Abstract
We use a monoclonal antibody against the C-terminal of oxyntomodulin (OXM) to investigate enteroendocrine cells (EEC) in mouse, rat, human and pig. This antibody has cross-reactivity with the OXM precursor, glicentin (Gli) but does not recognise glucagon. The antibody stained EEC in the jejunum and colon of each species. We compared OXM/Gli immunoreactivity with that revealed by antibodies against structurally related peptides, GLP-1 and glucagon and against GIP and PYY that are predicted to be in some EEC that express OXM/Gli. We used super-resolution to locate immunoreactive vesicles. In the pancreas, OXM/Gli was in glucagon cells but was located in separate storage vesicles to glucagon. In jejunal EEC, OXM/Gli and GIP were in many of the same cells but often in separate vesicles, whereas PYY and OXM/Gli were commonly colocalised in the same storage vesicles of colonic EEC. When binding of anti-GLP-1 to the structurally related GIP was removed by absorption with GIP peptide, GLP-1 and OXM/Gli immunoreactivities were contained in the same population of EEC in the intestine. We conclude that anti-OXM/Gli is a more reliable marker than anti-GLP-1 for EEC expressing preproglucagon products. Storage vesicles that were immunoreactive for OXM/Gli were almost always immunoreactive for GLP-1. OXM concentrations, measured by ELISA, were highest in the distal ileum and colon. Lesser concentrations were found in more proximal parts of small intestine and pancreas. Very little was in the stomach. In EEC containing GIP and OXM/Gli, these hormones are packaged in different secretory vesicles. Separate packaging also occurred for OXM and glucagon, whereas OXM/Gli and PYY and OXM/Gli and GLP-1 were commonly contained together in secretory vesicles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The proglucagon gene (Gcg) product, preproglucagon, is the precursor to several circulating hormones that have important roles in glucose metabolism, including glucagon, GLP-1 and oxyntomodulin (OXM). OXM is a dual GLP-1 receptor and glucagon receptor agonist. This combination of actions has led to interest in oxyntomodulin mimetics being used to reduce food intake, by acting through both receptors, while offsetting the diabetogenic potential of glucagon receptor agonism by OXM action on the GLP-1 receptor (Pocai 2012; Pocai 2014; Holst et al. 2018). In addition to its effects on feeding and the pancreatic islets, oxyntomodulin decreases acid secretion when applied to isolated gastric glands and when infused in vivo.
Gcg transcripts are translated into preproglucagon, from which a 20-amino acid signal peptide is removed to form the 160 amino-acid proglucagon, which then undergoes tissue-specific processing in the pancreas, intestine and brain. The differential cleavage of proglucagon is thought to be due to selective activity of prohormone convertases (PC), with PC2 action resulting in the formation of glucagon, miniglucagon, glicentin-related polypeptide (GRPP) and a major proglucagon fragment (MPGF) in the pancreas, whereas PC1/3 produces glucagon-like peptide 1 (GLP-1), GLP-2, OXM and glicentin in the brain and intestine (Bataille 2007) (Fig. 1). However, the expression patterns of each individual proglucagon product have been difficult to study due to problems in producing antibodies specific for each peptide. OXM contains the same sequence as glucagon but with 8 more amino acids at the C-terminus and glicentin is identical to OXM at their common C-terminus but has an additional N-terminus region, 33 amino acids in length (Bataille 2007). Furthermore, GLP-1, GLP-2 and glucagon arose from exon duplication events and share a high degree of sequence homology (Lopez et al. 1984) and GIP appears to have been derived from a Gcg/Gip gene duplication (Mullen et al. 1992) and has many similarities to GLP-1 in particular (Fig. 1d). In the absence of tools to distinguish the peptides, it has generally been assumed that every proglucagon cell in the intestine produces the same set of proglucagon products, GLP-1, GLP-2, glicentin, GRPP and OXM and in the pancreas glucagon, MPGF and GRPP but not OXM or glicentin (Pocai 2012).
Proglucagon derived peptides: sequences, cleavage sites and sorting signals. Colour coding in a is followed in (b) and (c). a Full length proglucagon sequence. b Glicentin (N-terminal) domain of proglucagon. c Major proglucagon fragment (MPGF; C-terminal) domain of proglucagon. Intestinal and pancreatic products indicated above and below the sequence respectively (b, c). Amino acid position numbers above the start of each segment. Regions containing α-helical sequences underlined. GLP, glucagon-like peptide; GRPP, glicentin-related pancreatic polypeptide; IP, intervening peptide; CPE, carboxypeptidase-E; PC, prohomone convertase; NRDc, N-arginine dibasic convertase; ApB, aminopeptidase B. d Comparison of sequences of GLP-1 and GIP. Yellow indicates identical residues, blue indicates similar residues
Enteroendocrine cells (EEC) exhibit a substantial degree of complexity in their patterns of hormone expression, including many examples of hormone co-localisation (Egerod et al. 2012; Habib et al. 2012; Fothergill et al. 2017). Advances in super-resolution microscopy that enables investigation of the storage of hormones at a subcellular level has shown that many coexpressed hormones are stored in separate secretory vesicles. For example, in cells containing both GLP-1 and CCK in the mouse duodenum, only 20% of the subcellular area stained by GLP-1 and CCK was overlapping (Fothergill et al. 2017). Separate sub-cellular storage of GLP-1 and PYY was demonstrated in human, pig, rat and mouse intestinal tissue (Cho et al. 2014). Neurotensin has also been shown to be coexpressed but differentially stored in cells containing GLP-1 or PYY (Grunddal et al. 2015). On the other hand, GLP-1 and GLP-2 were co-stored in secretory vesicles in chicken EEC (Nishimura et al. 2013) and in human tissue alongside glicentin-like immunoreactivity (Varndell et al. 1985). Given the complexities in EEC expression patterns, it is possible that the storage of hormones at a subcellular level could differ in different subpopulations of cells.
Considering the limitations in producing specific antibodies against proglucagon-derived peptides, the localisation of OXM has not been explored in detail at either a cell or subcellular level. In this study, we use antibodies raised against the C-terminal of human OXM, a sequence in common with glicentin but not glucagon, GLP-1 or GLP-2. We also make comparisons with peptides from different genes, namely GIP and PYY, which are frequently coexpressed with GLP-1 and have therapeutic interest as GIP is an incretin and PYY promotes satiety; both effects in common with OXM.
Materials and methods
Tissue sources and preparation
All procedures were conducted according to the National Health and Medical Research Council of Australia guidelines and were approved by the University of Melbourne Animal Experimentation Ethics Committee (animal tissue), the Ethics and Research Committee of the Federal University of Minas Gerais (human colon) and the Human Research Ethics Committee (Austin Health, human jejunum). Human colon samples (male and female) were obtained from patients undergoing surgery to remove an adjacent region of colon because of trauma or cancer. Human jejunum was obtained from patients (2 male, 1 female) undergoing the Whipple’s procedure to remove cancerous or diseased pancreas. Male C57BL/6 mice aged 8–10 weeks and rats aged 5–9 weeks (male and female) were housed in the Biomedical Animal Facility at the University of Melbourne and were provided standard chow and water ad libitum. Female pigs were from the University of Melbourne School of Agriculture and Food. Mice and rats were anaesthetised with isoflurane and killed by decapitation while pigs were sedated with ketamine and killed by cardiac injection of pentobarbital sodium.
Tissues for immunohistochemistry were removed, opened along the mesenteric border and pinned mucosa up. Segments were washed with phosphate buffered saline (PBS; 0.15 mol L−1 NaCl in 0.01 mol L−1 sodium phosphate buffer, pH 7.2) and fixed at 4 °C overnight with Zamboni’s fixative (2% w/v formaldehyde and 0.2% w/v picric acid in 0.1 mol L−1 sodium phosphate buffer, pH 7.2). Tissues were washed three times with dimethyl sulfoxide and three times with PBS, before being stored in PBS-sucrose-azide (0.1% w/v sodium azide and 30% w/v sucrose in PBS) at 4 °C. Human jejunum was fixed with 4% paraformaldehyde as described previously (Cho et al. 2014). Samples were placed in PBS-sucrose-azide and OCT compound (Tissue Tek, Elkhart, IN, USA) in a 1:1 ratio before being embedded in 100% OCT and frozen in isopentane cooled with liquid nitrogen. Mouse intestinal and pancreatic tissues for ELISA were snap-frozen in liquid nitrogen and stored at − 80 °C. At least three animals or patient samples were used for all analyses in this study.
Immunohistochemistry
Sections of 8-μm thickness were cut and air dried for 1 h on SuperFrostPlus® microscope slides (Menzel-Glaser; Thermo Fisher, Scoresby, Vic, Australia). They were then covered with normal horse serum (10% v/v with triton-X in PBS) for 30 min at room temperature and incubated with mixtures of primary antibodies (Table 1) overnight at 4 °C. The preparations were then washed three times with PBS before a 1-h incubation with mixtures of secondary antibodies (Table 1) at room temperature. Sections were washed three times with dH2O and incubated with Hoechst 33258 solution (10 μg/mL Bisbenzimide-Blue in dH2O; Sigma) for 5 min. Slides were washed three times with distilled water before mounting coverslips with non-fluorescent mounting medium (Prolong diamond, Thermo Fisher). Fluorescent TetraSpeck™ microspheres (100 nm; Molecular Probes, Mulgrave, VIC, Australia) were also mounted onto slides with the same mounting media and coverslips for assessing chromatic aberration.
Monoclonal antibody producing cells were derived from a mouse immunised with the full human oxyntomodulin sequence (1-37). Clones were selected for their binding to OXM in an ELISA assay and the absence of detectable competition of binding of oxyntomodulin by related peptides, particularly the absence of glucagon binding, the glucagon sequence being fully included in the OXM amino acid sequence. The OXM preferring clones were further screened for effectiveness in an immunohistochemistry screen using mouse small intestine. The monoclonal antibody used in this study has no detectable binding to glucagon in immunohistochemistry studies (see Results).
The ability of oxyntomodulin (OXM) peptide to quench immunoreactivity was tested by pre-absorbing the OXM antibody (1:2000) with OXM peptide (0 μM, 0.1 μM, 1 μM, and 10 μM) overnight at 4 °C. The antibody-peptide mixture was used in place of the primary antibody in the methods for immunohistochemistry as detailed above. Similarly, the anti-GLP-1 antibody, 8912 (1:2000), was incubated with GIP (10−4 M) to quench interaction between the antibody and GIP. OXM and GIP antibodies were incubated overnight the day after tissue incubation with GLP-1 antibody ± GIP peptide.
Image acquisition and analysis
Fluorescence was visualised by high-resolution confocal microscopy (LSM880 and LSM800, Carl Zeiss, Sydney Australia). Quantification of the abundance and coexpression of GLP-1, OXM/Gli, PYY and GIP was analysed with Fiji (imagej.nih.gov/ij/) using tile scan images taken with a × 20 air objective. Cells were selected manually for each hormone and were considered positive if the fluorescence intensity was greater than 2 standard deviations above the average background intensity of the epithelium. Cell counts were standardised to epithelial surface area.
Super resolution z-stack images were taken on the LSM880 using the Airyscan mode with a × 63 oil objective and deconvolved using the Zen 3D Airyscan processing function (Zeiss, Sydney, Australia). Quantification of vesicle density and colocalisation was as described previously (Fothergill et al. 2017). Briefly, super-resolution images were corrected for chromatic aberration, as determined by imaging Tetraspeck fluorescent beads and images 0.3 μm thick were maximally projected using Fiji. Vesicles were detected by thresholding the images, segregating thresholded regions between local maxima and excluding regions smaller than 0.002 μm2 using an automated script in Fiji. When quantifying relative vesicle densities, a single threshold was chosen for each colour to exclude background fluorescence. In contrast, images were thresholded individually for each colour when assessing vesicle colocalisation to only select bright, distinct vesicles. Measurements were taken from Fiji and analysed using Prism 5.0 (GraphPad Software, San Diego, CA, USA). Data are presented as mean ± SEM. Vesicle densities less than 0.5 vesicles/μm2 were considered negative for that hormone when expressing percentage colocalisation.
ELISA
Samples of mouse corpus, duodenum, jejunum, distal ileum, proximal colon, distal colon and pancreas were sonicated in ice-cold lysis buffer (50 mM Tris buffer [pH 7.5], 5 mM EDTA, 1 mM DTT, 50 μM phenylmethylsulfonyl fluoride) and centrifuged at 14,000g for 15 min at 4 °C to remove tissue debris. Supernatants were collected and protein concentrations were determined using the Bradford assay. Five microliters of diluted samples and known concentrations of bovine serum albumin were loaded into a 96-well plate with 250 μL Quick Start™ Bradford 1× Dye Reagent (no. 5000205, Biorad) per well. Absorbance at 595 was measured.
The OXM ELISA kit (Anshlabs, Webster, Texas, USA) had no detectable binding of glucagon (1-29), GLP-1 (7-36), GLP-1 (9-36), GLP-2 (1-34), GRPP, MPGF-2, or insulin. Excess glicentin, 250 pg/mL, caused 5% displacement of OXM (1 pg/mL), indicating that the ELISA has low but detectable, affinity for glicentin. The assay was run according to manufacturer’s instructions, with the exception that tissues were diluted in lysis buffer not sample diluent. Optimal protein concentrations to load in the ELISA were determined by Bradford assay in preliminary tests. Known concentrations of OXM (3–290 pg/mL) were fitted with a polynomial cubic regression curve with a R2 value > 0.999. Control samples of OXM (18 pg/mL and 69 pg/mL) were used in each assay in duplicate and concentrations predicted by the standard curve were close to expected values (18.8 ± 0.4 pg/mL and 69.7 ± 1.1 pg/mL).
Results
Efficacy of oxyntomodulin (OXM/Gli) antibody across species
The OXM/Gli antibody stained EEC in mouse, rat, pig and human jejunum and colon (Fig. 2). Binding of the monoclonal OXM/Gli antibody (oxyntomodulin 323-AO010, Anshlabs) in mouse distal ileum was prevented by preincubating the primary antibody with 1-μM OXM peptide. In the small intestine, anti-GLP-1 also revealed the structurally related GIP and cells colocalised by both the GIP and GLP-1 antibodies but not revealed by the OXM/Gli antibody were observed (Fig. 3). When GIP peptide was used to compete with endogenous GIP for binding of anti-GLP-1, there were no longer GLP-1 immunoreactive cells that were OXM/Gli negative, almost all cells with OXM/Gli having GLP-1 immunoreactivity; some of these double-labelled EEC were also GIP immunoreactive in the small intestine. Double-labelling immunohistochemistry of OXM with GLP-1 revealed nearly complete colocalisation in colonic EEC (Fig. 4b, d), where cell counts were not confounded by anti-GLP-1 binding to GIP, because GIP cells were extremely few.
Examples of cells immunoreactive for GLP-1 (green; a, b, c, d) and OXM/Gli (magenta; a′, b′, c′, d′) in mouse (a), rat (b), pig (c) and human (d) colon. Cells immunoreactive for both hormones are indicated by an arrow. Colour merge images also show Hoechst nuclear staining in blue (a′′, b′′, c′′, d′′). Scale bars are 10 μm
Peptide absorption control. Cells immunoreactive for GLP-1 (a), GIP (a′′) and OXM/Gli (a′; arrow a) and cells positive for GLP-1 and GIP but not OXM/Gli were revealed (arrow b). When GLP-1 antibody was preabsorbed with 10−4 M GIP peptide (b), every GLP-1 positive cell was immunoreactive for OXM/Gli (b′; indicated by arrows). OXM/Gli and GIP antibodies were incubated overnight the day after tissue incubation with GLP-1 antibody ± GIP peptide. Scale bars are 40 μm
Quantitative assessment of hormone colocalization at a cell level. Colocalisation of OXM/Gli with GIP or PYY was investigated in mouse jejunum (a) and colon (b) and human jejunum (c) and colon (d) and expressed as cells per cm of mucosal surface length. Colocalisation of OXM/Gli, PYY and GLP-1 was assessed in the colon only. (e) The proportion of OXM/Gli cells immunoreactive for GIP, PYY and GLP-1 is also presented as a percentage of OXM/Gli immunoreactive cells. n = 3 mouse or human samples
Colocalisation of glucagon-gene products, OXM and GLP-1, compared to GIP and PYY at a cellular level in jejunum and colon from humans and mice
Quantification of OXM/Gli colocalisation with GIP and PYY in mouse and human samples of jejunum and distal colon and GLP-1 in the colon was determined at a cellular level using high-resolution tile scans in human and mouse samples of jejunum and distal colon. In mouse jejunum, 50 ± 11% of OXM/Gli cells were immunoreactive for GIP and 17 ± 7% were immunoreactive for PYY (Fig. 4a, e). Similarly, in human jejunum 40 ± 12% of OXM/Gli cells contained GIP and 15 ± 6% contained PYY (Fig. 4c, e). GIP was very rare in the mouse and human colon (0.5 ± 0.3 cells/cm and 1.4 ± 1.1 cells/cm), as was colocalisation with OXM/Gli (Fig. 4b, d). There was nearly a complete overlap of oxyntomodulin and GLP-1 in the colons of both species (Fig. 4b, d, e; 96 ± 2% OXM/Gli cells were GLP-1 immunoreactive in human and 98 ± 2% in mouse, pooled data). In mouse and human colon, the majority of PYY cells contained GLP-1 and OXM/Gli (Fig. 4 b, d, e); 95 ± 3% and 94 ± 3% of mouse and human colon PYY cells respectively).
Coexpression of OXM/Gli, GLP-1 and PYY at a subcellular level in human colon
GLP-1 cells in human colon were imaged using super-resolution Airyscan microscopy, without reference, when cells were initially chosen, to whether other hormones were also present. These images were analysed at a subcellular level to quantify relative vesicle abundance of PYY and OXM/Gli in GLP-1 cells from human colon (Fig. 5).
Relative vesicle abundance (vesicles/μm2) of OXM/Gli, GLP-1 and PYY in human colon. Cells were chosen for their GLP-1 immunoreactivity and OXM/Gli and GIP were determined after three colour imaging. a Sets of points connected by grey lines are results from the same cell. Scatterplots (b, d) compare vesicle densities (vesicles/μm2) of each pair of hormones. Linear regression lines are based only on cells containing both hormones (defined as greater than 0.5 vesicles/μm2, denoted by dotted line). n = 40 cells
All GLP-1 cells in the human colon contained OXM/Gli vesicles and 65% contained PYY vesicles (defined as cells containing greater than 0.5 vesicles/μm2). Vesicle densities were positively correlated between all combinations of GLP-1, OXM/Gli and PYY (Fig. 5b–d; r ≥ 0.5, n = 26–40, P < 0.01).
OXM expression in mouse tissues by ELISA
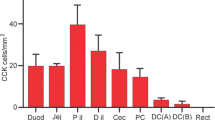
Extracts of mouse gastric corpus, duodenum, jejunum, distal ileum, proximal and distal colon and pancreas were assayed for OXM by ELISA and all regions showed some immunoreactivity (Fig. 6). The gastric corpus had the lowest concentration of OXM of all regions tested (17 ± 1 pg OXM per mg protein), corresponding to very rare OXM-containing cells identified with immunohistochemistry. OXM concentration generally increased distally throughout the intestine, with 276 ± 131 pg/mg in the duodenum and 5189 ± 434 pg/mg in the distal colon.
Concentrations of oxyntomodulin (OXM) determined by ELISA in mouse tissues. Extracts of gastric corpus, duodenum, jejunum, distal ileum, proximal and distal colon and pancreas were assayed for OXM immunoreactivity and results are shown as μg OXM per g protein. n = 3 mice for most regions, 4 mice for duodenum
Comparison of OXM/Gli and glucagon staining in the pancreas and intestine
Due to the detection of OXM using the ELISA kit, localisation of immunoreactivity was investigated in the pancreas with immunohistochemistry. OXM/Gli was fully colocalised with glucagon at a cell level in mouse pancreatic islets and not colocalised with insulin (Fig. 7a). In the intestine, glucagon immunoreactivity was extremely weak or absent in EEC that contained OXM/Gli (Fig. 7b, c), indicative of a low degree of anti-glucagon antibody cross reactivity with proglucagon products in the intestine and/or a very low level of glucagon storage. Low levels of glucagon have been reported in the gastrointestinal tract (Holst et al. 1983) and it has been recently shown that glucagon is released from extra-pancreatic tissues, most probably intestinal L cells, in patients after total pancreatectomy (Lund et al. 2016).
Examples of cells immunoreactive for insulin and glucagon colocalised with OXM/Gli (row a), oxyntomodulin cells with weak or no glucagon immunoreactivity in mouse jejunum (row b), and colon (row c). Arrows indicate cells immunoreactive for both oxyntomodulin (a′′, b, c) and glucagon (a′, b′, c′), although very weakly glucagon immunoreactive in (b′) and (c′). Colour merge images also show Hoechst nuclear staining in blue (a′′′, b′′, c′′)
Storage and co-storage of hormones in vesicles
Co-storage of hormones at a vesicular level was investigated in cells containing two or more hormones (i.e., greater than 0.5 vesicles/μm2) and assessed by the area of vesicular overlap. Examples of separate vesicular storage of OXM/Gli and GIP could be observed in jejunal EEC (Fig. 8a, d). In colonic EEC, there was a high level of vesicular overlap between GLP-1, OXM/Gli and PYY (Fig. 8c, f). Despite 100% overlap of OXM/Gli and glucagon at a cell level in pancreatic islets, immunoreactivity for the two proglucagon-derived products was infrequently colocalised at a vesicular level in α-cells (Fig. 8b, e). Strongly OXM/Gli-positive vesicles that lacked glucagon immunoreactivity often appeared as large donut-shaped structures (Fig. 8e arrow a) and were generally located deep with the cell cytoplasm. Electron microscope studies have found that α-cell vesicles contain an electron dense core, predominantly immunoreactive for glucagon, surrounded by a less electron dense halo containing glicentin-like immunoreactivity (Ravazzola and Orci 1980; Ali Rachedi et al. 1984; Varndell et al. 1985). It is possible that the donut-shaped OXM/Gli vesicles observed in α-cells correspond to the halo observed in the electron microscope studies.
Subcellular analysis of vesicular overlap in EECs from human jejunum (a), mouse pancreas (b) and human colon (c). Non-overlapping and colocalized areas are displayed as a percentage of the total area occupied by vesicles (a–c). Cells were analysed if colocalised for both hormones at a cell level and the numbers of cells chosen is shown above each group of columns. Example of a region from a jejunal EEC containing GIP (d) and OXM/Gli (d′). Arrow a shows a vesicle containing OXM/Gli only, arrow b shows a GIP-predominant vesicle. Pancreatic α-cell containing OXM/Gli (e′; arrow a) and glucagon (e; arrow b) in separate vesicles. Region of a colonic EEC containing PYY (f), OXM/Gli (f′) and GLP-1 (f′′) with arrows indicating that all three hormones were colocalised in each vesicle. Scale bars are 1 μm
Discussion
The anti-oxyntomodulin/glicentin (OXM/Gli) antibody used in the present study effectively stained mouse, rat, pig and human EEC in both small and large intestinal tissue. The antibody was directed against the OXM C-terminal and our observations in pancreas, where it did not recognise glucagon, confirm its specificity for the OXM/glicentin C-terminal. OXM/Gli and GLP-1, products of PC1/3 processing of proglucagon, were almost always colocalised within colonic EEC. The overlap between OXM/Gli, GIP and PYY was characterised in mouse and human. GIP cells were more abundant in the jejunum and PYY cells were more abundant in the colon, consistent with the literature (Jorsal et al. 2018).
In human colon, GLP-1, PYY and OXM/Gli were generally colocalised at both a cell and subcellular level and the vesicle abundance of these hormones was positively correlated. Similarly, Varndell et al. (1985) showed with electron microscopy that GLP-1, GLP-2 and glicentin immunoreactivity were colocalised within electron dense cores of secretory vesicles in human colon. When assessed at a super-resolution level, there was a very low level of vesicular overlap between GIP and OXM/Gli in cells that contained both peptides. It is possible that the segregation of GIP and OXM/Gli into separate vesicles is not absolute and that in some vesicles, one of the peptides was present at a level below the detection threshold.
OXM has overlapping and differential effects when compared to GLP-1, GIP and PYY. OXM, GLP-1 and GIP all promote glucose-stimulated insulin release (the incretin effect) (Holst et al. 2018), while OXM and PYY additively reduced food intake in overweight and obese humans (Field et al. 2010). A specific OXM receptor has yet to be identified and may not exist; however, OXM acts at the GLP-1 receptor (GLP-1R) with about one fortieth the potency of GLP-1 and at the glucagon receptor (GCGR), where it is about 14 times less potent (Henderson et al. 2016). OXM is a full agonist at the GLP-1 receptor in activating cAMP in parietal cells (Gros et al. 1995); however, using a BRET assay, Jorgensen et al. (2007) demonstrated that OXM is a partial agonist in recruiting β-arrestin and G protein-coupled receptor kinase 2 to the GLP-1R. The authors suggest that the biased signalling of OXM at the GLP-1R could result in differential physiological effects compared to GLP-1. The complex patterns of overlap between GLP-1, OXM, GIP and PYY may allow for a tailored response to environmental stimuli in the GI tract.
The OXM ELISA kit revealed little or no immunoreactivity in extracts of gastric corpus, consistent with rare OXM/Gli positive EEC being found using immunohistochemistry. OXM concentrations increased distally throughout the mouse intestine. This mirrors recent work by Wewer Albrechtsen et al. (2016) who assessed GLP-1, GLP-2 and C-terminal glicentin/OXM by RIA and found that tissue concentrations increase distally in the mouse intestine. Interestingly, we detected OXM-like immunoreactivity by ELISA in the mouse pancreas and localised it to glucagon cells. OXM and/or glicentin are not conventionally considered products of α-cells in the pancreas; however, they are intermediaries in the production of glucagon (Bataille 2007) and OXM has been observed in a pancreatic islet α-cell line using mass spectrometry (Buchanan et al. 2007). The present immunohistochemistry study revealed OXM and/or glicentin in glucagon-containing α-cells in pancreatic islets, in separate vesicular stores to glucagon.
General conclusions
The traditional nomenclature, which states that K cells contain GIP and L cells contain GLP-1, oxyntomodulin, glicentin and PYY, is incorrect. Separate vesicular storage of several other EEC hormones has been shown previously; however, in the present work, OXM/Gli immunoreactivity generally coincided with GLP-1 and/or PYY at a subcellular level. By contrast, when OXM/Gli and GIP were both present in a cell, they rarely overlapped at the level of vesicular stores. This raises the possibility that proglucagon-derived peptides and GIP could be differentially released from individual cells, as well as from separate populations of cells.
References
Ali Rachedi A, Varndell IM, Adrian TE, Gapp DA, Van Noorden S, Bloom SR, Polak JM (1984) Peptide YY PYY immunoreactivity is co-stored with glucagon related immunoreactants in endocrine cells of the gut and pancreas. Histochemistry 80:487–491
Bataille D (2007) Pro-protein convertases in intermediary metabolism: islet hormones, brain/gut hormones and integrated physiology. J Mol Med 85:673–684
Buchanan CM, Malik AS, Cooper GJS (2007) Direct visualisation of peptide hormones in cultured pancreatic islet alpha- and beta-cells by intact-cell mass spectrometry. Rapid Commun Mass Spectrom 21:3452–3458
Cho H-J, Robinson ES, Rivera LR, McMillan PJ, Testro A, Nikfarjam M, Bravo DM, Furness JB (2014) Glucagon-like peptide 1 and peptide YY are in separate storage organelles in enteroendocrine cells. Cell Tissue Res 357:63–69
Egerod KL, Engelstoft MS, Grunddal KV et al (2012) A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153:5782–5795
Field BCT, Wren AM, Peters V, Baynes KCR, Martin NM, Patterson M, Alsaraf S, Amber V, Wynne K, Ghatei MA, Bloom SR (2010) PYY3–36 and oxyntomodulin can be additive in their effect on food intake in overweight and obese humans. Diabetes 59:1635–1639
Fothergill LJ, Callaghan B, Hunne B, Bravo DM, Furness JB (2017) Costorage of enteroendocrine hormones evaluated at the cell and subcellular levels in male mice. Endocrinology 158:2113–2123
Gros L, Hollande F, Thorens B, Kervran A, Bataille D (1995) Comparative effects of GLP-1-(7-36) amide, oxyntomodulin and glucagon on rabbit gastric parietal cell function. Eur J Pharmacol Mol Pharmacol 228:319–327
Grunddal KV, Ratner CF, Svendsen B et al (2015) Neurotensin is co-expressed, co-released and acts together with GLP-1 and PYY in enteroendocrine control of metabolism. Endocrinology 157:176–194
Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CAM, Parker HE, Morley TCE, Yeo GSH, Reimann F, Gribble FM (2012) Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 153:3054–3065
Henderson SJ, Konkar A, Hornigold DC et al (2016) Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes Metab 18:1176–1190
Holst JJ, Aggestrup S, Loud FB, Olesen M (1983) Content and gel filtration profiles of glucagon-like and somatostatin-like immunoreactivity in human fundic mucosa. J Clin Endocrinol Metab 56:729–732
Holst JJ, Albrechtsen NJW, Gabe MBN, Rosenkilde MM (2018) Oxyntomodulin: actions and role in diabetes. Peptides 100:48–53
Jorgensen R, Kubale V, Vrecl M, Schwartz TW, Elling CE (2007) Oxyntomodulin differentially affects glucagon-like peptide-1 receptor β-arrestin recruitment and signaling through Gαs. J Pharmacol Exp Ther 322:148–154
Jorsal T, Rhee NA, Pedersen J et al (2018) Enteroendocrine K and L cells in healthy and type 2 diabetic individuals. Diabetologia 61:284–294
Lopez LC, Li W-H, Frazier ML, Luo C-C, Saunders GF (1984) Evolution of glucagon genes. Mol Biol Evol 1:335–344
Lund A, Bagger JI, Albrechtsen NJW et al (2016) Evidence of extrapancreatic glucagon secretion in man. Diabetes 65:585–597
Mullen RJ, Buck CR, Smith AM (1992) NeuN, a neuronal specific nuclear protein in vertebrates. Development 116:201–211
Nishimura K, Hiramatsu K, Monir MM, Takemoto C, Watanabe T (2013) Ultrastructural study on colocalization of glucagon-like peptide (GLP)-1 with GLP-2 in chicken intestinal L-cells. J Vet Med Sci 75:1335–1339
Pocai A (2012) Unraveling oxyntomodulin, GLP1’s enigmatic brother. J Endocrinol 215:335–346
Pocai A (2014) Action and therapeutic potential of oxyntomodulin. Mol Metab 3:241–251
Ravazzola M, Orci L (1980) Glucagon and glicentin immunoreactivity are topologically segregated in the α granule of the human pancreatic α cell. Nature 284:66–67
Varndell IM, Bishop AE, Sikri KL, Uttenthal LO, Bloom SR, Polak JM (1985) Localization of glucagon-like peptide (GLP) immunoreactants in human gut and pancreas using light and electron microscopic immunocytochemistry. J Histochem Cytochem 33:1080–1086
Wewer Albrechtsen NJ, Kuhre RE, Toräng S, Holst JJ (2016) The intestinal distribution pattern of appetite and glucose regulatory peptides in mice, rats and pigs. BMC Res Notes 9:60
Acknowledgements
Insulin and glucagon antibodies were kindly provided by Prof Len Harrison. Confocal imaging was performed at the Biological Optical Microscopy Platform (BOMP), The University of Melbourne (www.microscopy.unimelb.edu.au). Ellie Cho (applications specialist, BOMP) provided valuable advice on experimental design and image analysis.
Funding
This work was supported by an Australian Government Research Training Program Scholarship to LJF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fothergill, L.J., Ringuet, M.T., Sioras, E. et al. Cellular and sub-cellular localisation of oxyntomodulin-like immunoreactivity in enteroendocrine cells of human, mouse, pig and rat. Cell Tissue Res 375, 359–369 (2019). https://doi.org/10.1007/s00441-018-2921-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2921-z