Abstract
Recent studies reveal substantial species and regional differences in enteroendocrine cell (EEC) populations, including differences in patterns of hormone coexpression, which limit extrapolation between animal models and human. In this study, jejunal samples, with no histologically identifiable pathology, from patients undergoing Whipple’s procedure were investigated for the presence of gastrointestinal hormones using double- and triple-labelling immunohistochemistry and high-resolution confocal microscopy. Ten hormones (5-HT, CCK, secretin, proglucagon-derived peptides, PYY, GIP, somatostatin, neurotensin, ghrelin and motilin) were localised in EEC of the human jejunum. If only single staining is considered, the most numerous EEC were those containing 5-HT, CCK, ghrelin, GIP, motilin, secretin and proglucagon-derived peptides. All hormones had some degree of colocalisation with other hormones. This included a population of EEC in which GIP, CCK and proglucagon-derived peptides are costored, and four 5-HT cell populations, 5-HT/GIP, 5-HT/ghrelin, 5-HT/PYY, and 5-HT/secretin cell groups, and a high degree of overlap between motilin and ghrelin. The presence of 5-HT in many secretin cells is consistent across species, whereas lack of 5-HT and CCK colocalisation distinguishes human from mouse. It seems likely that the different subclasses of 5-HT cells subserve different roles. At a subcellular level, we examined the vesicular localisation of secretin and 5-HT, and found these to be separately stored. We conclude that hormone-containing cells in the human jejunum do not comply with a one-cell, one-hormone classification and that colocalisations of hormones are likely to define subtypes of EEC that have different roles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transcriptional profiling of reporter mice over about the past 7 years has revealed that gene expression for multiple hormones occurs in most enteroendocrine cells (EECs) in the small intestine (Egerod et al. 2012, Habib et al. 2012, Sykaras et al. 2014, Grunddal et al. 2015, Fothergill et al. 2017). Moreover, the hormone products of these genes are commonly colocalised in EEC (Fothergill et al. 2017), although there are discrepancies between transcript and gene product localisation. An example of discrepancy comes from investigation of CCK cells isolated from transgenic mice expressing enhanced green fluorescent protein (eGFP) under the control of the CCK promoter (Egerod et al. 2012) in which ghrelin RNA copy numbers in CCK cells were high (at about 25% the copy number of CCK), whereas minimal or no immunohistochemical coexpression of ghrelin with CCK was observed (Egerod et al. 2012; Sykaras et al. 2014). Consistent with this immunohistochemical observation, proghrelin peptides were not detected above the noise level in LC-MS analysis of FACS-separated CCK-eGFP cells. In another example, while secretin precursor RNA was at similar levels to the CCK RNA copy number, only small amounts of prosecretin peptides were detected in the LC-MS analysis of CCK cells, although secretin was colocalised with CCK positive cells in mouse small intestine and human tissue samples (Egerod et al. 2012). Also in mouse, FACS purified proglucagon and GIP reporter cells expressed both precursor RNAs and immunoreactive peptides corresponding to CCK, GIP and secretin (Habib et al. 2012).
Examples of differential distribution of enteroendocrine hormones across species include the pattern of expression and colocalisation of GLP-1, GIP and PYY in mouse and pig (Cho et al. 2015). All combinations of GIP, GLP-1 and PYY were found in EEC, with different gradients of occurrence along the gastrointestinal tract of the two species. Approximately 50% of all cells revealed by triple-labelling for GLP-1, GIP and PYY in the pig duodenum express PYY or PYY plus GLP-1, whereas no duodenal EEC express PYY in the mouse (Mortensen et al. 2003; Cho et al. 2015). While the absolute numbers of EEC that could be classified as K cells (GIP containing) or L cells (GLP-1 or PYY containing) per mm2 of mucosa were similar in the small intestine of the two species, in the pig jejunum, GLP-1 and GIP overlap was observed in only a small subset (< 10%) of K/L cells, whereas in the mouse small intestine, the hormones were costored in ~ 25% of GIP and GLP-1 cells (Cho et al. 2015; Fothergill et al. 2019).
Currently, there is little known about combinations of hormones in human EEC and no region of human small intestine has been studied in detail for hormone colocalisation.
Materials and methods
Tissue sources
Normal human jejunum (n = 3) discarded during Whipple’s procedures was used (Cho et al. 2014b). Samples were from a 69 year old male (patient 1, pancreatic adenocarcinoma), a 38-year-old male (patient 2, chronic pancreatitis) and an 86-year-old female (patient 3, pancreatic tumour). The tissue samples were obtained from The University of Melbourne Department of Surgery at the Austin Hospital (Austin Health). No intestinal tissue pathology was detected by histology of tissue adjacent to the samples used in this study (Cho et al. 2014b). All procedures carried out in this study were conducted according to the National Health and Medical Research Council of Australia guidelines and were approved by the Human Research Ethics Committee (Austin Health). All tissue samples were obtained in the morning.
Immunohistochemistry
Tissue was fixed in 4% paraformaldehyde and processed as previously described (Cho et al. 2014b). Processed tissue pieces were placed in PBS-sucrose azide and OCT compound (Tissue Tek, Elkhart, IN, USA) in a ratio of 1:1 for 24 h at 4 °C before being trimmed and embedded in 100% OCT compound and frozen in isopentane cooled with liquid nitrogen. Sections of 10 μm thickness were cut, air dried for 1 h on microscope slides (SuperFrostPlus®; Menzel-Glaser #1.5 from Thermo Fisher, Scoresby, Vic, Australia) and incubated with 10% normal horse serum for 30 min. Sections were then incubated with mixtures of primary antibodies (Table 1) for double or triple staining at 4 °C, overnight. Tissue was washed three times in PBS and incubated in secondary antibody (Table 1) for 1 h at room temperature. For staining nuclei, preparations were washed once with PBS then twice with distilled water and incubated for 5 min in Hoechst bisbenzamide 33258 solution (diluted to 10 μg/mL in dH2O) and then washed 3 times with distilled water before mounting with fluorescence mounting medium (Dako Corporation, Carpinteria, CA, USA). The glucagon gene encodes for preproglucagon, from which the hormones GLP-1, GLP-2 and oxyntomodulin (OXM) are cleaved. GLP-1 and GIP have substantial correspondence of sequences of amino acids and for this reason antibodies against one of these hormones can recognise the other (Musson et al. 2011; Fothergill et al. 2019). This is a particular problem with antibodies to GLP-1 because there is a sequence in GIP that has homology to the full sequence of GLP-1. Anti-GIP antibodies usually present a lesser problem because the C-terminal 12 amino acids of GIP are not shared with GLP-1. We have used a monoclonal anti-OXM antibody to ensure that cross-reactivity with GIP did not occur (Fothergill et al. 2019). In that study, all oxyntomodulin positive cells contained GLP-1.
A slide to estimate background fluorescence was prepared in the same way as the test slides with antibody diluent in place of the primary antibodies. Slides containing only one of the primary antibodies, but all of the secondary antibodies for all triple antibody combinations were also prepared as control for inappropriate secondary antibody binding or bleed through of fluorescence between detection channels. No inappropriate secondary binding or bleed through was encountered. In order to have antibody combinations suited to different triple stains, primary antibodies raised in different species against the same hormone were used (Table 1). Antibodies raised against the same hormone were tested together in double stains to confirm that they revealed the same populations of EEC, which they did in all cases.
Microscopy
Fluorescence was visualised by high-resolution confocal microscopy (LSM800 and LSM880, Carl Zeiss, Sydney, Australia). Images were obtained from tile scans taken at × 20 magnification in four channels: 350, 488, 568 and 647 nm on the LSM800. Super resolution Z-stack images were taken on the LSM880 using the Airyscan mode with a × 63 oil objective and deconvolved using the Zen 3D Airyscan processing function (Zeiss). TetraSpeck microspheres were imaged and deconvolved to determine the relative direction and magnitude of chromatic aberration for each channel. Super-resolution images were corrected for chromatic aberration, and misaligned slices of the Z-stack were removed. To further reduce errors arising from chromatic aberration in the Z-axis, images were then maximally projected using Fiji imaging (Fothergill et al. 2017).
Analysis
Images were exported and analysed offline using ImageJ (imagej.nih.gov/ij/). Cells were selected manually for each hormone and were considered positive if the fluorescence intensity was greater than 2 standard deviations above the average background intensity of the epithelium. For quantitation of immunoreactive cells, counts are expressed as numbers of cells per measured length of epithelium from which EEC were counted. Large areas of each tissue sample were examined to reduce selection bias. The average length of epithelium analysed per tissue sample was 11.8 ± 3.7 cm.
An average of 121 ± 19.5 cells was counted from patient 1 for each of the 14 combinations examined, 200 ± 25.2 cells from patient 2 and 166 ± 21.9 cells from patient 3. The total numbers of cells counted were for 3246, 2366 and 2123, respectively. The frequencies of occurrence of EEC types were calculated as the average of the numbers counted for each patient sample (n = 3). Where an EEC type was analysed in more than one immunohistochemical analysis, an average of the numbers of cells for each immunohistochemical analysis was determined, then an average from all patient samples was calculated. Quantification of mono, di- or triple-labelled EEC cells was analysed using Prism 5.0 (GraphPad Software, San Diego, CA, USA). Data is presented as mean ± SEM. Images were imported into CorelDraw (Corel Corporation, Ottawa, Canada) for final preparation of figures. A general linear model was applied to data with subsequent Bonferroni comparisons to evaluate differences in frequencies of occurrence of each type of EEC between patient samples.
Results
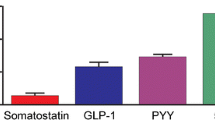
The frequencies of occurrence of cells containing each of the known major enteroendocrine hormones were quantified in the human jejunum, independent of whether they contained other hormones (Fig. 1). Of all the hormones examined, the largest population of EEC cells contained 5-HT (17.6 ± 1.8 cells/cm mucosal surface length). CCK containing cells were the second most abundant EEC (12.0 ± 1.3 cells/cm mucosa), followed by ghrelin, GIP, motilin, secretin and glucagon-gene products (revealed by anti-oxyntomodulin and designated OXM-GLP-1). The smallest populations of EEC cells contained PYY, neurotensin or somatostatin (3.5 ± 1.0 cells/cm mucosa for PYY, 3.5 ± 1.0 cells/cm for neurotensin and 3.5 ± 0.1 cells/cm mucosa for somatostatin). No significant differences were found between patient samples for total numbers of cells containing a particular hormone, with the exception of neurotensin cells. A significant difference (P = 0.03) in the total number of cells containing neurotensin occurred between the samples from a patient with adenocarcinoma and one with acute pancreatitis, more cells occurring in acute pancreatitis. The number of neurotensin immunoreactive cells in all jejunal samples was low (Fig. 1), and variations of about 2 cells/cm resulted in this apparent significant difference.
Frequencies of occurrence of all the immunoreactive EEC types in the human jejunum, per cm of mucosal surface length, without taking into account colocalisation. 5-HT 5-hydroxytryptamine, CCK cholecystokinin, Ghr ghrelin, GIP glucose-dependent insulinotropic peptide, Motil motilin, Secr secretin, OXM oxyntomodulin, GLP-1 glucagon-like peptide 1, PYY peptide tyrosine tyrosine, NTS neurotensin, SST somatotstatin. Anti-oxyntomodulin was used to locate cells with glucagon gene products (GLP-1). Data is mean ± SEM, tissue from n = 3 subjects (121 to 200 cells per combination per subject sample)
Patterns of colocalisation of hormones
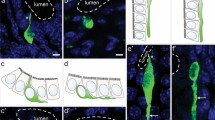
Colocalisation of hormones was investigated in triple and double-labelled sections, examined in tile scans. Immunoreactivity for two hormones, and sometimes three hormones, in the same EEC was common (Fig. 2). Where two different cells were adjacent to each other, the separate cells could be distinguished (Fig. 2a, a”, a”’). By examining the Z-stacks of various triple hormone stains, we observed that hormones were sometimes concentrated in different parts of the same cell (Fig. 2b, b’, b”, b”’). EEC appeared to be more numerous per cm of epithelium in the crypts than the villi, except for cells containing secretin or ghrelin, which were fewer in the crypts (Supplementary Fig. 1).
Examples of hormone colocalisation. The positions of individual cells are indicated by arrows or asterisks that are repeated in micrographs of identical fields in each row. (a, a’, a”, a”’) Localisation of 5-HT, CCK and secretin. 5-HT was commonly colocalised with secretin, and less commonly with CCK. (b, b’, b”, b”’) Localisation of ghrelin, 5-HT and OXM/GLP-1. Ghrelin and 5-HT are colocalised in the cells marked with asterisks. An arrow indicates a cell that is only immunoreactive for OXM/GLP-1. Hormones can have different subcellular localisations (cell indicated by arrowhead). In this cell, ghrelin is basally located and 5-HT is both basally and apically located. (c, c’, c”, c”’) Localisation of CCK, OXM/GLP-1 and GIP. Arrows indicate cells that were immunoreactive for only CCK. Double asterisks indicate cells with immunoreactivity for three hormones, single asterisks indicate immunoreactivity for two hormones, and arrows indicate where only one hormone is revealed
Extent of colocalisation of hormones in 5-HT cells
In order to investigate colocalisation with 5-HT, triple staining was conducted for 5-HT plus secretin plus CCK, 5-HT plus NTS plus PYY, 5-HT plus ghrelin plus motilin, and 5-HT plus ghrelin plus oxyntomodulin, and double staining for 5-HT plus GIP. Triple-labelling immunohistochemistry revealed costorage of 5-HT with secretin but very rarely with CCK (Fig. 2a). Of 5-HT cells, 12.2 ± 1.9% were immunopositive for secretin (Fig. 3a), and 47.9 ± 0.4 of secretin cells were immunopositive for 5-HT (Fig. 3b). There was a small degree of co-localisation of CCK and secretin: 12.4 ± 3.9% of CCK cells were secretin positive (Fig. 3b). These EEC were 5-HT negative.
Proportions of EEC with colocalised hormones for the eight most commonly occurring jejunal hormones. Proportions of cells with colocalised hormones are for the hormones indicated above each histogram. The left column of each histogram is 100% for the designated EEC type. The other columns represent proportions of EEC containing this hormone that are immunoreactive for the hormone indicted under the column. Data presented as mean plus SEM
Several hormones were prominently colocated in 5-HT containing cells: GIP, ghrelin and PYY. Immunoreactivity for GIP occurred in 46.9 ± 8.3% of 5-HT cells (Fig. 3a). About half of 5-HT cells (44.7 ± 9.9%) were immunoreactive for ghrelin. Of ghrelin cells, 57.7 ± 3.9% were immunoreactive for motilin (Fig. 4c); however, fewer than 1% of 5-HT cells were immunoreactive for motilin, indicating that two separate populations of ghrelin-containing cells exist, Ghr/5-HT and Ghr/motilin (Fig. 3c). This was confirmed by ghrelin, motilin plus 5-HT triple staining in which fewer than 1% of immunoreactive cells were triple stained and no cells contained ghrelin alone. The other significant overlap was with PYY, which was in 20.2 ± 6.4% of 5-HT cells. This represents about 75.6 ± 4.6% of PYY cells (Fig. 3h). Thus, multiple classes of 5-HT cells have been identified, 5-HT/secretin, 5-HT/GIP, 5-HT/ghrelin and 5-HT/PYY. CCK, motilin, oxyntomodulin and neurotensin were very rarely colocated with 5-HT (< 2%). The rarity of 5-HT/CCK cells (1.4 ± 0.4% of 5-HT cells) contrasts with the mouse small intestine where a significant proportion of CCK cells are 5-HT immunopositive, about 40% of jejunal CCK cells (Fakhry et al. 2017).
Colocalisation of CCK, GIP and OXM-GLP-1. The plain coloured columns show numbers of cells with only one of the 3 hormones: 9.5 ± 3.3 cells/cm with only GIP, 6.7 ± 2.0 cells/cm with only CCK, and 1.5 ± 0.3 cells/cm with only OXM-GLP-1. GIP and OXM-GLP-1 were colocalised in 4.5 ± 0.4 cells/cm; 1 ± 0.5 cells/cm were immunoreactive for both CCK and OXM-GLP-1 and 1.6 ± 0.6 cells/cm were immunoreactive for both CCK and GIP. All three hormones occurred in 1.5 ± 0.4 cells/cm. Data are mean plus SEM
While it is difficult to accurately deduce colocalisation patterns of hormones beyond the specific triple and double stains tested, this analysis informs the identity of various clusters of hormones. For example, GIP and 5-HT were frequently colocalised (50.6 ± 4.7% of GIP cells), as were GIP and CCK (24.4 ± 1.9% of GIP cells). As 5-HT and CCK were very rarely colocalised, this data suggests three populations of GIP cells: those containing 5-HT, those containing CCK and a population containing neither 5-HT nor CCK. Similarly, the rarity of GIP and motilin colocalisation (> 1% of GIP cells contained motilin) in cells implies that there are at least two populations of cells containing ghrelin, oxyntomodulin or CCK.
Colocalisation patterns of CCK, preproglucagon-derived hormones and GIP
These 3 hormones had significant colocalisation, as illustrated in Fig. 4, which is derived from analysis of triple staining for the three hormones. Triple hormone colocalisation was observed more frequently in this combination of hormones than in any other triple stain tested. All three hormones occurred in 1.5 ± 0.4 cells/cm, accounting for approximately 17.4 ± 3.4% of oxyntomodulin cells. OXM-containing cells frequently contained either GIP or CCK, with only around 18% of OXM-containing cells not containing at least one of the other hormones.
Other hormones
Ghrelin and motilin are related hormones and both have prokinetic actions in the gastrointestinal tract, although through different mechanisms, and both inhibit nausea (Sanger and Furness 2016). We found that 79.8 ± 7.9% of motilin cells contained ghrelin (Fig. 3e). In fact, 2 separate populations, Ghr/5-HT and Ghr/motilin cells accounted for almost 100% of ghrelin cells (see above). Only around 6% of the ghrelin population colocalised with neurotensin. Somatostatin positive cells were the least numerous EEC type located in the human jejunum with 3.5 ± 0.2 cells/cm mucosa. Of somatostatin-positive cells, 13.8 ± 4.7% were found to be immunoreactive for GIP. Somatostatin and PYY were rarely colocalised in cells, and only 2.6 ± 1.5% of somatostatin cells were positive for PYY.
Super-resolution microscopy
Super-resolution microscopy studies in samples from laboratory animals have revealed that hormones are commonly located in separate storage vesicles in EEC that contain peptide and amine hormones in the same cell (Cho et al. 2014b; Grunddal et al. 2015; Fothergill et al. 2017). To test whether hormones are separately stored at a subcellular level in human EEC, we investigated cells containing GIP and OXM, and a peptide and amine pair, secretin and 5-HT (Fig. 5). Most vesicles that store OXM and GIP, which both modulate blood glucose levels, were separate, although the hormones appeared to be costored in a minority of vesicles (Fig. 5a, a’, a”). This apparent colocalisation may be a result of vesicles containing different peptides that are located in close proximity in the z-axis not being resolved. By contrast, vesicles immunoreactive for 5-HT or secretin were almost completely separate in cells in which both were found (Fig. 5b, b’, b”).
Vesicular locations of hormones present in the same EEC. (a, a’, a”) GIP and OXM immunoreactivities are in some cases in separate vesicles (2, OXM only; 3, GIP only), or both immunoreactivities are seen in the same vesicle (example indicated by 1). (b, b’, b”) 5-HT and secretin are contained in separate vesicles: 1, example of a vesicle only immunoreactive for 5-HT; 2, example of a vesicle only immunoreactive for secretin
Discussion
Overview
Colocalisation of multiple gut hormones in individual intestinal EEC has been revealed by recent studies in animal models, primarily in mouse (Egerod et al. 2012, Habib et al. 2012, Sykaras et al. 2014, Cho et al. 2015, Grunddal et al. 2015, Fothergill et al. 2017). The present study reveals a comparably extensive colocalisation of hormones in EEC of the human jejunum that was previously unknown. The degree of colocalisation may even be under-estimated, because the hormones were sometimes in different parts of EEC, as previously reported (Grunddal et al. 2015), and in the 10 μm sections that were taken parts of some cells would not be present. Colocalisations discovered include a population of EEC in which GIP, CCK and preproglucagon-derived peptides are costored, and four 5-HT cell populations. Unlike in mouse (Cho et al. 2014a; Reynaud et al. 2016), in human jejunum 5-HT was rarely colocalised with CCK, although a CCK/secretin cell group was identified. As recent authors have pointed out, these common and extensive patterns of colocalisation require a revision of the way in which EEC are classified, in particular it means that the single letter code (one cell-one hormone) adhered to in text books and even by some recent reviews, must be revised (Helander and Fändriks 2012; Engelstoft et al. 2013; Gribble and Reimann 2016; Fothergill and Furness 2018). Although the histology and gross appearance of the samples of jejunum were normal, it is feasible the pancreatic cancer could affect jejunal endocrine cell populations. Sah et al. (2013) showed that pancreatic EEC populations in regions away from the cancer differ from normal, and it is reasonable to assume that pancreatic cancer could also affect EEC populations in the jejunum. It should also be pointed out that the analysis was from jejunum of only three patients. Nevertheless, the patterns of colocalisation were consistent.
Illustration of major overlaps
Patterns of major hormone colocalisation determined from triple-stained sections are depicted in the diagrams of Fig. 6. This shows that proportions of secretin cells have colocalisation with 5-HT or CCK, but not with both hormones (Fig. 6a), that some of the 5-HT/secretin cells are PYY immunoreactive (Fig. 6b). There are substantial overlaps between GIP, OXM and CCK, with some EEC expressing all three (Fig. 6c). Most ghrelin cells are either motilin or 5-HT positive, and the majority of motilin cells are ghrelin immunoreactive, but not 5-HT positive (Fig. 6d).
Diagrammatic representation of patterns of colocalisation of hormones, based on triple labelling for the groups of hormones that are represented. Quantitative data and variances of hormone colocalisation is in Figs. 3 and 4 and in the text. a Relationship of populations of EEC immunoreactive for 5-HT, CCK and secretin. As in most mammals, there were cells containing both secretin and 5-HT, but unlike some other species, 5HT and CCK did not overlap. b Patterns of colocalisation of 5-HT, PYY and secretin. c Colocalisation of hormones affecting appetite and metabolism, GIP, glucagon-gene products (OXM/GLP-1) and CCK. There is a complex pattern of colocalisation of the two incretins and CCK. d: There are two major ghrelin cell populations, one almost entirely coincident with motilin and the other a subpopulation of the 5-HT cells
Classes of 5-HT cells
EEC that contain 5-HT (enterochromaffin cells) have previously been considered separate from EEC that contain peptide hormones (Gershon 2013; Mawe and Hoffman 2013; Diwakarla et al. 2017). However, we find multiple classes of peptide hormone containing 5-HT cells in the human jejunum, 5-HT/secretin (12.2 ± 3.4% of 5-HT cells; Fig. 6a), 5-HT/GIP (46.9 ± 14.3% of 5-HT cells), 5-HT/ghrelin (44.7 ± 14.3% of 5-HT cells) 5-HT/PYY (20.19 ± of 5-HT cells; Fig. 6b), some of which overlap, and 5-HT cells containing no (as yet) identified second hormone. This reflects the diversity of roles of 5-HT of gastrointestinal origin (Berger et al. 2009; Martin et al. 2017).
The presence of EEC that contains both 5-HT and secretin has been previously reported. In mouse, the majority of secretin cells contain 5-HT, and all secretin cells in the duodenum and jejunum of bovine, cat and guinea-pig contain 5-HT (Roth and Gordon 1990; Lopez et al. 1995). About 70% of secretin cells of human duodenum are immunoreactive for 5-HT (Cetin 1990). In the current work, 48% of human jejunal secretin cells contained 5-HT. 5-HT mimics the effect of secretin to release bicarbonate; low doses of 5-HT (20–200 nmol/kg/h) infused into the vasculature of the rat duodenum (Säfsten et al. 2006) or direct application of 5-HT to the isolated duodenum from mice (Tuo and Isenberg 2003) both increase bicarbonate secretion. Thus, there are synergistic effects of 5-HT and secretin released from the same cells. It can therefore be proposed that 5-HT/secretin cells are well conserved across mammalian species and that these are a specific functional subtype of 5-HT cells. They would be predicted to have receptors for acidic conditions, for example to express acid-sensitive ion channels.
We found that 20% of 5-HT cells in the human jejunum contained PYY (Fig. 6b). Colocalisation of 5-HT with PYY has been previously found in human colon, where approximately 25% of 5-HT cells also contain PYY (Hörsch et al. 1994; Martins et al. 2017). Both 5-HT and PYY suppress appetite; thus, the products of these cells may also act in synergy.
So far, as we are aware, colocalisation of 5-HT and GIP in human proximal small intestine has not been previously reported. However, in mice, tryptophan hydroxylase-1 expressing cells (marking 5-HT producing cells) in the proximal small intestine of GLU-Venus mice have been reported to express low levels of Gip (marking GIP producing cells) (Glass et al. 2017). 5-HT and GIP have different roles, and why they should be colocalised in some cells is not obvious. In particular, in contrast to GIP, 5-HT appears to inhibit insulin secretion, in that increased expression of 5-HT receptors in pancreatic β-cells has been observed to inhibit insulin secretion (Zhang et al. 2013).
Therefore, it seems likely that the different subclasses of 5-HT cells subserve different roles and are likely to express different receptors that control release of their hormonal products. The 5-HT/secretin cells could be anticipated to be acid sensing, 5-HT/ GIP cells carbohydrate sensing and 5-HT/ PYY cells may detect fats and other nutrients.
CCK, GIP and proglucagon-derived peptide colocalisation
In the human jejunum we found numerous GIP, but fewer OXM/ GLP-1 cells. GIP and GLP-1 cells are also common in human duodenum, with GLP-1 cells being a slightly larger population (Theodorakis et al. 2006). Theodorakis et al. (2006) reported that 38% of GIP cells contained GLP-1 in the duodenum and we found 26% of GIP cell to contain OXM/ GLP-1 in the jejunum. This contrasts with mouse in which only approximately 5% of GIP cells express GLP-1 in the proximal small intestine (Svendsen et al. 2016), whereas in the porcine mid-jejunum the proportion of GLP-1 and GIP cells that were immunoreactive for both peptides was around 60% of the cells (Mortensen et al. 2003). As is discussed below, this is one of several substantial species differences. Our subcellular analysis suggests that OXM/ GLP-1 and GIP are sometimes stored in the same vesicles. Thus, in human, it might be expected that GIP and OXM/ GLP-1 could be released from the same cells and act synergistically as incretins on the pancreatic islets.
In our study, a high proportion of the OXM/ GLP-1 cells also contained CCK (Figs. 4 and 6). By immunohistochemistry, it was previously reported that most GIP cells of the human duodenum were immunoreactive for CCK (Egerod et al. 2012). The presence of CCK in the same cells as GIP and OXM/GLP-1 is consistent with data from mouse, in which CCK cells, identified by a CCK reporter, had greater gene copy numbers for the GIP and GLP-1 genes than gene copy numbers for CCK (Egerod et al. 2012). CCK and GIP have been detected by mass spectrometry analysis in preproglucagon cells purified from mouse proximal small intestine (Glass et al. 2017). By immunohistochemistry, a high proportion of GLP-1 positive cells was positive for CCK, and CCK/GIP costorage was also observed (Egerod et al. 2012). Also in mouse, analysis of GLP-1 cells in the proximal small intestine showed that most contained CCK and some of these also contained GIP (Habib et al. 2012). In a further study, analysis of CCK cells in the mouse duodenum showed that 37% expressed GIP and 14% were positive for proglucagon, the GLP-1 precursor (Sykaras et al. 2014). Thus, in both mouse and human, it is consistently shown that there is colocalisation of CCK, GIP and GLP-1. Although the roles of CCK that are most commonly emphasised are to release pancreatic enzymes, contract the gall bladder and increase satiety, it also stimulates insulin secretion (Lo et al. 2011) which may be partly indirect, through the activation of a vagal reflex (Cheung et al. 2009). Thus, release of hormones from EEC that contain CCK, GIP and GLP-1 may increase insulin levels. Selective stimulation of these cells may thus have anti-diabetic effects.
PYY
Amongst the EEC containing GIP, GLP-1 and/or PYY in the proximal intestine, PYY cells are in a high proportion in pig (about 45% of GIP/GLP-1 cells), are slightly fewer in human (about 24% of these cells), but are rare in mouse (about 4% of the cells). The high amount in the proximal small intestine of the pig is consistent with PYY having been originally discovered and sequenced from this region of this species (Tatemoto 1982). Thus, release of PYY from the upper small intestine in human (and pig) may have a role in satiety that it does not have in mouse. There is nevertheless a gradient along the gastrointestinal tract in human with PYY concentrations of 5–6 pmol/g in the duodenum and jejunum, 84 pmol/g in the terminal ileum, 196 pmol/g in the sigmoid colon and 480 pmol/g in the rectum (Adrian et al. 1985). In the human jejunum, 59.5 ± 13.3% of PYY immunoreactive EEC were also immunoreactive for OXM/GLP-1 and 19.1 ± 4.2% of OXM/GLP-1 immunoreactive EEC were also immunoreactive for PYY This is in contrast to the human distal colon where over 95% of GLP-1 immunoreactive EEC were also immunoreactive for PYY and about 70% of PYY cells contained GLP-1 immunoreactivity (Martins et al. 2017).
Ghrelin and motilin
We found that 80% of motilin cells also contained ghrelin, which is consistent with the high degrees of colocalisation of these hormones that have been reported in human intestine by other authors (Wierup et al. 2007; Egerod et al. 2012). Gastric emptying is enhanced by both ghrelin (Levin et al. 2006) and motilin (Peeters et al. 1992). Exogenous administration of motilin initiates premature phase III contractions in the human stomach (Deloose et al. 2012). Ghrelin, which is considered the principal hunger hormone, is also able to induce premature gastric phase III activity upon exogenous administration in human (Tack et al. 2006; Deloose et al. 2012). However, unlike motilin, plasma ghrelin levels do not fluctuate in synchrony with phase III contractions in human (Deloose et al. 2015). Thus, in human, ghrelin and motilin are likely to act together to increase gastric emptying and propulsive activity in the upper small intestine and to promote feeding. In mice and rats, motilin and its receptor are only present as pseudogenes (He et al. 2010).
Differential subcellular storage
The recent advent of high-resolution confocal and super-resolution microscopy has allowed separate storage of peptide hormones in EEC to be resolved. Colocalisation studies using antibodies to GLP-1 and PYY showed that these hormones to be primarily located in separate vesicles in mouse, rat, human and pig EEC (Cho et al. 2014b; Grunddal et al. 2015). Separate vesicular stores of ghrelin and nefstatin-1 were found in gastric EEC (Stengel et al. 2009). In the human and mouse small intestine, separation of vesicular stores for GLP-1 and neurotensin and for PYY and neurotensin was discovered (Grunddal et al. 2015). This contrasted with GLP-1 and PYY, which were sometimes colocalised. As we found in the present study, the degree of vesicular colocalisation or separation differs between hormone pairs. In the present study, vesicular stores for 5-HT and secretin were separate, whereas OXM/GLP-1 and GIP were occasionally in the same storage organelles. The significance of separate and common subcellular storage of hormones has yet to be determined, but it is feasible that separately stored vesicles might be independently secreted, whereas this seems unlikely for hormones in the same storage vesicles, such as vesicles containing both OXM and GIP in the human jejunum.
In conclusion, enteric hormones are commonly colocalised at a cellular level in the human jejunum, the patterns of colocalisation defining more cell types than would occur if hormones were all stored in separate cells. It is probable that the same hormone, released from separate cell populations in company with different hormones, can subserve a range of distinct physiological roles.
References
Adrian TE, Ferri G-L, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR (1985) Human distribution and release of a putative’ new gut hormone, peptide YY. Gastroenterology 89:1070–1077
Berger M, Gray JA, Roth BL (2009) The expanded biology of serotonin. Annu Rev Med 60:355–366
Buchan AMJ, Doyle AD, Accili EA (1990) Canine jejunal submucosa cultures: characterization and release of neural somatostatin. Can J Physiol Pharmacol 68:705–710
Cetin Y (1990) Secretin-cells of the mammalian intestine contain serotonin. Histochemistry 93:601–606
Cheung GWC, Kokorovic A, Lam CKL, Chari M, Lam TKT (2009) Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab 10:99–109
Cho H-J, Callaghan B, Bron R, Bravo DM, Furness JB (2014a) Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell Tissue Res 356:77–82
Cho H-J, Robinson ES, Rivera LR, McMillan PJ, Testro A, Nikfarjam M, Bravo DM, Furness JB (2014b) Glucagon-like peptide 1 and peptide YY are in separate storage organelles in enteroendocrine cells. Cell Tissue Res 357:63–69
Cho H-J, Kosari S, Hunne B, Callaghan B, Rivera LR, Bravo DM, Furness JB (2015) Differences in hormone localisation patterns of K and L type enteroendocrine cells in the mouse and pig small intestine and colon. Cell Tissue Res 359:693–698
Deloose E, Janssen P, Depoortere I, Tack J (2012) The migrating motor complex: control mechanisms and its role in health and disease. Nat Rev Gastroenterol Hepatol 9:271–285
Deloose E, Vos R, Corsetti M, Depoortere I, Tack J (2015) Endogenous motilin, but not ghrelin plasma levels fluctuate in accordance with gastric phase III activity of the migrating motor complex in man. Neurogastroenterol Motil 27:63–71
Diwakarla S, Fothergill LJ, Fakhry J, Callaghan B, Furness JB (2017) Heterogeneity of enterochromaffin cells within the gastrointestinal tract. Neurogastroenterol Motil 29:e13101
Egerod KL, Engelstoft MS, Grunddal KV, Nøhr MK, Secher A, Sakata I, Pedersen J, Windeløv JA, Füchtbauer E-M, Olsen J, Sundler F, Christensen JP, Wierup N, Olsen JV, Holst JJ, Zigman JM, Poulsen SS, Schwartz TW (2012) A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153:5782–5795
Engelstoft MS, Egerod KL, Lund ML, Schwartz TW (2013) Enteroendocrine cell types revisited. Curr Opin Pharmacol 13:912–921
Fakhry J, Wang J, Martins P, Fothergill LJ, Hunne B, Prieur P, Shulkes A, Rehfeld JF, Callaghan B, Furness JB (2017) Distribution and characterisation of CCK containing enteroendocrine cells of the mouse small and large intestine. Cell Tissue Res 369:245–253
Fothergill LJ, Furness JB (2018) Diversity of enteroendocrine cells investigated at cellular and subcellular levels: the need for a new classification scheme. Histochem Cell Biol 150:693–702
Fothergill LJ, Callaghan B, Hunne B, Bravo DM, Furness JB (2017) Costorage of enteroendocrine hormones evaluated at the cell and subcellular levels in male mice. Endocrinology 158:2113–2123
Fothergill LJ, Ringuet MT, Sioras E, Hunne B, Fazio Coles TE, Martins P, Furness JB (2019) Cellular and sub-cellular localisation of oxyntomodulin-like immunoreactivity in enteroendocrine cells of human, mouse, pig, and rat. Cell Tissue Res 375:359–369
Furness JB, Hunne B, Matsuda N, Yin L, Russo D, Kato I, Fujimiya M, Patterson M, McLeod J, Andrews ZB, Bron R (2011) Investigation of the presence of ghrelin in the central nervous system of the rat and mouse. Neuroscience 193:1–9
Gershon MD (2013) 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Op Endoc Diab Obes 20:14–21
Glass LL, Calero-Nieto FJ, Jawaid W, Larraufie P, Kay RG, Göttgens B, Reimann F, Gribble FM (2017) Single-cell RNA-sequencing reveals a distinct population of proglucagon-expressing cells specific to the mouse upper small intestine. Mol Metab 6:1296–1303
Gribble FM, Reimann F (2016) Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol 78:277–299
Grunddal KV, Ratner CF, Svendsen B, Sommer F, Engelstoft MS, Madsen AN, Pedersen J, Nøhr MK, Egerod KL, Nawrocki AR, Kowalski T, Howard AD, Poulsen SS, Offermanns S, Bäckhed F, Holst JJ, Holst B, Schwartz TW (2015) Neurotensin is co-expressed, co-released and acts together with GLP-1 and PYY in enteroendocrine control of metabolism. Endocrinology 157:176–194
Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CAM, Parker HE, Morley TCE, Yeo GSH, Reimann F, Gribble FM (2012) Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 153:3054–3065
He J, Irwin DM, Chen R, Zhang YP (2010) Stepwise loss of motilin and its specific receptor genes in rodents. J Mol Endocrinol 44:37–44
Helander HF, Fändriks L (2012) The enteroendocrine “letter cells” – time for a new nomenclature? Scand J Gastroenterol 47:3–12
Hörsch D, Fink T, Göke B, Arnold R, Büchler M, Weihe E (1994) Distribution and chemical phenotypes of neuroendocrine cells in the human anal canal. Regul Pept 54:527–542
Kovacs TOG, Walsh JH, Maxwell V, Wong HC, Azuma T, Katt E (1989) Gastrin is a major mediator of the gastric phase of acid secretion in dogs: proof by monoclonal antibody neutralization. Gastroenterology 97:1406–1413
Levin F, Edholm T, Schmidt PT, Grybäck P, Jacobsson H, Degerblad M, Höybye C, Holst JJ, Rehfeld JF, Hellström PM, Näslund E (2006) Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab 91:3296–3302
Lo C-M, Obici S, Dong HH, Haas M, Lou D, Kim DH, Liu M, D’Alessio D, Woods SC, Tso P (2011) Impaired insulin secretion and enhanced insulin sensitivity in cholecystokinin-deficient mice. Diabetes 60:2000–2007
Lopez MJ, Upchurch BH, Rindi G, Leiter AB (1995) Studies in transgenic mice reveal potential relationships between secretin-producing cells and other endocrine cell types. J Biol Chem 270:885–891
Martin AM, Young RL, Leong L, Rogers GB, Spencer NJ, Jessup CF, Keating DJ (2017) The diverse metabolic roles of peripheral serotonin. Endocrinology 158:1049–1063
Martins P, Fakhry J, Chaves de Oliveira E, Hunne B, Fothergill LJ, Ringuet M, d’Ávila Reis D, Rehfeld JF, Callaghan B, Furness JB (2017) Analysis of enteroendocrine cell populations in the human colon. Cell Tissue Res 367:361–368
Mawe GM, Hoffman JM (2013) Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 10:473–486
Mortensen K, Christensen LL, Holst JJ, Orskov C (2003) GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept 114:189–196
Musson MC, Jepeal LI, Finnerty JR, Wolfe MM (2011) Evolutionary expression of glucose-dependent-insulinotropic polypeptide (GIP). Regul Pept 171:26–34
Patterson M, Murphy KG, le Roux CW, Ghatei MA, Bloom SR (2005) Characterization of ghrelin-like immunoreactivity in human plasma. J Clin Endocrinol Metab 90:2205–2211
Peeters TL, Muls E, Janssens J, Urbain JL, Bex M, Van Cutsem E, Bouillon R (1992) Effect of motilin on gastric emptying in patients with diabetic gastroparesis. Effect of motilin on gastric emptying in patients with diabetic gastroparesis. Gastroenterology 102:97–101
Reynaud Y, Fakhry J, Fothergill L, Callaghan B, Ringuet MT, Hunne B, Bravo DM, Furness JB (2016) The chemical coding of 5-hydroxytryptamine containing enteroendocrine cells in the mouse gastrointestinal tract. Cell Tissue Res 364:489–497
Roth KA, Gordon JI (1990) Spatial differentiation of the intestinal epithelium: analysis of enteroendocrine cells containing immunoreactive serotonin, secretin, and substance P in normal and transgenic mice. Proc Natl Acad Sci U S A 87:6408–6412
Säfsten B, Sjöblom M, Flemström G (2006) Serotonin increases protective duodenal bicarbonate secretion via enteric ganglia and a 5-HT4-dependent pathway. Scand J Gastroenterol 41:1279–1289
Sah RP, Nagpal SJS, Mukhopadhyay D, Chari ST (2013) New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol 10:423–433
Sanger GJ, Furness JB (2016) Ghrelin and motilin receptors as drug targets for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol 19:38–48
Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, Tache Y, Scachs G, Lambrecht NWG (2009) Identification and characterization of Nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology 150:232–238
Svendsen B, Pais R, Engelstoft MS, Milev NB, Richards P, Christiansen CB, Egerod KL, Jensen SM, Habib AM, Gribble FM, Schwartz TW, Reimann F, Holst JJ (2016) GLP1- and GIP-producing cells rarely overlap and differ by bombesin receptor-2 expression and responsiveness. J Endocrinol 228:39–48
Sykaras AG, Demenis C, Cheng L, Pisitkun T, Mclaughlin JT, Fenton RA, Smith CP (2014) Duodenal CCK cells from male mice express multiple hormones including ghrelin. Endocrinology 155:3339–3351
Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, Janssens J, Peeters T (2006) Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut 55:327–333
Tatemoto K (1982) Isolation and characterization of peptide YY (PYY), a candidate gut hormone that inhibits pancreatic exocrine secretion. Proc Natl Acad Sci U S A 79:2514–2518
Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM (2006) Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 290:E550–E559
Tuo B-G, Isenberg JI (2003) Effect of 5-hydroxytryptamine on duodenal mucosal bicarbonate secretion in mice. Gastroenterology 125:805–814
Wierup N, Björkqvist M, Westrӧm B, Pierzynowski S, Sundler F, Sjölund K (2007) Ghrelin and motilin are cosecreted from a prominent endocrine cell population in the small intestine. J Clin Endocrinol Metab 92:3573–3581
Yanaihara N, Yanaihara C, Nagai K, Sato H, Shimizu F, Yamaguchi K, Abe K (1980) Motilin-like immunoreactivity in porcine, canine, human and rat tissues. Biomed Res 1:76–83
Zhang Q, Zhu Y, Zhou W, Gao L, Yuan L, Han X (2013) Serotonin receptor 2C and insulin secretion. PLoS One 8:e54250
Acknowledgments
This work was supported by NIH (SPARC) grant ID # OT2OD023847 (PI Terry Powley) to JBF. We thank Josiane Fakhry for helpful comments on the manuscript. Confocal imaging was performed at the Biological Optical Microscopy Platform, University of Melbourne.
Funding
NIH (SPARC) grant ID # OT2OD023847 (PI Terry Powley) to JBF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed consent
Informed consent was obtained.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Procedures were approved by the Human Research Ethics Committee of Austin Health
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary figure 1
This figure shows the counts of EEC obtained in the villi and crypts of human jejunum. The numbers are similar in the two regions, except for ghrelin and secretin, which were fewer in the crypts. c = crypts, v = villi. (PNG 432 kb)
Rights and permissions
About this article
Cite this article
Fazio Coles, T.E., Fothergill, L.J., Hunne, B. et al. Quantitation and chemical coding of enteroendocrine cell populations in the human jejunum. Cell Tissue Res 379, 109–120 (2020). https://doi.org/10.1007/s00441-019-03099-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-019-03099-3