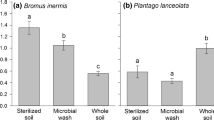
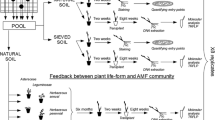
Abstract
Mycorrhizal fungi are essential to the establishment of the vast majority of plant species but are often conceptualized with contradictory roles in plant community assembly. On the one hand, host-specific mycorrhizal fungi may allow a plant to be competitively dominant by enhancing growth. On the other hand, host-specific mycorrhizal fungi with different functional capabilities may increase nutrient niche partitioning, allowing plant species to coexist. Here, to resolve the balance of these two contradictory forces, we used a controlled greenhouse study to manipulate the presence of two main types of mycorrhizal fungus, ectomycorrhizal fungi and arbuscular mycorrhizal fungi, and used a range of conspecific and heterospecific competitor densities to investigate the role of mycorrhizal fungi in plant competition and coexistence. We find that the presence of arbuscular mycorrhizal fungi equalizes fitness differences between plants and stabilizes competition to create conditions for host species coexistence. Our results show how below-ground mutualisms can shift outcomes of plant competition and that a holistic view of plant communities that incorporates their mycorrhizal partners is important in predicting plant community dynamics.




Similar content being viewed by others
Data availability
All data are available via the Dryad Digital Repository at https://doi.org/10.5061/dryad.rxwdbrvjb.
Code availability
All code for analysis is available via Github at https://github.com/ClaireWilling/MycorrhizaCoexist.git.
References
Vandenkoornhuyse, P., Quaiser, A., Duhamel, M., Le Van, A. & Dufresne, A. The importance of the microbiome of the plant holobiont. N. Phytol. 206, 1196–1206 (2015).
Smith, S. E. & Read, D. J. Mycorrhizal Symbiosis (Academic Press, 2010).
Steidinger, B. S. et al. Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature 569, 404–408 (2019).
van der Heijden, M. G. A., van der, Martin, F. M., Selosse, M.-A. & Sanders, I. R. Mycorrhizal ecology and evolution: the past, the present, and the future. N. Phytol. 205, 1406–1423 (2015).
Kakouridis, A. et al. Routes to roots: direct evidence of water transport by arbuscular mycorrhizal fungi to host plants. N. Phytol. 236, 210–221 (2022).
Pellitier, P. T. & Zak, D. R. Ectomycorrhizal fungi and the enzymatic liberation of nitrogen from soil organic matter: why evolutionary history matters. N. Phytol. 217, 68–73 (2018).
Hodge, A. & Fitter, A. H. Microbial mediation of plant competition and community structure. Funct. Ecol. 27, 865–875 (2013).
Peay, K. G. Timing of mutualist arrival has a greater effect on Pinus muricata seedling growth than interspecific competition. J. Ecol. 106, 514–523 (2018).
Wagg, C., Jansa, J., Stadler, M., Schmid, B. & van der Heijden, M. G. A. Mycorrhizal fungal identity and diversity relaxes plant–plant competition. Ecology 92, 1303–1313 (2011).
Scheublin, T. R., Van Logtestijn, R. S. P. & Van Der Heijden, M. G. A. Presence and identity of arbuscular mycorrhizal fungi influence competitive interactions between plant species. J. Ecol. 95, 631–638 (2007).
Hoeksema, J. D. et al. Ectomycorrhizal plant–fungal co-invasions as natural experiments for connecting plant and fungal traits to their ecosystem consequences. Front. Glob. Change 3, 84 (2020).
Corrales, A., Mangan, S. A., Turner, B. L. & Dalling, J. W. An ectomycorrhizal nitrogen economy facilitates monodominance in a neotropical forest. Ecol. Lett. 19, 383–392 (2016).
Lu, M. & Hedin, L. O. Global plant–symbiont organization and emergence of biogeochemical cycles resolved by evolution-based trait modelling. Nat. Ecol. Evol. 3, 239–250 (2019).
Averill, C. et al. Alternative stable states of the forest mycobiome are maintained through positive feedbacks. Nat. Ecol. Evol. 6, 375–382 (2022).
Laliberté, E., Lambers, H., Burgess, T. I. & Wright, S. J. Phosphorus limitation, soil-borne pathogens and the coexistence of plant species in hyperdiverse forests and shrublands. N. Phytol. 206, 507–521 (2015).
Bever, J. D. et al. Rooting theories of plant community ecology in microbial interactions. Trends Ecol. Evol. 25, 468–478 (2010).
Bever, J. D., Platt, T. G. & Morton, E. R. Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu. Rev. Microbiol. 66, 265–283 (2012).
Hart, S. P. How does facilitation influence the outcome of species interactions? J. Ecol. 111, 2094–2104 (2023).
Peay, K. G. The mutualistic niche: mycorrhizal symbiosis and community dynamics. Annu. Rev. Ecol. Evol. Syst. 47, 143–164 (2016).
Van Nuland, M. E. & Peay, K. G. Symbiotic niche mapping reveals functional specialization by two ectomycorrhizal fungi that expands the host plant niche. Fungal Ecol. 46, 100960 (2020).
Chomicki, G., Weber, M., Antonelli, A., Bascompte, J. & Kiers, E. T. The impact of mutualisms on species richness. Trends Ecol. Evol. 34, 698–711 (2019).
Tedersoo, L., Bahram, M. & Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 367, eaba1223 (2020).
Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366 (2000).
Westoby, M. & Wright, I. J. Land-plant ecology on the basis of functional traits. Trends Ecol. Evol. 21, 261–268 (2006).
Kraft, N. J. B., Godoy, O. & Levine, J. M. Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl Acad. Sci. USA 112, 797–802 (2015).
Barabás, G., D’Andrea, R. & Stump, S. M. Chesson’s coexistence theory. Ecol. Monogr. 88, 277–303 (2018).
Song, C., Barabás, G. & Saavedra, S. On the consequences of the interdependence of stabilizing and equalizing mechanisms. Am. Nat. 194, 627–639 (2019).
Kandlikar, G. S., Johnson, C. A., Yan, X., Kraft, N. J. B. & Levine, J. M. Winning and losing with microbes: how microbially mediated fitness differences influence plant diversity. Ecol. Lett. 22, 1178–1191 (2019).
Ke, P.-J. & Wan, J. Effects of soil microbes on plant competition: a perspective from modern coexistence theory. Ecol. Monogr. 90, e01391 (2020).
Averill, C., Turner, B. L. & Finzi, A. C. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505, 543–545 (2014).
Bever, J. D. Negative feedback within a mutualism: host-specific growth of mycorrhizal fungi reduces plant benefit. Proc. Biol. Sci. 269, 2595–2601 (2002).
van der Heijden, M. G. A. et al. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396, 69–72 (1998).
Bever, J. D. Soil community feedback and the coexistence of competitors: conceptual frameworks and empirical tests. N. Phytol. 157, 465–473 (2003).
Afkhami, M. E., McIntyre, P. J. & Strauss, S. Y. Mutualist-mediated effects on species’ range limits across large geographic scales. Ecol. Lett. 17, 1265–1273 (2014).
Yan, X., Levine, J. M. & Kandlikar, G. S. A quantitative synthesis of soil microbial effects on plant species coexistence. Proc. Natl Acad. Sci. USA 119, e2122088119 (2022).
Crawford, K. M. et al. When and where plant–soil feedback may promote plant coexistence: a meta‐analysis. Ecol. Lett. 22, 1274–1284 (2019).
Xi, N. et al. Relationships between plant–soil feedbacks and functional traits. J. Ecol. 109, 3411–3423 (2021).
Beiler, K. J., Durall, D. M., Simard, S. W., Maxwell, S. A. & Kretzer, A. M. Architecture of the wood-wide web: Rhizopogon spp. genets link multiple Douglas-fir cohorts. N. Phytol. 185, 543–553 (2010).
Hart, M. M., Reader, R. J. & Klironomos, J. N. Plant coexistence mediated by arbuscular mycorrhizal fungi. Trends Ecol. Evol. 18, 418–423 (2003).
Endlweber, K. & Scheu, S. Interactions between mycorrhizal fungi and Collembola: effects on root structure of competing plant species. Biol. Fertil. Soils 43, 741–749 (2007).
Guo, Y. et al. The interspecific competition presents greater nutrient facilitation compared with intraspecific competition through AM fungi interacting with litter for two host plants in karst soil. J. Plant Ecol. 15, 399–412 (2022).
Hartnett, D. C., Hetrick, B. A. D., Wilson, G. W. T. & Gibson, D. J. Mycorrhizal influence on intra- and interspecific neighbour interactions among co-occurring prairie grasses. J. Ecol. 81, 787–795 (1993).
Marler, M. J., Zabinski, C. A. & Callaway, R. M. Mycorrhizae indirectly enhance competitive effects of an invasive forb on a native bunchgrass. Ecology 80, 1180–1186 (1999).
Moora, M. & Zobel, M. Effect of arbuscular mycorrhiza on inter- and intraspecific competition of two grassland species. Oecologia 108, 79–84 (1996).
Schroeder-Moreno, M. S. & Janos, D. P. Intra- and inter-specific density affects plant growth responses to arbuscular mycorrhizas. Botany 86, 1180–1193 (2008).
Kandlikar (गौरव कांडिलकर), G. S., Yan (严心怡), X., Levine, J. M. & Kraft, N. J. B. Soil microbes generate stronger fitness differences than stabilization among california annual plants. Am. Nat. 197, E30–E39 (2021).
Forrestel, A. B., Moritz, M. A. & Stephens, S. L. Landscape-scale vegetation change following fire in Point Reyes, California, USA. Fire Ecol. 7, 114–128 (2011).
Dickie, I. A. & Reich, P. B. Ectomycorrhizal fungal communities at forest edges. J. Ecol. 93, 244–255 (2005).
Peay, K. G. & Bruns, T. D. Spore dispersal of basidiomycete fungi at the landscape scale is driven by stochastic and deterministic processes and generates variability in plant–fungal interactions. N. Phytol. 204, 180–191 (2014).
Smith, G. R., Steidinger, B. S., Bruns, T. D. & Peay, K. G. Competition–colonization tradeoffs structure fungal diversity. ISME J. 12, 1758–1767 (2018).
Harvey, B. J. & Holzman, B. A. Divergent successional pathways of stand development following fire in a California closed-cone pine forest. J. Veget. Sci. 25, 88–99 (2014).
Teste, F. P. et al. Plant–soil feedback and the maintenance of diversity in Mediterranean-climate shrublands. Science 355, 173–176 (2017).
Simha, A., Pardo-De la Hoz, C. J. & Carley, L. N. Moving beyond the ‘diversity paradox’: the limitations of competition-based frameworks in understanding species diversity. Am. Nat. 200, 89–100 (2022).
Horton, T. R., Cázares, E. & Bruns, T. D. Ectomycorrhizal, vesicular–arbuscular and dark septate fungal colonization of bishop pine (Pinus muricata) seedlings in the first 5 months of growth after wildfire. Mycorrhiza 8, 11–18 (1998).
Wagg, C., Antunes, P. M. & Peterson, R. L. Arbuscular mycorrhizal fungal phylogeny-related interactions with a non-host. Symbiosis 53, 41–46 (2011).
Hobbie, E. A., Macko, S. A. & Shugart, H. H. Insights into nitrogen and carbon dynamics of ectomycorrhizal and saprotrophic fungi from isotopic evidence. Oecologia 118, 353 (1999).
Banerjee, S., Schlaeppi, K. & van der Heijden, M. G. A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 16, 567–576 (2018).
Peh, K. S.-H., Lewis, S. L. & Lloyd, J. Mechanisms of monodominance in diverse tropical tree-dominated systems. J. Ecol. 99, 891–898 (2011).
Mangan, S. A. et al. Negative plant–soil feedback predicts tree-species relative abundance in a tropical forest. Nature 466, 752–755 (2010).
Bennett, J. A. et al. Plant–soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 355, 181–184 (2017).
Enright, D. J., Frangioso, K. M., Isobe, K., Rizzo, D. M. & Glassman, S. I. Mega-fire in redwood tanoak forest reduces bacterial and fungal richness and selects for pyrophilous taxa that are phylogenetically conserved. Mol. Ecol. 31, 2475–2493 (2022).
Klein, T., Siegwolf, R. T. & Körner, C. Belowground carbon trade among tall trees in a temperate forest. Science 352, 342–344 (2016).
Karst, J., Jones, M. D. & Hoeksema, J. D. Positive citation bias and overinterpreted results lead to misinformation on common mycorrhizal networks in forests. Nat. Ecol. Evol. https://doi.org/10.1038/s41559-023-01986-1 (2023).
Kretzer, A. M., Dunham, S., Molina, R. & Spatafora, J. W. Microsatellite markers reveal the below ground distribution of genets in two species of Rhizopogon forming tuberculate ectomycorrhizas on Douglas fir. N. Phytol. 161, 313–320 (2004).
Bergemann, S. E. & Miller, S. L. Size, distribution, and persistence of genets in local populations of the late-stage ectomycorrhizal basidiomycete, Russula brevipes. N. Phytol. 156, 313–320 (2002).
Averill, C., Bhatnagar, J. M., Dietze, M. C., Pearse, W. D. & Kivlin, S. N. Global imprint of mycorrhizal fungi on whole-plant nutrient economics. Proc. Natl Acad. Sci. USA 116, 23163–23168 (2019).
Grime, J. P. Vegetation classification by reference to strategies. Nature 250, 26–31 (1974).
Collier, F. A. & Bidartondo, M. I. Waiting for fungi: the ectomycorrhizal invasion of lowland heathlands. J. Ecol. 97, 950–963 (2009).
Thirkell, T. J., Cameron, D. D. & Hodge, A. Resolving the ‘nitrogen paradox’ of arbuscular mycorrhizas: fertilization with organic matter brings considerable benefits for plant nutrition and growth. Plant Cell Environ. 39, 1683–1690 (2016).
Shah, F. et al. Ectomycorrhizal fungi decompose soil organic matter using oxidative mechanisms adapted from saprotrophic ancestors. N. Phytol. 209, 1705–1719 (2016).
Terrer, C., Vicca, S., Hungate, B. A., Phillips, R. P. & Prentice, I. C. Mycorrhizal association as a primary control of the CO2 fertilization effect. Science 353, 72–74 (2016).
Phillips, R. P., Brzostek, E. & Midgley, M. G. The mycorrhizal-associated nutrient economy: a new framework for predicting carbon–nutrient couplings in temperate forests. N. Phytol. 199, 41–51 (2013).
Herman, D. J., Firestone, M. K., Nuccio, E. E. & Hodge, A. Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiol. Ecol. 80, 236–247 (2012).
Abbott, K. C., Eppinga, M. B., Umbanhowar, J., Baudena, M. & Bever, J. D. Microbiome influence on host community dynamics: conceptual integration of microbiome feedback with classical host–microbe theory. Ecol. Lett. 24, 2796–2811 (2021).
Jiang, J. et al. Pathogens and mutualists as joint drivers of host species coexistence and turnover: implications for plant competition and succession. Am. Nat. 195, 591–602 (2020).
Collins, C. D., Bever, J. D. & Hersh, M. H. Community context for mechanisms of disease dilution: insights from linking epidemiology and plant–soil feedback theory. Ann. N. Y. Acad. Sci. 1469, 65–85 (2020).
Letten, A. D. & Stouffer, D. B. The mechanistic basis for higher-order interactions and non-additivity in competitive communities. Ecol. Lett. 22, 423–436 (2019).
Fernández, N., Knoblochová, T., Kohout, P., Janoušková, M. & Rydlová, J. Asymmetric interaction between two mycorrhizal fungal guilds and consequences for the establishment of their host plants. Front. Plant Sci. 13, 873204. (2022).
Letten, A. D., Ke, P.-J. & Fukami, T. Linking modern coexistence theory and contemporary niche theory. Ecol. Monogr. 87, 161–177 (2017).
Ellner, S. P., Snyder, R. E., Adler, P. B. & Hooker, G. Toward a ‘modern coexistence theory’ for the discrete and spatial. Ecol. Monogr. 92, e1548 (2022).
Van Der Heijden, M. G. A. & Horton, T. R. Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. J. Ecol. 97, 1139–1150 (2009).
Delavaux, C. S. et al. Mycorrhizal feedbacks influence global forest structure and diversity. Commun. Biol. 6, 1–11 (2023).
Chang, C.-Y., Bajić, D., Vila, J. C. C., Estrela, S. & Sanchez, A. Emergent coexistence in multispecies microbial communities. Science 381, 343–348 (2023).
Peay, K. G., Schubert, M. G., Nguyen, N. H. & Bruns, T. D. Measuring ectomycorrhizal fungal dispersal: macroecological patterns driven by microscopic propagules. Mol. Ecol. 21, 4122–4136 (2012).
Peay, K. G., Bruns, T. D., Kennedy, P. G., Bergemann, S. E. & Garbelotto, M. A strong species–area relationship for eukaryotic soil microbes: island size matters for ectomycorrhizal fungi. Ecol. Lett. 10, 470–480 (2007).
Dawson, T. E. Fog in the California redwood forest: ecosystem inputs and use by plants. Oecologia 117, 476–485 (1998).
Hart, S. P., Freckleton, R. P. & Levine, J. M. How to quantify competitive ability. J. Ecol. 106, 1902–1909 (2018).
Ke, P.-J. & Wan, J. A general approach for quantifying microbial effects on plant competition. Plant Soil 485, 1–14 (2022).
Bruns, T. D., Hale, M. L. & Nguyen, N. H. Rhizopogon olivaceotinctus increases its inoculum potential in heated soil independent of competitive release from other ectomycorrhizal fungi. Mycologia 111, 936–941 (2019).
Gardes, M. & Bruns, T. D. ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol. Ecol. 2, 113–118 (1993).
Duhamel, M. et al. Plant selection initiates alternative successional trajectories in the soil microbial community after disturbance. Ecol. Monogr. 89, e01367 (2019).
Phillips, J. M. & Hayman, D. S. Improved procedures for clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55, 158-IN18 (1970).
Giovannetti, M. & Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. N. Phytol. 84, 489–500 (1980).
Smith, W. K., Schoettle, A. W. & Cui, M. Importance of the method of leaf area measurement to the interpretation of gas exchange of complex shoots. Tree Physiol. 8, 121–127 (1991).
Anderson, C. J. R. & Rosas-Anderson, P. J. Leafscan (Version 1.3.21). https://itunes.apple.com/app/id1254892230 (2017).
Rueden, C. T. et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18, 1–26 (2017).
R: a language and environment for statistical computing (R Foundation for Statistical Computing, R Core Team, 2022).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Chesson, P. & Kuang, J. J. The interaction between predation and competition. Nature 456, 235–238 (2008).
Rousselet, G. A., Pernet, C. R. & Wilcox, R. R. The percentile bootstrap: a primer with step-by-step instructions in R. Adv. Meth. Pract. Psychol. Sci. 4, 2021.
Terry, J. C. D. & Armitage, D. W. Widespread analytical pitfalls in empirical coexistence studies and a checklist for improving their statistical robustness. Methods Ecol. Evol. 15, 594–611 (2024).
Johnson, C. A., Dutt, P. & Levine, J. M. Competition for pollinators destabilizes plant coexistence. Nature 607, 721–725 (2022).
Acknowledgements
We thank K. N. Chin and J. M. M. Ferré for their help in planting seedlings for this experiment. Additionally, we thank K. N. Chin for her help in creating art for this manuscript. We thank L. D. L Anderegg for his input on the study design and feedback on early versions of this manuscript. K.G.P. is a Canadian Institute for Advanced Research (CIFAR) Fellow in the programme Fungal Kingdom: Threats and Opportunities and is supported by a United States Department of Energy (DOE) Award DE-SC0023661. C.E.W., J.J.Y., A.M.C. and K.G.P were all supported by the United States National Science Foundation (NSF) Faculty Early Career (CAREER) Award 1845544 for this work, which was awarded to K.G.P.
Author information
Authors and Affiliations
Contributions
C.E.W. and K.G.P. planned and designed the research. C.E.W. and K.G.P. conducted field work. C.E.W., J.J.Y. and A.M.C. conducted the laboratory work. C.E.W. analysed and interpreted the data with critical contributions from J.W., J.J.Y., A.M.C. and K.G.P. The manuscript was written by C.E.W. and all co-authors provided important contributions and critical revisions. All authors approve of the final version of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2 and Figs. 1–3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Willing, C.E., Wan, J., Yeam, J.J. et al. Arbuscular mycorrhizal fungi equalize differences in plant fitness and facilitate plant species coexistence through niche differentiation. Nat Ecol Evol (2024). https://doi.org/10.1038/s41559-024-02526-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41559-024-02526-1
- Springer Nature Limited