant roots from the microbial wash and whole soil treatments (Fig. S2d), implying that with the exception of the presence of AM fungi, there were no other biotic differences between the whole soil and microbial wash treatments.
A limitation of the present study is that competitive interactions were assessed on only two species, and it is not known whether the results are representative of competition between other species that differ in their response to AM fungi. This sampling limitation is relatively common in the literature because the number of replicates required to quantify competition in additive response surface experimental designs increases exponentially with the number of species used, and experiments with more than a few species can, therefore, become logistically difficult to do (Levine et al. 2017). As a result, a large proportion of competition studies on species that differ in response to AM fungi have focused on species pairs (Fitter 1977; Hetrick and Wilson 1989; Hamel et al. 1992; Hartnett et al. 1993; Hetrick et al. 1994; West 1996). Because two species were used, it is possible that the differences we observed were caused by functional differences between B. inermis and P. lanceolata that are unrelated to mycorrhizal response, such as growth rate, growth form and nutrient or water uptake ability (Grime 1977; Casper and Jackson 1997; Aerts 1999). We also note that B. inermis and P. lanceolata are introduced, though naturalized plant species (Cavers et al. 1980; Otfinowski et al. 2007), and it is possible that different results would have been obtained if native species had been used in our experiments. Future studies on plants with different growth forms and from a variety of geographic regions will be necessary to determine whether our findings are applicable to a larger and more diverse sample of species.
In conclusion, we found that for the species pair we studied, differences in growth response to AM fungi are associated with the outcome of competition, such that there is stronger self-limitation of positively responding species and weaker self-limitation of negatively responding species in soil containing AM fungi. This is ecologically significant because it suggests that plant–soil interactions can contribute to stabilizing mechanisms of species coexistence not only through relationships between plants and their pathogens (Bever et al. 2012; Chung and Rudgers 2016), but also through relationships between plants and their microbial mutualists. Few studies have focused on how plants respond to AM fungi at higher planting densities (Koide and Dickie 2002), and most of these studies have used designs that do not allow full separation of intraspecific and interspecific competition because they do not manipulate conspecific and heterospecific density simultaneously (Hartnett et al. 1993; Moora and Zobel 1996). Thus, future studies employing a design that allows for quantification of both intraspecific and interspecific competition can improve our understanding of the full effects of plant response to AM fungi on the mechanisms that influence species coexistence. We also note that competition assessed using biomass may not necessarily predict population dynamics among competing species, and thus the likelihood of coexistence (Levine et al. 2017). Therefore, quantifying how variation in mycorrhizal growth response influences demography (e.g., Salguero-Gómez et al. 2018; Maherali 2020) will also be necessary to project whether differences in plant response to AM fungi influence long-term species persistence in plant communities.
References
Adler PB, HilleRisLambers J, Levine JM (2007) A niche for neutrality. Ecol Lett 10:95–104. https://doi.org/10.1111/j.1461-0248.2006.00996.x
Adler PB, Ellner SP, Levine JM (2010) Coexistence of perennial plants: an embarrassment of niches. Ecol Lett 13:1019–1029. https://doi.org/10.1111/j.1461-0248.2010.01496.x
Aerts R (1999) Interspecific competition in natural plant communities: mechanisms, trade-offs and plant-soil feedbacks. J Exp Bot 50:29–37. https://doi.org/10.1093/jxb/50.330.29
Alexander HM, Holt RD (1998) The interaction between plant competition and disease. Perspect Plant Ecol Evol Syst 1:206–220. https://doi.org/10.1078/1433-8319-00059
Ames RN, Mihara KL, Bethlenfalvay GJ (1987) The establishment of microorganisms in vesicular-arbuscular mycorrhizal and control treatments. Biol Fertil Soils 3:217–223. https://doi.org/10.1007/BF00640633
Bever JD (1994) Feedback between plants and their soil communities in an old field community. Ecology 75:1965–1977. https://doi.org/10.2307/1941601
Bever JD, Dickie IA, Facelli E, Facelli JM, Klironomos J, Moora M, Rillig MC, Stock WD, Tibbett M, Zobel M (2010) Rooting theories of plant community ecology in microbial interactions. Trends Ecol Evol 25:468–478. https://doi.org/10.1016/j.tree.2010.05.004
Bever JD, Platt TG, Morton ER (2012) Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu Rev Microbiol 66:265–283. https://doi.org/10.1146/annurev-micro-092611-150107
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77. https://doi.org/10.1007/s11104-008-9877-9
Casper BB, Jackson RB (1997) Plant competition underground. Annu Rev Ecol Syst 28:545–570. https://doi.org/10.1146/annurev.ecolsys.28.1.545
Cavers PB, Bassett IJ, Crompton CW (1980) The biology of Canadian weeds. 47. Plantago lanceolata L. Can J Plant Sci 60:1269–1282. https://doi.org/10.4141/cjps80-180
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Evol Syst 31:343–366. https://doi.org/10.1146/annurev.ecolsys.31.1.343
Chung YA, Rudgers JA (2016) Plant-soil feedbacks promote negative frequency dependence in the coexistence of two aridland grasses. Proc R Soc B 283:e20160608. https://doi.org/10.1098/rspb.2016.0608
Cumming G, Finch S (2005) Inference by eye: confidence intervals and how to read pictures of data. Am Psychol 60:170–180. https://doi.org/10.1037/0003-066X.60.2.170
Derelle D, Courty P, Dajoz I, Declerck S, van Aarle IM, Carmignac D, Genet P (2015) Plant identity and density can influence arbuscular mycorrhizal fungi colonization, plant growth, and reproduction investment in coculture. Botany 93:405–412. https://doi.org/10.1139/cjb-2014-0180
Fitter AH (1977) Influence of mycorrhizal infection on competition for phosphorus and potassium by two grasses. New Phytol 79:119–125. https://doi.org/10.1111/j.1469-8137.1977.tb02187.x
Freckleton RP, Watkinson AR (2000) Designs for greenhouse studies of interactions between plants: an analytical perspective. J Ecol 88:386–391. https://doi.org/10.1046/j.1365-2745.2000.00467.x
George E, Marschner H, Jakobsen I (1995) Role of arbuscular mycorrhizal fungi in uptake of phosphorus and nitrogen from soil. Crit Rev Biotechnol 15:257–270. https://doi.org/10.3109/07388559509147412
Grime JP (1977) Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 111:1169–1194. https://doi.org/10.1086/283244
Hamel C, Furlan V, Smith DL (1992) Mycorrhizal effects on interspecific plant competition and transfer in legume-grass mixtures. Crop Sci 32:991–996. https://doi.org/10.2135/cropsci1992.0011183X003200040032x
Hartnett DC, Hetrick BAD, Wilson GWT, Gibson DJ (1993) Mycorrhizal influence on intra- and interspecific neighbour interactions among co-occurring prairie grasses. J Ecol 81:787–795. https://doi.org/10.2307/2261676
Heinze J, Bergmann J, Rillig MC, Joshi J (2015) Negative biotic soil-effects enhance biodiversity by restricting potentially dominant plant species in grasslands. Perspect Plant Ecol Evol Syst 17:227–235. https://doi.org/10.1016/j.ppees.2015.03.002
Hetrick BAD, Wilson GWT (1989) Relationship between mycorrhizal dependence and competitive ability of two tallgrass prairie grasses. Can J Bot 67:2608–2615. https://doi.org/10.1139/b89-337
Hetrick BAD, Hartnett DC, Wilson GWT, Gibson DJ (1994) Effects of mycorrhizae, phosphorus availability, and plant density on yield relationships among competing tallgrass prairie grasses. Can J Bot 72:168–176. https://doi.org/10.1139/b94-023
HilleRisLambers J, Adler PB, Harpole WS, Levine JM, Mayfield MM (2012) Rethinking community assembly through the lens of coexistence theory. Annu Rev Ecol Evol Syst 43:227–248. https://doi.org/10.1146/annurev-ecolsys-110411-160411
Hodge A, Fitter AH (2013) Microbial mediation of plant competition and community structure. Funct Ecol 27:865–875. https://doi.org/10.1111/1365-2435.12002
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC, Wilson GW, Klironomos JN, Umbanhowar J (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:395–407. https://doi.org/10.1111/j.1461-0248.2009.01430.x
Husáková I, Weiner J, Münzbergová Z (2018) Species traits and shoot-root biomass allocation in 20 dry-grassland species. J Plant Ecol 11:273–285. https://doi.org/10.1093/jpe/rtw143
Inouye BD (2001) Response surface experimental designs for investigating interspecific competition. Ecology 82:2696–2706. https://doi.org/10.2307/2679954
Janos DP (2007) Plant responsiveness to mycorrhizas differs from dependence upon mycorrhizas. Mycorrhiza 17:75–91. https://doi.org/10.1007/s00572-006-0094-1
Jiang J, Moore JAM, Priyadarshi A, Classen AT (2017) Plant-mycorrhizal interactions mediate plant community coexistence by altering resource demand. Ecology 98:187–197. https://doi.org/10.1002/ecy.1630
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70. https://doi.org/10.1038/417067a
Koide RT, Dickie IA (2002) Effects of mycorrhizal fungi on plant populations. Plant Soil 244:307–317. https://doi.org/10.1023/A:1020204004844
Koide RT, Li M (1989) Appropriate controls for vesicular-arbuscular mycorrhizal research. New Phytol 111:35–44. https://doi.org/10.1111/j.1469-8137.1989.tb04215.x
Laliberté E, Lambers H, Burgess T, Wright SJ (2015) Phosphorus limitation, soil-borne pathogens and the coexistence of plant species in hyperdiverse forests and shrublands. New Phytol 206:507–521. https://doi.org/10.1111/nph.13203
Law R, Watkinson AR (1987) Response-surface analysis of two-species competition: an experiment on Phleum arenarium and Vulpia fasciculata. J Ecol 75:871–886. https://doi.org/10.2307/2260211
Levine JM, HilleRisLambers J (2009) The importance of niches for the maintenance of species diversity. Nature 461:254–258. https://doi.org/10.1038/nature08251
Levine JM, Bascompte J, Adler PB, Allesina S (2017) Beyond pairwise mechanisms of species coexistence in complex communities. Nature 546:56–64. https://doi.org/10.1038/nature22898
Liang M, Liu X, Etienne RS, Huang F, Wang Y, Yu S (2015) Arbuscular mycorrhizal fungi counteract the Janzen-Connell effect of soil pathogens. Ecology 96:562–574. https://doi.org/10.1890/14-0871.1
Maherali H (2020) Mutualism as a plant functional trait: linking variation in the mycorrhizal symbiosis to climatic tolerance, geographic range and population dynamics. Int J Plant Sci 181:9–19. https://doi.org/10.1086/706187
McGonigle T, Miller M, Evans D, Fairchild G, Swan J (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:68–73. https://doi.org/10.1111/j.1469-8137.1990.tb00476.x
Moora M, Zobel M (1996) Effect of arbuscular mycorrhiza on inter- and intraspecific competition of two grassland species. Oecologia 108:79–84. https://doi.org/10.1007/BF00333217
Otfinowski R, Kenkel NC, Catling PM (2007) The biology of Canadian weeds. 134. Bromus inermis Leyss. Can J Plant Sci 87:183–198. https://doi.org/10.4141/P06-071
Salguero-Gómez R, Violle C, Gimenez O, Childs D (2018) Delivering the promises of trait-based approaches to the needs of demographic approaches, and vice versa. Funct Ecol 32:1424–1435. https://doi.org/10.1111/1365-2435.13148
Scheublin TR, van Logtestijn RSP, van der Heijden MGA (2007) Presence and identity of arbuscular mycorrhizal fungi influence competitive interactions between plant species. J Ecol 95:631–638. https://doi.org/10.1111/j.1365-2745.2007.01244.x
Sherrard ME, Maherali H (2012) Local adaptation across a fertility gradient is influenced by soil biota in the invasive grass, Bromus inermis. Evol Ecol 26:529–544. https://doi.org/10.1007/s10682-011-9518-2
Siefert A, Zillig AW, Friesen ML, Strauss SY (2019) Mutualists stabilize the coexistence of congeneric legumes. Am Nat 193:200–212. https://doi.org/10.1086/701056
Spitters CJT (1983) An alternative approach to the analysis of mixed cropping experiments, 1: estimation of competition effects. Neth J Agric Sci 31:1–11
Stanescu S, Maherali H (2017) Mycorrhizal feedback is not associated with the outcome of competition in old-field perennial plants. Oikos 126:248–258. https://doi.org/10.1111/oik.03580
Stürmer SL, Bever JD, Morton JB (2018) Biogeography of arbuscular mycorrhizal fungi (Glomeromycota): a phylogenetic perspective on species distribution patterns. Mycorrhiza 28:587–603. https://doi.org/10.1007/s00572-018-0864-6
Thompson KA, Husband BC, Maherali H (2015) No influence of water limitation on the outcome of competition between diploid and tetraploid Chamerion angustifolium (Onagraceae). J Ecol 103:733–741. https://doi.org/10.1111/1365-2745.12384
Urcelay C, Diaz S (2003) The mycorrhizal dependence of subordinates determines the effect of arbuscular mycorrhizal fungi on plant diversity. Ecol Lett 6:388–391. https://doi.org/10.1046/j.1461-0248.2003.00444.x
van der Putten WH, Peters BAM (1997) How soil-borne pathogens may affect plant competition. Ecology 78:1785–1795. https://doi.org/10.1890/0012-9658(1997)078%5b1785:hsbpma%5d2.0.co;2
Van TK, Wheeler GS, Center TD (1999) Competition between Hydrilla verticillata and Vallisneria americana as influenced by soil fertility. Aquat Bot 62:225–233. https://doi.org/10.1016/S0304-3770(98)00100-4
Veresoglou SD, Rillig MC, Johnson D (2018) Responsiveness of plants to mycorrhiza regulates coexistence. J Ecol 106:1864–1875. https://doi.org/10.1111/1365-2745.13026
Vierheilig H, Coughland A, Wyss U, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007
Watkinson AR, Freckleton RP (1997) Quantifying the impact of arbuscular mycorrhiza on plant competition. J Ecol 85:541–545. https://doi.org/10.2307/2960576
Wehner J, Antunes PM, Powell JR, Mazukatow J, Rillig MC (2010) Plant pathogen protection by arbuscular mycorrhizas: a role for fungal diversity? Pedobiol 53:197–201. https://doi.org/10.1016/j.pedobi.2009.10.002
West HM (1996) Influence of arbuscular mycorrhizal infection in competition between Holcus lanatus and Dactylis glomerata. J Ecol 84:429–438. https://doi.org/10.2307/2261204
Zar JH (1998) Biostatistical analysis, 4th edn. Pearson, Hoboken
Acknowledgements
We thank CM Caruso for helpful comments on the manuscript.
Funding
This research was supported by a University of Guelph College of Biological Science Stephen and Henrietta Stobie Summer Research Assistantship to MBM and a Natural Sciences and Engineering Research Council of Canada Discovery research Grant to HM (RGPIN 261300-2013).
Author information
Authors and Affiliations
Contributions
MBM and HM designed the study, MBM carried out the experiments, MBM and HM analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Data accessibility
Data are available in electronic supplementary material.
Additional information
Communicated by Catherine Gehring.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McHaffie, M.B., Maherali, H. Variation in mycorrhizal growth response influences competitive interactions and mechanisms of plant species coexistence. Oecologia 192, 755–765 (2020). https://doi.org/10.1007/s00442-020-04609-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-020-04609-9