Abstract
Biotic soil factors, such as fungi, bacteria and herbivores affect resource acquisition and fitness in plants, yet little is known of their role as agents of selection. Evolutionary responses to these selective agents could be an important mechanism that explains the success of invasive species. In this study, we tested whether populations of the invasive grass Bromus inermis are adapted to their home soil environment, and whether biotic factors influence the magnitude of this adaptation. We selected three populations growing at sites that differed in soil fertility and grew individuals from each population in each soil. To assess whether biotic factors influence the magnitude of adaptation, we also grew the same populations in sterilized field soil. To further examine the role of one element of the soil biota (fungi) in local adaptation, we measured colonization by arbuscular mycorrhizal (AM) and septate fungi, and tested whether the extent of colonization differed between local and foreign plants. In non-sterilized (living) soil, there was evidence of a home site advantage because local plants produced significantly more biomass than at least one of the two populations of foreign plants in all three soil origins. By contrast, there was no evidence of a home site advantage in sterilized soil because local plants never produced significantly more biomass than either population of foreign plants. Fungal colonization differed between local and foreign plants in the living soil and this variation corresponded with biomass differences. When local plants produced more biomass than foreign plants, they were also less intensively colonized by AM fungi. Colonization by septate fungi did not vary between local and foreign plants. Our results suggest that biotic soil factors are important causes of plant adaptation, and that selection for reduced interactions with mycorrhizae could be one mechanism through which adaptation to a novel environment occurs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The strength of selection on phenotypic traits is highly dependent on the surrounding biotic and abiotic environment (Endler 1986; Wade and Kalisz 1990), and consequently, populations occupying different habitats often experience divergent natural selection. Over time, these contrasting selection patterns should promote genetically differentiated populations, each adapted to the characteristics of their home environment. Identifying the causes of natural selection is a major goal of evolutionary ecology because it allows one to both understand why certain phenotypes have evolved and to predict phenotypic changes over subsequent generations (Endler 1986). In plants, evidence for adaptation to a local environment is common (e.g., Clausen et al. 1948; Antonovics and Bradshaw 1970; Hereford 2009) and thought to be an important mechanism through which genetic variation is maintained across populations (Felsenstein 1976). However, identifying the specific environmental gradients to which plants adapt (i.e. the agents of selection causing local adaptation) is rarely done (Endler 1986; Macel et al. 2007; Hereford 2009).
Abiotic and biotic factors both promote fine-scale local adaptation in plants. For example, reciprocal transplant experiments have shown that plants adapt to abiotic factors such as heavy metal tolerance and water availability (e.g., Antonovics and Bradshaw 1970; Bennington and McGraw 1995) and biotic factors such as competition and leaf herbivory (e.g., Turkington and Harper 1979; Schemske 1984; reviewed in Linhart and Grant 1996; Leimu and Fischer 2008). Though field-based reciprocal transplants are able to capture natural environmental variation and realistic selection pressures from these ecological factors, they often do not isolate and identify the specific factors causing adaptation (Linhart and Grant 1996; Hereford 2009). Fewer than half of reciprocal transplant studies directly measure an environmental variable and these account for only 4% of variation in the magnitude of local adaptation (Hereford 2009). Direct environmental manipulations under controlled conditions are therefore necessary to isolate the influence of particular agents of selection (e.g., Snaydon and Bradshaw 1962; Lenssen et al. 2004).
Of the potential agents of selection in the soil environment, abiotic factors have received considerably more attention than biotic factors. Nutrient availability, for example, is thought to be one of the primary factors driving evolutionary diversification in plants. In support of this hypothesis, species from low nutrient environments typically have slower relative growth rates, smaller growth forms and more conservative resource use than species from high nutrient environments (Grime 1977, 2001; Chapin 1980; Lambers and Poorter 1992). There is also evidence that plants adapt to the phosphorus (e.g., Snaydon and Bradshaw 1962) and nitrogen concentrations (e.g., McGraw and Chapin 1989) of their home soil, and that environments with different nutrient availability exert contrasting selection on functional traits (e.g., Donovan et al. 2009). By contrast, few studies have tested whether biotic soil factors influence local adaptation in plants (Pregitzer et al. 2010). Arbuscular mycorrhizal (AM) fungi, soil herbivores and fungal soil pathogens all influence plant fitness (e.g., Maron 1998; Wolfe et al. 2005), suggesting that these factors will also shape the evolution of plant function. Biotic soil factors could also modify aspects of the abiotic resource environment, and therefore alter selection exerted by abiotic soil factors. For example, in Andropogon gerardii (Poaceae), plants adapt to low nutrient soil by increasing their dependence on AM fungi for phosphorus and nitrogen uptake (Johnson 1993; Schultz et al. 2001; Johnson et al. 2010). Consequently, abiotic and biotic factors could be equally important drivers of plant adaptation to soil.
Adaptation in response to selection pressures exerted by novel soil environments could contribute to evolutionary divergence during biological invasions. Many invasive species display morphological (Buswell et al. 2011) and genetic (Maron et al. 2004) differentiation across moisture, nutrient and latitudinal gradients in their introduced range, suggesting that adaptation to these selective agents has caused evolutionary divergence. Because most plants are colonized by AM fungi (Brundrett 2009), these organisms may be important agents of selection on plant populations during biological invasions as well. Though ecological interactions with mycorrhiza are expected to influence invasion (Pringle et al. 2009), few studies have considered whether the interactions between plants and fungi evolve during invasion and range expansion (Seifert et al. 2009).
Our first goal was to determine whether plants were locally adapted to their home soil, and to assess whether biotic factors influence the magnitude of this adaptation. To test whether plants were adapted to their home soil, we grew three populations of the invasive perennial grass, Bromus inermis from different nutrient environments in their home and two away field soils in a greenhouse. To isolate the role of biotic factors in plant adaptation to soil, we grew these same populations in sterilized field soil from each site. Within each soil origin, we predicted that the population from that environment (local plants) would produce more biomass than the populations originating from the two other environments (foreign plants), and that the presence of soil biota would increase the magnitude of this home site advantage.
Our second goal was to assess the role of one element of the soil biota (fungi) in plant adaptation. We measured colonization by AM and septate fungi and compared the extent of colonization between populations and soil origins. For B. inermis, associations with AM fungi can either be mutualistic or parasitic depending on the interacting fungal species. For example, when grown in association with 10 different species of AM fungi, B. inermis displayed increased growth with half the species and decreased growth with half; however, positive associations yielded the largest growth responses (Klironomos 2003). Assuming that AM fungi, on average, benefit local plants, and plants adapt to their home AM fungal community, we predicted that local plants would be more intensively colonized by AM fungi than foreign plants. Like AM fungi, septate fungi generally increase plant biomass under controlled condition (Newsham 2011). Assuming that septate fungi also benefit plants in the wild, we predicted that local plants would be more intensively colonized by septate hyphae than foreign plants.
Materials and methods
Study species
Bromus inermis Leyss. (smooth brome; Poaceae) is an invasive perennial grass (Otfinowski et al. 2007). It was originally introduced from Central Eurasia in 1888 (Newell and Keim 1943) and is now a widely distributed throughout North America. This widespread distribution indicates that B. inermis can potentially adapt to contrasting soil environments. It is generally self-sterile (McKone 1985) and reproduces sexually through outcrossed wind pollination and clonally through an underground rhizome. It is a dominant species in most old-field communities because of high freezing and drought tolerance (Otfinowski et al. 2007).
Soil and seed source
In 2007, we collected soil and seeds from three naturalized B. inermis populations in Southern Ontario. The populations were located at: (1) the Long-Term Mycorrhizae Research Site (LTMRS) in the University of Guelph Arboretum in Guelph, Ontario (43° 32′ N, 80° 13′ W) (2) the rare charitable research reserve (rare) in Cambridge, Ontario (43° 22′ N, 80° 20′ W) and (3) the Koffler Scientific Reserve at Joker’s Hill (KSR) in Newmarket, Ontario (44° 03′ N, 79° 29′ W).
To incorporate site heterogeneity in measurements of soil conditions and seed collections, we set up a grid of 125 positions that were 5 m apart on a 55 m × 50 m plot at each site. To achieve this, we placed 12 transects 5 m apart and flagged positions at 5 m intervals along each transect. On the first 11 transects, we flagged 11 positions and on the 12th transect we flagged 4 positions. To assess nutrient availability, we collected soil cores (15–30 cm) at 20 random positions within our plot at each site. Cores were analyzed for % nitrate and ammonium using potassium chloride extraction and for % phosphorus using sodium bicarbonate extraction (University of Guelph Laboratory Services, Guelph, ON). We collected seed from 125 maternal seed families at each site by harvesting the closest inflorescence to each flag. Of these 375 families, we randomly selected 90 families (30 from each population) for use in this experiment.
At each site, we collected 250 L of soil from 15 randomly selected positions. Soil was collected to a depth of 30 cm, where most B. inermis roots are located (Gist and Smith 1948). Within each site, soil was homogenized and all stones and large roots (>2 mm diameter) were removed. To assess whether plants were adapted to their home soil and whether biotic factors influence the magnitude of this adaptation, we used two different soil treatments. Half of the soil was left intact and stored at 4°C prior to use and the other half was sterilized through gamma-irradiation (McMaster Nuclear Reactor, Hamilton, ON). The sterilized soil was exposed to a 32 kGy (±10%) dose of gamma-irradiation from a Cobalt-60 source over a period of 7 days.
Gamma-irradiation is an effective technique for eliminating soil biota and was used preferentially over autoclaving because it causes fewer changes in soil chemistry and structure (Salonius et al. 1967; McNamara et al. 2003; Berns et al. 2008). As with any method of sterilization however, gamma-irradiation still increases the initial nutrient availability of the soil and can modify other abiotic soil properties as well (Troelstra et al. 2001). This increase in initial nutrient availability could explain the increased size of plants in the sterilized relative to the living soil (Fig. 1). But even though sterilization resulted in size disparity among treatments, it did not compromise a test of whether plants were adapted solely to the nutrient availability of their home soils. This is because gamma-irradiation did not change to the relative ranking of nutrient status of the soil origins, as indicated by a non-significant effect of sterilization on plant biomass across soil origins (soil condition × soil origin term NS; see Table 2 in the Results).
Experimental design
Between December 6, 2007 and March 7, 2008 (93 days), we grew 30 maternal families from each of the three populations (KSR, LTMRS and rare) in six common garden soil environments in a greenhouse. Each population was grown in soil collected from each site (soil origin: KSR, LTMRS and rare) that had either been sterilized or not (soil condition: sterilized, living) resulting in a full factorial design with 18 treatment combinations (2 soil conditions × 3 soil origins × 3 populations). To synchronize germination and ensure we would have at least 12 viable seeds from each line (two seeds for each of the six soil treatment combinations), we stratified 30 seeds by placing them in darkness on moist filter paper for 96 h at 4°C. After refrigeration, the seeds were returned to room temperature but were left in the dark for 24 h. We planted two germinated seedlings from each line into an 857 mL mini treepot (Part # MT2510, Stuewe and Sons Inc., Tangent, OR, USA) containing 1 of the 6 soil condition × soil origin treatment combinations and placed them on a greenhouse bench in a randomized position. All pots were thinned to one seedling 4–6 days after planting for a total of 30 plants within each of 18 treatment combinations (n = 540). We top-watered the developing seedlings for 22 days to promote establishment.
After the 22-day establishment period, we watered the plants using drip irrigation and provided them with supplemental light. We adjusted light, temperature and water conditions to simulate the average monthly values in June, July and August, obtained from the three nearest weather stations (http://www.climate.weatheroffice.ec.gc.ca) to each population. To simulate June weather for 30 days, we provided supplemental light to maintain 13.5-h days, adjusted greenhouse temperatures to fluctuate diurnally between 11 and 23.2°C, and provided 10.8 mL of water throughout the day. To simulate July weather for 31 days, we provided supplemental light to maintain 14-h days, adjusted greenhouse temperatures to fluctuate diurnally between 14 and 26°C, and provided 12 mL of water throughout the day. To simulate August weather for the final 10 days, we provided supplemental light to maintain 13-h days, adjusted greenhouse temperatures to fluctuate diurnally between 14 and 25.3°C, and provided 12 mL of water throughout the day. Throughout the experiment, germinating non-focal plants were removed from pots containing living soil. Plants were not fertilized during the experiment.
Biomass and plant size
Because plants did not flower in the greenhouse, we used aboveground biomass as an estimate of fitness. In a three-year field study (Sherrard 2010), we found that aboveground biomass was strongly and positively correlated with seed production for these populations, making it an appropriate estimate of plant fitness. We harvested aboveground biomass after 90 treatment days (days 91–93). Plants were harvested below the rhizome, including all ramets but excluding all root material. Tissue was dried in an oven at 72°C for a period of at least 48 h, until it reached a constant dry weight. The root material from the pots was collected 3 days later, sub-sampled, and stored in 50% ethanol for fungal colonization analysis.
To assess how plant size changed over time, we measured the total length of the aboveground vegetative tissue as the sum of all leaves at seven approximately equal time intervals during the first 70 days.
Arbuscular mycorrhizal (AM) and septate fungal colonization
To assess fungal colonization in the living soil, we randomly selected 15 root samples from each of the nine (population × soil origin) treatment combinations. To assess the efficacy of the sterilization treatment, we also randomly selected 5 root samples from each of the nine (population × soil origin) treatment combinations in the sterilized soil. To prepare root samples for colonization assessment, we cleared the root using 10% KOH at 90°C for 1 h, stained the roots using 0.03% chlorazol black E, in a 1:1:1 solution of glycerol, lactic acid and water at 90°C for 3 h, and then de-stained the roots in 50% glycerol for 24 h (Brundrett 1994). We randomly selected stained root fragments, then cut and placed twelve 1 cm fragments onto microscope slides. We assessed fungal colonization for all samples using the magnified intersection method (McGonigle et al. 1990). For arbuscular mycorrhizal (AM) fungi, we scored the presence of hyphae, arbuscules, and vesicles at the intersection of the ocular crosshair on 200 × magnification. For each AM fungal structure, we assessed colonization as the fraction of 150 random crosshair assessments per plant, and calculated total AM fungal colonization as the sum of these three fractional values. To assess colonization by other fungi (non AM), we scored the presence of septate hyphae at the intersection of the ocular crosshair. We present colonization by septate fungi as the fraction of 150 random crosshair assessments per plant.
Testing for local adaptation
In this study, we tested for local adaptation using the ‘local versus foreign’ criterion (Kawecki and Ebert 2004; Leimu and Fischer 2008; Hereford 2009). The ‘local versus foreign’ criterion is satisfied when local plants have higher fitness than foreign plants in their home habitat (Kawecki and Ebert 2004). Some reciprocal transplant and common garden studies test for local adaptation using the alternative ‘home versus away’ criterion, which is satisfied when local plants have higher fitness in their home habitat than in different (away) habitats (Kawecki and Ebert 2004). Nevertheless, only the ‘local versus foreign’ criterion provides definitive support for local adaptation because it is consistent with the role of natural selection acting within a habitat. By contrast, the ‘home versus away’ criterion confounds natural selection within a habitat with resource differences between habitats (Kawecki and Ebert 2004).
Statistical analysis
To test whether biomass differed among treatment combinations, we used three-way analysis of variance (ANOVA) with soil condition, soil origin, and population as fixed factors. We tested whether sterilization affected the magnitude of plant adaptation to soil, which would be indicated by a significant soil condition × soil origin × population term. We also assessed whether sterilization caused any asymmetric changes in nutrient availability among the three soil origins, which would be indicated by a significant soil condition × soil origin term.
To interpret a potentially significant soil condition × soil origin × population interaction, we partitioned the term using planned orthogonal 1 degree of freedom (df) contrasts (Sokal and Rohlf 1995). These contrasts were used to test specific predictions of whether biomass differences among populations satisfied the ‘local versus foreign’ criterion of local adaptation (Kawecki and Ebert 2004). Specifically, we tested whether: (1) KSR plants produced more biomass than LTMRS plants in KSR soil, (2) KSR plants produced more biomass than rare plants in KSR soil, (3) LTMRS plants produced more biomass than KSR plants in LTMRS soil, (4) LTMRS plants produced more biomass than rare plants in LTMRS soil, (5) rare plants produced more biomass than LTMRS plants in rare soil, and (6) rare plants produced more biomass than KSR plants in rare soil. We performed these six contrasts in both the sterilized and living soil. Because the 1-df contrasts are orthogonal, the comparisons are independent of one another, and thus, no statistical correction for multiple comparisons was made.
To determine whether the sterilization treatment effectively reduced fungal colonization, we used one-way ANOVAs with soil condition as a fixed factor and colonization by AM fungi and septate hyphae as dependent variables. To test whether the extent of fungal colonization differed among treatment combinations in living soil, we used two-way ANOVAs with soil origin and population as fixed factors and colonization by AM fungi and septate hyphae as dependent variables. In addition to two-way ANOVAs, we used 1-df contrasts to test for differences in colonization among populations; this analysis was identical to the ‘local versus foreign’ comparison used for biomass. Performing the same contrasts for fungal colonization and biomass allowed us to assess whether variation in colonization corresponded with patterns of local adaptation.
To test whether fungal colonization influenced plant biomass across living soils, we calculated Pearson product moment correlations between biomass and both fungal colonization measures across soil origins. For these correlations, soil origin was included as a covariate to account for differences in biomass among soil origins.
For each ANOVA, we tested the assumption of homogeneous residual variance using Levene’s test and the assumption of normally distributed residual variance using the Shapiro–Wilk test and by visually inspecting the distribution of residuals. Biomass data were log-transformed to correct heteroscedasticity and fungal colonization data did not violate the assumption of homoscedastic residual variance. Mortality was more common in living soil, but did not occur more frequently in any one soil origin, population, or family. Plants that died (n = 9) were excluded from ANOVAs. Though maternal families were nested within each population, low within family replication meant that there were insufficient degrees of freedom to include this term as both a main effect and in the interaction terms of the ANOVA, and as a result, this effect was excluded from the analysis. However, in a one-way ANOVA, maternal family had no significant effect on biomass in either soil condition. Orthogonal 1-df contrasts were performed in SPSS (version 18.0, SPSS Inc., Chicago, Ill). All other statistical analyses were performed in JMP (v. 8 SAS Institute, Cary, NC).
Results
Nutrient differences among sites
LTMRS was the lowest nutrient soil, having 72% lower phosphorus availability than rare and KSR soil (Table 1). LTMRS soil also had 49% lower NO3 availability than rare soil. rare was the highest nutrient soil, having comparable phosphorus to KSR but 48% higher NO3 availability (Table 1).
Variation in biomass
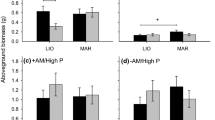
Plants grew faster and produced more biomass in sterilized soil than living soil (Fig. S1 in Supplementary Materials, Fig. 1; Table 2). Plants also produced more biomass in KSR and rare soil than in LTMRS soil (Fig. 1), as indicated by a significant soil origin term (Table 2). Mean biomass did not differ among populations (Table 2).
Testing for local adaptation
The effect of soil origin on the populations differed between the living soil and sterilized soil, as indicated by a significant soil condition × soil origin × population term (Table 2). In living soil, local plants produced significantly more biomass than at least one of the two populations of foreign plants in all three soil origins (Fig. 1b; Table 3). Specifically, LTMRS plants produced more biomass than rare plants in LTMRS soil (Fig. 1b; Table 3). There was also a reciprocal home site advantage for rare and KSR plants. Rare plants produced more biomass than KSR plants in rare soil, and KSR plants produced more biomass than rare plants in KSR soil (Fig. 1b; Table 3). In sterilized soil, local plants never produced significantly more biomass than either population of foreign plants (Fig. 1a; Table 3).
Variation in arbuscular mycorrhizal (AM) and septate fungal colonization
Gamma-irradiation significantly reduced fungal colonization in the sterilized soil. AM fungal colonization was 264% higher in living (mean = 0.466) than sterilized (mean = 0.128) soil (F ratio = 116.62, P < 0.0001) and colonization by septate hyphae was 353% higher in living (mean = 0.196) than sterilized (mean = 0.043) soil (F ratio = 40.21, P < 0.0001). In the living soil, colonization by AM fungi and septate hyphae differed significantly among soil origins. AM fungal colonization was 45% higher in LTMRS and rare soil than in KSR soil (Fig. 2a), as indicated by a significant soil origin term (Table 4), and colonization by septate hyphae was 58% higher in LTMRS soil than in KSR and rare soil (Fig. 2b; Table 4).
The extent of mean (±1 SE) fungal colonization for each population in each soil origin in living soil. Bars represent a mean of 15 root samples. Panels represent total colonization by arbuscular mycorrhizal (AM) fungi (i.e., arbuscules + vesicles + hyphae) (a) and colonization by septate fungal hyphae (b). Tukey’s HSD test was used to assess whether the extent of colonization differed among soil origins and letters indicate significant differences
Testing whether fungal colonization differs among local and foreign plants, and whether it affects plant biomass
In living soil, differences in the extent of AM fungal colonization among populations varied depending on soil origin, as indicated by a significant soil origin × population term (Table 4). The extent of colonization by AM fungi differed significantly between local and foreign plants in two cases. KSR plants were less colonized by AM fungi than rare plants in KSR soil (Fig. 2a; Table 5) and rare plants were less colonized by AM fungi than KSR plants in rare soil (Fig. 2a; Table 5). Colonization by septate hyphae did not differ among populations in any soil origin (Fig. 2b; Table 4 and 5).
After accounting for differences in biomass among soil origins, plant biomass was negatively correlated with the extent of colonization by AM fungi (r = −0.133, P = 0.047) in the living soil, but not with the extent of colonization by septate hyphae (r = −0.060, P = 0.459).
Discussion
Biotic soil factors such as fungi, herbivores and bacteria influence resource uptake and fitness in plants (e.g., Newsham et al. 1994; Maron 1998; Wolfe et al. 2005), but their role as potential causes of adaptation is poorly understood. By growing three populations reciprocally in their soils in a greenhouse, we found that plants were locally adapted to their home soil environment. Specifically, we found that local plants produced more biomass than at least one of the two populations of foreign plants in all three soil origins in the living soil (Fig. 2b; Table 3). This pattern was best exemplified by the reciprocal home-site advantage for KSR and rare plants, each of which produced more biomass in their home soil than the foreign population (Fig. 2b; Table 3). By contrast, we did not find evidence of local adaptation when the same populations were grown in sterilized soil, as local plants never produced more biomass than either population of foreign plants in this soil condition (Fig. 2a; Table 3). Our results therefore suggest that biotic factors are an important cause of plant adaptation to soil, and can be stronger promoters of local adaptation than differences in nutrient availability.
Differences in fungal communities among soil origins may have been one cause of local adaptation in the living soil. For example, rare and KSR plants, which each produced more biomass in their home soil than the foreign population (Fig. 1b; Table 3), originate from habitats with markedly different AM fungal communities. In particular, total AM fungal colonization was 45% higher in rare soil than KSR soil (Fig. 2a), suggesting that AM fungi are less abundant in KSR soil. In addition to differing in abundance, our results suggest that there was selection for reduced interactions between AM fungal communities and their associated B. inermis populations. KSR and rare plants each incurred less AM-colonization in their home soil than the foreign population (Fig. 2a; Table 5). Also, we found that biomass was negatively correlated with AM-colonization across populations and soil origins (r = −0.133, P = 0.047). If colonization intensity is indicative of fungal carbon demands (Gange and Ayres 1999), then our results indicate that these demands exceed the benefits provided by the fungus to B. inermis plants. This hypothesis is consistent with previous studies of interactions between AM fungi and B. inermis, which found that mycorrhizal inoculation reduced plant growth relative to a non-AM control (Bildusas et al. 1986). Our results could also suggest that the soils used for this study contain a greater proportion of parasitic than mutualistic AM fungal species for B. inermis (Klironomos 2003). As a result, invading B. inermis populations may adapt to novel soils by reducing their susceptibility to AM fungal colonization and its associated carbon demands.
The hypothesis that B. inermis evolves weaker interactions with AM fungi during invasion is consistent with previous observations that many invasive species are either non-mycorrhizal or facultatively mycorrhizal (Pringle et al. 2009), suggesting that independence from AM fungi facilitates their range expansion (Pringle et al. 2009). Though we did not measure the degree to which B. inermis populations respond to AM fungi at our study sites, the evolution of reduced mycorrhizal dependence was observed in introduced versus native populations of Hypericum perforatum, another European species that has become naturalized in North America (Seifert et al. 2009). This reduction in mycorrhizal responsiveness could continue with the evolution of an invasive species as it expands its range. If reduced AM fungal colonization in local populations of B. inermis results in reduced responsiveness to AM fungi, we predict that local plants grown with their own AM fungi will show weaker growth responses than foreign plants grown with the same fungi.
Unlike rare and KSR plants, local adaptation in the LTMRS population could have been caused by evolution in response to non-AM fungi. For instance, LTMRS plants may have produced more biomass than rare plants in their home soil (Fig. 1b; Table 3) because of adaptation to the extent of colonization by septate fungi. Septate hyphal colonization was 72% higher in LTMRS soil than rare soil (Fig. 2b). If the carbon demands imposed by septate fungi mirror those of AM fungi, then LTMRS plants may have evolved greater resistance to colonization by septate fungi than rare plants. This evolutionary divergence is analogous to the way contrasting patterns of herbivory cause different degrees of resistance to evolve in plant populations (Schemske 1984; Rausher and Simms 1989). Because colonization by septate hyphae did not differ significantly among populations, or reduce plant biomass (r = −0.060, P = 0.459), our conclusion is somewhat circumstantial; however, rare and LTMRS plants produced equal biomass in sterilized LTMRS soil (Fig. 1a; Table 3), suggesting that at least one element of the soil biota was responsible for the superior performance of local plants in living LTMRS soil.
Another element of the soil biota that could have driven the patterns of local adaptation observed in living soil is pathogens. Soil pathogens can have large negative effects on plant performance and could therefore exert strong selection pressures on their associated plant populations. For example, in Prunus serotina, removal of soil pathogens increases seedling survival by as much as 136% (Packer and Clay 2000; Reinhart et al. 2005). Accumulation of soil pathogens over time can also limit species abundance in their home range, while initial release from pathogens could account for the dominance of invasive species in novel ranges (Klironomos 2002). If populations adapt to the pathogens in their home soil by reducing their susceptibility to attack, then local plants should experience less of a reduction in fitness than foreign plants when exposed to these pathogens.
Support for local adaptation was not universal in the living soil (i.e. local plants did not produce significantly more biomass than foreign plants in every pairwise comparison); however, the frequency of home site advantage was consistent with that observed in past reciprocal transplant studies. A recent meta-analysis found that local plants have higher fitness than foreign plants in 71% of pairwise comparisons (Leimu and Fischer 2008), and another meta-analysis found that the mean fitness advantage of local populations in reciprocal transplant studies is 45% (Hereford 2009). In the living soil, we found that local plants produced more biomass than foreign plants in three of six comparisons (50%; Table 3), and the magnitude of their fitness advantage was 27% (Fig. 1b). Nevertheless, our results may suggest that these three populations are still evolving and have not yet reached local fitness optima. This interpretation is consistent with the fairly recent introduction of B. inermis to North America (Newell and Keim 1943). Alternatively, because this species is widespread and wind-pollinated, gene flow from adjacent populations could be preventing the evolution of strong local adaptation among populations.
A strength of our experiment relative to most local adaptation studies is that populations were selected without a priori hypotheses of local adaptation. Many reciprocal transplants are performed over steep elevational and resource gradients, or across distances greatly exceeding the maximum dispersal of the study species (Hereford 2009). The goal of this approach is to minimize gene flow and genetic drift, which can erode local adaptation (Schluter 2000). In contrast, we selected three populations within a narrow range in southern Ontario that display few phenotypic differences (Sherrard 2010). Although the magnitude of local adaptation is positively correlated with environmental divergence (Hereford 2009), we found that plants adapt to their home soil at finer spatial scales and between sites lacking extensive resource variation. This evidence for adaptation to soil, even in the face of possible gene flow, suggests that biotic factors are strong agents of selection in plants.
While our results indicate that plants are adapted to their home soil, and suggest that biotic factors influence the magnitude of this adaptation, we cannot identify whether biotic factors cause these patterns independently or in combination with abiotic soil factors. For example, plants may adapt to their home soil through the evolution of resistance to a particular soil pathogen or herbivore. Alternatively, plants may adapt to the nutrient availability of their home soil by shifting their dependence on AM fungi to maximize nutrient uptake relative to carbon loss (Schultz et al. 2001; Johnson et al. 2010). Another way of isolating the role of biotic factors, and a potential avenue of future research, would be to reciprocally transplant populations into soil with identical abiotic properties that has been inoculated with the contrasting biota of the two populations.
Our results suggest that the ecological causes of plant adaptation could be misinterpreted in reciprocal transplant studies that only consider the role of abiotic factors. In particular, had we not isolated the effect of nutrient availability by sterilizing the soil, we would have falsely concluded that nutrient levels were responsible for adaptation. However, if adaptation to a low nutrient soil was responsible for the higher biomass of LTMRS plants than rare plants in LTMRS soil, then this effect would have also been observed in sterilized soil. The absence of consistent responses between living and sterilized soils indicates that biotic factors in the soil were the most likely agents of selection, and differences in root colonization suggest that AM fungi were one contributing factor. Adaptation to novel soil biota could be an important cause of rapid range expansion during biological invasions. For instance, B. inermis was first observed in southern Ontario in the early 1900s and is now found throughout the province (Otfinowski et al. 2007), suggesting that the evolutionary responses we observed among study populations occurred in less than 100 years. Identifying the agents that cause adaptation is a major goal in evolutionary ecology because it allows us to make long-term predictions of phenotypic responses to environmental change (Endler 1986; Kawecki and Ebert 2004). Though many studies focus exclusively on abiotic factors as causes of adaptation, we show that soil biotic factors can be as important as abiotic factors for causing plant adaptation.
References
Antonovics J, Bradshaw AE (1970) Evolution in closely adjacent plant populations. VIII. Clinal patterns at a mine boundary. Heredity 25:349–362
Bennington CC, McGraw JB (1995) Natural selection and ecotypic differentiation in Impatiens pallida. Ecol Monogr 65:303–324
Berns AE, Philipp H, Narres H-D et al (2008) Effect of gamma-sterilization and autoclaving on soil organic matter structure as studied by solid state NMR, UV and fluorescence spectroscopy. Eur J Soil Sci 59:540–550
Bildusas IJ, Dixon RK, Pfleger FL et al (1986) Growth, nutrition and gas exchange of Bromus inermis inoculated with Glomus fasciculatum. New Phytol 102:303–311
Brundrett M (1994) Clearing and staining mycorrhizal roots. In: Brundrett M, Melville L, Peterson L (eds) Practical methods in mycorrhizal research. Mycologue Publications, Waterloo, pp 42–46
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77
Buswell JM, Moles At, Hartlet S (2011) Is rapid evolution common in introduced plant species? J Ecol 99:214–224
Chapin FS III (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Clausen J, Keck DD, Heisey WM (1948) Experimental studies on the nature of species. III. Environmental responses of climactic races of Achillea. Carnegie Institute of Washington Publication 581:129
Donovan LA, Ludwig F, Rosenthal DM et al (2009) Phenotypic selection on leaf ecophysiological traits in Helianthus. New Phytol 183:868–879
Endler JA (1986) Natural selection in the wild. Princeton University Press, Princeton
Felsenstein J (1976) The theoretical population genetics of variable selection and migration. Annu Rev Genet 10:253–280
Gange AC, Ayres RL (1999) On the relation between arbuscular mycorrhizal colonization and plant ‘benefit’. Oikos 87:615–621
Gist GR, Smith RM (1948) Root development of several common forage grasses to a depth of eighteen inches. J Am Soc Agron 40:1036–1042
Grime PJ (1977) Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 111:1169–1194
Grime PJ (2001) Plant strategies, vegetation processes and ecosystem properties, 2nd edn. Wiley, Chichester
Hereford J (2009) A quantitative survey of local adaptation and fitness trade-offs. Am Nat 173:579–588
Johnson N (1993) Can fertilization of soil select less mutualistic mycorrhizae? Ecol Appl 3:749–757
Johnson NC, Wilson GWT, Bowker MA et al (2010) Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc Natl Acad Sci USA 107:2093–2098
Kawecki TJ, Ebert D (2004) Conceptual issues in local adaptation. Ecol Let 7:1225–1241
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301
Lambers H, Poorter H (1992) Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. In: Begon M, Fitter AH (eds) Advances in Ecological Research Volume 23. Academic Press Ltd., London, pp 187–261
Leimu R, Fischer M (2008) A meta-analysis of local adaptation in plants. PLoS ONE 3:e4010
Lenssen JPM, van Kleunen M, Fischer M et al (2004) Local adaptation of the clonal plant Ranunculus reptans to flooding along a small-scale gradient. J Ecol 92:696–706
Linhart YB, Grant MC (1996) Evolutionary significance of local genetic differentiation in plants. Annu Rev Ecol Syst 27:237–277
Macel M, Lawson CS, Mortimer SR et al (2007) Climate vs. soil factors in local adaptation of two common plant species. Ecology 88:424–433
Maron JL (1998) Individual and joint effects of below- and above-ground insect herbivory on perennial plant fitness. Ecology 79:1281–1293
Maron JL, Vilà M, Bommarco R et al (2004) Rapid evolution of an invasive plant. Ecol Mono 74:261–280
McGonigle TP, Miller MH, Evans DG et al (1990) A method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
McGraw JB, Chapin FS III (1989) Competitive ability and adaptation to fertile and infertile soils in two Eriophorum species. Ecology 70:736–749
McKone MJ (1985) Reproductive biology of several bromegrasses (Bromus): breeding system, pattern of fruit maturation, and seed set. Am J Bot 72:1334–1339
McNamara NP, Black HIJ, Beresford NA et al (2003) Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Appl Soil Ecol 24:117–132
Newell LC, Keim FD (1943) Field performance of bromegrass strains from different regional seed sources. J Am Soc Agron 35:420–434
Newsham KK (2011) A meta-analysis of plant responses to dark septate root endophytes. New Phytol 190:783–793
Newsham KK, Fitter AH, Watkinson AR (1994) Arbuscular mycorrhiza protect an annual grass from root pathogenic fungi in the field. J Ecol 83:991–1000
Otfinowski R, Kenkel NC, Catling PM (2007) The biology of Canadian weeds. 134. Bromus inermis Leyss. Can J Plant Sci 87:183–198
Packer A, Clay K (2000) Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature 404:278–281
Pregitzer CC, Bailey JK, Hart SC et al (2010) Soils as agents of selection: feedbacks between plants and soils alter seedling survival and performance. Evol Ecol 24:1045–1059
Pringle A, Bever JD, Gardes M et al (2009) Mycorrhizal symbioses and plant invasions. Annu Rev Ecol Syst 40:699–715
Rausher MD, Simms EL (1989) The evolution of resistance to herbivory in Ipomoea purpurea I. attempts to detect selection. Evolution 43:563–572
Reinhart KO, Royo AA, van der Putten WH et al (2005) Soil feedback and pathogen activity in Prunus serotina throughout its native range. J Ecol 93:890–898
Salonius PO, Robin JB, Chase FE (1967) A comparison of autoclaved and gamma-irradiated soils as media for microbial colonization experiments. Plant Soil 27:239–248
Schemske DW (1984) Population structure and local selection in Impatiens pallida (Balsaminaceae), a selfing annual. Evolution 38:817–832
Schluter D (2000) The ecology of adaptive radiation. Oxford University Press, New York
Schultz PA, Miller RM, Jastrow JD et al (2001) Evidence of a mycorrhizal mechanism for the adaptation of Andropogon gerardii (Poaceae) to high- and low-nutrient prairies. Am J Bot 88:1650–1656
Seifert EK, Bever JD, Maron JL (2009) Evidence for the evolution of reduced mycorrhizal dependence during plant invasion. Ecology 90:1055–1062
Sherrard ME (2010) Physiological adaptation to biotic and abiotic soil factors in Bromus inermis. Dissertation, University of Guelph, Guelph, Canada
Snaydon RW, Bradshaw AD (1962) Differences between natural populations of Trifolium repens L. in response to mineral nutrients. J Exp Bot 13:422–434
Sokal RR, Rohlf FJ (1995) Biometry. Freeman, New York
Troelstra SR, Wagenaar R, Smant W et al (2001) Interpretation of bioassays in the study of interactions between soil organisms and plants: involvement of nutrient factors. New Phytol 150:697–706
Turkington R, Harper JL (1979) The growth, distribution and neighbour relationships of Trifolium repens in a permanent pasture. IV. Fine-scale biotic differentiation. J Ecol 67:245–254
Wade MJ, Kalisz S (1990) The causes of natural selection. Evolution 44:1947–1955
Wolfe BE, Husband BC, Klironomos JN (2005) Effects of a belowground mutualism on an aboveground mutualism. Ecol Let 8:218–223
Acknowledgments
We thank C. M. Caruso, J. A. Newman and J. N. Klironomos for helpful discussions and comments on earlier versions of this manuscript. We thank M. Arcand, A. Clark, R. Germain, A. Lambert, L. MacDonald and M. Mucci for assistance in the greenhouse, field and lab. This work was supported by the Natural Science and Engineering Research Council of Canada and grants from the Canadian Foundation for Innovation and the Ontario Innovation Trust.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sherrard, M.E., Maherali, H. Local adaptation across a fertility gradient is influenced by soil biota in the invasive grass, Bromus inermis . Evol Ecol 26, 529–544 (2012). https://doi.org/10.1007/s10682-011-9518-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-011-9518-2