Abstract
Recent studies have shown that patterns of colocalisation of hormones in enteroendocrine cells are more complex than previously appreciated and that the patterns differ substantially between species. In this study, the human sigmoid colon is investigated by immunohistochemistry for the presence of gastrointestinal hormones and their colocalisation. The segments of colon were distant from the pathology that led to colectomy and appeared structurally normal. Only four hormones, 5-hydroxytryptamine (5-HT), glucagon-like peptide 1 (GLP-1), peptide YY (PYY) and somatostatin, were common in enteroendocrine cells of the human colon. Cholecystokinin, present in the colon of some species, was absent, as were glucose-dependent insulinotropic peptide, ghrelin and motilin. Neurotensin cells were extremely rare. The most numerous cells were 5-HT cells, some of which also contained PYY or somatostatin and very rarely GLP-1. Almost all GLP-1 cells contained PYY. It is concluded that enteroendocrine cells of the human colon, like those of other regions and species, exhibit overlapping patterns of hormone colocalisation and that the hormones and their patterns of expression differ between human and other species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent re-evaluations of enteroendocrine cell (EEC) populations in experimental animals have shown that the one hormone, one cell dogma, which has tended to dominate descriptions of EEC, is not correct and that there are in fact extensive and in some cases quite complex, patterns of colocalisation of gut hormones in EEC (Helander and Fändriks 2012; Gribble and Reimann 2015). For example, when cells expressing a reporter transgene under CCK promotor control in mice are isolated and molecularly analysed, it is found that CCK gene transcripts are co-expressed with secretin, GIP, GLP-1, PYY and neurotensin transcripts and that co-expression of the peptide hormones is confirmed by mass spectrometry and immunohistochemistry (Egerod et al. 2012). Isolation of GIP-expressing and GLP-expressing EEC and correlated immunohistochemical analysis, also in mice, confirms overlaps in expression of GIP, GLP-1, CCK, PYY and secretin (Habib et al. 2012). Quantitative immunohistochemical analysis of colocalisation of the K cell marker, GIP and the L cell markers, GLP-1 and PYY in the mouse and pig gastrointestinal tract, shows that all possible combinations of these three hormones occur in EEC and that there are considerable regional differences between these species (Cho et al. 2015). An example of regional difference is that about 50 % of K/L cells in the pig duodenum express PYY or PYY plus GLP-1, whereas no duodenal EEC express PYY in the mouse (Mortensen et al. 2003; Cho et al. 2015).
In human colon, there are reports of somatostatin-containing EEC (Alumets et al. 1977), PYY-containing EEC, which were also immunoreactive for GLP, identified as glicentin in these early papers (Böttcher et al. 1984, 1986) and 5-HT-containing EEC (Buffa et al. 1978). Neurotensin-containing EEC are rare (Helmstaedter et al. 1977; Sundler et al. 1977; Buffa et al. 1978) and secretin immunoreactive cells appear to be absent (Buffa et al. 1978). Cholecystokinin is found in numerous cells of the human duodenum and jejunum (Rehfeld 1978) but only rarely in the human colon (Polak et al. 1975). Interestingly, CCK cells appear to be common in the mouse colon (Egerod et al. 2012). Furthermore, microarray analysis has shown that a proportion of GLP-expressing cells in the mouse colon also express the gene for CCK (Habib et al. 2012).
In order to determine the patterns of hormone coexpression in human colon, we investigate colocalisation of the major human colonic EEC hormones, GLP, PYY, 5-HT and somatostatin and also investigate hormones that have been reported in small populations of human colonic EEC, neurotensin and CCK. GIP was investigated because of its frequent colocalisation with GLP (Mortensen et al. 2003) and its presence in a small proportion of colonic EEC in pig and mouse (Cho et al. 2015). Ghrelin and motilin were also investigated, as mRNA for ghrelin was detected by qPCR in the left and right human colon (Gnanapavan et al. 2002) and motilin receptor expression is detected in human colonic smooth muscle and mucosa by immunohistochemistry and PCR (Ter Beek et al. 2008). Low levels of ghrelin were previously detected by radioimmunoassay and sparse ghrelin immunopositive cells were reported in rat large intestine (Date et al. 2000).
Materials and methods
Sources and preparation of tissues
All resected tissue was obtained from surgical procedures at the Medical School of the Federal University of Goiás, Brazil. The study was approved by the Ethics and Research Committee of the Federal University of Minas Gerais, number: 04939212.9.0000.5149. Informed consent was obtained from the patients before tissue procurement. The samples were collected/ fixed in Goiás and sent to Melbourne University.
Samples were obtained from normal sigmoid colon that was removed from 4 patients in whom an adjacent region of colon was removed because of trauma or cancer. The segments were cleaned of contents, opened along the mesenteric attachment and pinned, mucosa up without stretching, to balsa wood sheets in ice-cold phosphate-buffered saline (PBS; 0.15 M NaCl in 0.01 M sodium phosphate buffer, pH 7.2). The tissue was then placed in fixative (2 % formaldehyde plus 0.2 % picric acid in 0.1 M sodium phosphate buffer, pH 7.2) overnight at 4 °C. The following day, tissues were cleared 3 times (10 min) in dimethyl sulfoxide (DMSO) and then washed 3 times (10 min) in PBS. Tissue was transferred to PBS-sucrose-azide (PBS containing 0.1 % sodium azide and 30 % sucrose as a cryoprotectant). It was stored in this solution for up to a month. Human jejunum was obtained from the Department of Surgery at Austin Hospital with informed consent (Cho et al. 2014b).
Immunohistochemistry
Tissue samples were placed in PBS-sucrose azide and OCT compound (Tissue Tek, Elkhart, IN, USA) in a ratio of 1:1 for a further 24 h before being trimmed and embedded in 100 % OCT and frozen in isopentane cooled with liquid nitrogen. Sections of 12 μm thickness were cut, air-dried for 1 h on microscope slides (SuperFrostPlus®; Menzel-Glaser #1.5; Thermo Fisher, Scoresby, Vic, Australia) and incubated with 10 % normal horse serum for 30 min. Sections were then incubated with mixtures of primary antibodies (Table 1) for double or triple staining at 4 °C overnight. Tissue was washed three times in PBS and incubated in secondary antibody (Table 2) for 1 h at room temperature. For staining nuclei, preparations were washed once with PBS then twice with distilled water and incubated for 5 min in Hoechst 33258 solution (Bisbenzimide–Blue, diluted to 10 μg/mL in distilled water) and then washed 3 times with distilled water before mounting with fluorescent mounting medium (Dako, Carpinteria, CA, USA).
Detection of CCK gene expression
Total RNA was extracted from the mucosa of fixed human colon and duodenum samples using the RNeasy FFPE kit (Qiagen, Melbourne, Australia) and 1 μg total RNA was reverse transcribed into cDNA using iScript (BioRad, Gladesville, NSW, Australia). Human CCK mRNA was amplified by polymerase chain reaction (PCR) using 5PRIME MasterMix (5PRIME, Hamburg, Germany) with intron-spanning primers. The forward primer for human CCK (accession no. NM 000794.8) was TATCGCAGAGAACGGATG and the reverse primer was AGGTTCTTAACGATGGACAT to produce a 111-bp product (van der Wielen et al. 2014). The primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which were used as a positive control, were forward CCTGCACCACCAACTGCTTAGC and the reverse GTGTCGCTGTTGAAGTCAGAGG producing a band of 418 bp. PCR amplification of CCK was performed with an initial step of 95 °C for 5 min, followed by 15 cycles of 95 °C for 40 s, annealing temperatures of 51 °C for 30 s and 72 °C for 30 s for extension. This step was followed by a final extension at 72 °C for 10 min. Reactions were also conducted without reverse transcriptase, which confirmed the absence of genomic DNA.
Image analysis
Slides were examined using an AxioImager microscope (Zeiss, Sydney, Australia) and high-resolution confocal microscopy (Zeiss Meta510 laser scanning confocal microscope and Zeiss LSM800 Airyscan). For quantitative analysis, images were captured using a V-Slide fluorescent slide scanner (Zeiss). Images were exported and analysed off-line using ImageJ (imagej.nih.gov/ij/). For quantitation of immunoreactive cells, counts were made from 3 sections from each sample and expressed as numbers of cells per 100 crypts. This analysis was repeated in samples from 3 patients. Images were imported into CorelDraw (Corel, Ottowa, Canada) for final preparation of figures.
Statistical analysis
Data were analysed using GraphPad Prism 5.0 (Graph-Pad Software, San Diego, CA, USA) and presented as mean ± SEM. Differences were evaluated with 2-tailed Students’ t tests.
Results and discussion
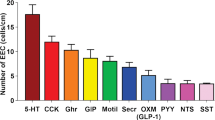
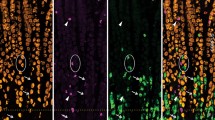
The most numerous EEC in the human colon were those immunoreactive for GLP-1, 5-HT, PYY and/or somatostatin (Fig. 1). Relative numbers were: GLP-1, 23 cells/100 crypts; 5-HT, 56 cells/100 crypts; PYY 29 cells/100 crypts; and somatostatin, 5 cells/100 crypts (Fig. 1). Neurotensin immunoreactive cells were strongly fluorescent when they were found but were very rare, fewer than 1 neurotensin cell per 100 crypts (Fig 2). In view of EEC with CCK-like immunoreactivity having been reported in the human colon (Polak et al. 1975) and CCK-expressing EEC being common in the mouse colon (Egerod et al. 2012), we used 4 different antibodies to investigate CCK presence in the human sigmoid colon. Each of these antibodies revealed EEC in the human jejunum but none revealed EEC in any of the samples of colon (Fig. 2). To further investigate CCK expression, we extracted RNA from colon mucosa and the mucosa of the human jejunum. PCR failed to amplify any cck transcripts in colon samples from 3 individuals. GAPDH was used as a positive control in these samples and cck expression was detected in fixed human jejunum samples (data not shown). GIP, ghrelin and motilin positive cells were not detected in the human distal colon, although they were present in the human jejunum.
Examples of single labeling of enteroendocrine cells in the human jejunum (JEJ) and colon (COL). Neurotensin (NT) immunoreactive EEC cells (a, a′) were rare and cholecystokinin immunoreactive cells could not be found in the human sigmoid colon. Both cell types were common in the human jejunum, which served as a positive control. Antibodies against the non-sulphated form of CCK (NS CCK; b, b′) and the biologically active, sulphated form (CCK; c, c′) failed to reveal cells in the colon. Two other anti-CCK antibodies, which also reveal gastrin, also failed to reveal EEC in the colon (see text). Scale bars 20 μm
Double staining for GLP-1 and PYY showed that over 95 % of GLP-1 immunoreactive EEC were also immunoreactive for PYY (Fig. 3). About 75 % of PYY cells contained GLP-1 immunoreactivity (21.7 ± 4.0 cells/100 glands, n = 4). About 14 % of 5-HT cells were immunreactive for PYY (7.5 ± 1.5 cells/100 glands, n = 4), whereas there was very rare colocalisation of 5-HT and GLP-1; fewer than 1 % of 5-HT cells were immunoreactive for GLP-1 and GLP-1 immunoreactivity in these cells was weak (Fig. 3c). From this, it can be deduced that there are two populations of EEC containing PYY: GLP-1/PYY and 5-HT/PYY cells. The population of somatostatin cells was smaller than the other cell types and about one-third of these were 5-HT immunoreactive (Figs. 3d, 4c). Somatostatin immunoreactive cells were not immunoreactive for either GLP-1 or PYY.
Double labeling of enteroendocrine cells in the human colon. Cells that were labeled for only one hormone are indicated by arrows and those immunoreactive for both by arrows with asterisks. Most GLP-1 immunoreactive cells (a) showed colocalisation with PYY, although there was a significant population of cells that were immunoreactive for PYY (a′) without GLP-1. The merged image is shown in a″. 5-HT (b) with PYY (b′) and merged in (b″). In almost all cases, 5-HT was not colocalised with GLP-1; in rare cases (arrows with asterisks) a 5-HT cell was faintly GLP-1 immunoreactive (c, c′, c″). 5-HT positive cells were more numerous than somatostatin (SOM) cells and about half of the somatostatin cells were 5-HT immunoreactive (d, d′, d″). Scale bars 20 μm
Quantitative assessment of colocalisation of hormones in enteroendocrine cell populations in human colon. a GLP-1 and PYY: 95 % of GLP-1 cells were PYY immunoreactive and about 75 % of PYY cells contained GLP-1. b PYY and 5-HT: 14 % of 5-HT cells were immunoreactive for PYY, 30 % of PYY cells were immunoreactive for 5-HT. c Of somatostatin cells, 62 % contained somatostatin alone and 38 % contained somatostatin and 5-HT. Data are from analysis of 3 sections in tissue samples from each of 4 individuals. /- indicates EEC that were immunoreactive for the indicated hormone without any of the other hormones investigated
The results indicate that more enteroendocrine cells in the human colon are immunoreactive for 5-HT than for any other enteric hormone; these were about 56 cells/100 crypts. About 14 % of 5-HT cells were PYY but not GLP-1 immunoreactive and a small number was immunoreactive for somatostatin. About 75 % of PYY cells were GLP-1 positive but not 5-HT immunoreactive and almost all GLP-1 positive EEC were also PYY positive. From these data, the percentages of EEC represented by the largest populations were 5-HT only, 57 %; GLP-1/PYY, 27 %; 5-HT/PYY, 9 % and SOM ± 5-HT, 6 %.
There are some interesting differences between species, for example CCK cells are found in mouse colon (Egerod et al. 2012) and GIP is found in small numbers of colonic EEC in both mice and pigs (Cho et al. 2015). Neither was found in enteroendocrine cells in the human sigmoid colon. Moreover, the proportional overlaps of GLP-1 and PYY are quite different in the colon across the three species: in mouse around 15 % of distal colon EEC contain GLP-1 alone and ∼5 % PYY alone, in pig colon 40 % contain GLP-1 alone and 7 % PYY alone (Cho et al. 2015), whereas in this study in the human distal colon we found that fewer than 1 % of EEC with GLP-1 and/or PYY contain GLP-1 alone and 25 % contain PYY without GLP-1.
5-HT, the hormone in the greatest number of cells, was found in three chemically distinct populations; those also containing PYY, those with somatostatin and those in which neither PYY nor somatostatin occurred. It is feasible that the subtypes of 5-HT cells have different functions. 5-HT differs from other enteroendocrine cell hormones in that it is a biogenic amine, not a peptide. In endocrine cells, its synthesis is regulated by tryptophan hydroxylase 1 (Tph1) whereas neuronal 5-HT synthesis requires Tph2 (Walther et al. 2003). Tph1 knock-out mice lacking EEC-derived 5-HT have significantly less weight gain, lower adiposity and lower expression of markers of adipose tissue inflammation when challenged with a high fat diet compared to wild-type mice (Crane et al. 2015). 5-HT stimulates movements of the colon and can initiate propulsion (Heredia et al. 2013). Moreover, in the tph-1 knock-out mouse, mucosal stimuli failed to elicit propulsive reflexes, although propulsive contractions could be elicited by distending the colon (Heredia et al. 2013). Thus, 5-HT released from EEC has a role in controlling motility and drugs acting on 5-HT receptors have been used to treat motility disorders (Andresen et al. 2008; Shin et al. 2014). 5-HT also activates nerve pathways that increase water and electrolyte secretion (Cooke et al. 1997). By contrast, PYY is an inhibitor of water and electrolyte secretion, both in human and mouse (Cox and Tough 2002; Hyland et al. 2003). Thus, it might be speculated that 5-HT/PYY EEC are involved in controlling functions other than secretion, for example motility, in which case effects of 5-HT on secretion may be neutralised by the co-release of PYY.
5-HT may also have other roles in the intestine, for example amplifying inflammation and stimulating growth and proliferation of enteric neurons (Liu et al. 2009; Margolis et al. 2014; Crane et al. 2015). In contrast to 5-HT, somatostatin is a potent anti-inflammatory agent (Helyes et al. 2001; Pintér et al. 2006). Thus, like the 5-HT/PYY cells, the 5-HT/somatostatin cells contain hormones with opposing actions at one of their possible sites of action. Therefore it might be suggested that activation of EEC containing 5-HT without somatostatin may be pro-inflammatory, whereas when 5-HT/somatostatin cells are activated the pro-inflammatory effect of 5-HT might be curtailed and other effects may be accentuated. Interestingly, somatostatin inhibited 5-HT secretion from human EC carcinoid cell lines in vitro (Kidd et al. 2008), which, if this effect occurs in the normal intestine, might also curtail the pro-inflammatory effect of 5-HT.
It is notable that the α-subunit of the taste-specific G protein, gustducin (Gαgust), is expressed by enteroendocrine cells that contain PYY or GLP-1 but not serotonin in human colon (Rozengurt et al. 2006), suggesting that nutrient-derived stimuli contribute to release of PYY or GLP-1 via nutrient receptors, whereas 5-HT is released by a range of mechanical stimuli (Grider et al. 1996; Bertrand 2006) and perhaps by inflammation.
Several studies suggest that there is a major developmental lineage for enteroendocrine differentiation in the mouse that leads to cells that express GLP-1 and 2, PYY, GIP, CCK, secretin and neurotensin and that other lineages exist, including one leading to EEC that express serotonin and substance P (Roth et al. 1992; Egerod et al. 2012; Habib et al. 2012). Based on their own studies and the literature, Egerod et al. (2012) proposed that there are 4 EEC lineages, one leading to the production of CCK, GLP-1, GIP, PYY, neurotensin and secretin, one leading to somatostatin-containing EEC, one to 5-HT and substance P EEC and one to ghrelin/motilin cells. Consistent with Egerod et al. (2012), Habib et al. (2012) also reported that GLP-1, GIP, CCK and secretin were in the same EEC cell lineage. They also reported that some cells of the CCK, GIP, GLP-1, PYY lineage express somatostatin. Our study found overlap of somatostatin and 5-HT but not somatostatin and CCK, GLP-1 or PYY in human colon. We also observed co-storage of 5-HT and PYY. Thus, there appear to be subtle differences between mouse and human in EEC lineages.
In conclusion, while the human sigmoid colon shows some similarities with other species in regard to the hormones produced and their co-storage, differences also occur. 5-HT represents the largest EEC population and colocalises with PYY and somatostatin but not with GLP-1. CCK is absent from EEC in the human colon in contrast to its presence in EEC of the mouse colon. The noticeable overlap seen between the two main hormones PYY and 5-HT in the colon raises questions regarding the role they play in health and possibly in pathology.
References
Agersnap M, Rehfeld JF (2014) Measurement of nonsulfated cholecystokinins. Scand J Clin Lab Invest 74:424–431
Alumets J, Sundler F, Håkanson R (1977) Distribution, ontogeny and ultrastructure of somatostatin immunoreactive cells in the pancreas and gut. Cell Tissue Res 185:465–479
Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M (2008) Effects of 5-hydroxytryptamine (Serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol 6:545–555
Bertrand PP (2006) Real-time measurement of serotonin release and motility in guinea pig ileum. J Physiol Lond 577(2):689–704
Böttcher G, Sjölund K, Ekblad E, Håkanson R, Schwartz TW, Sundler F (1984) Coexistence of peptide YY and glicentin immunoreactivity in endocrine cells of the gut. Regul Pept 8:261–266
Böttcher G, Alumets J, Håkanson R, Sundler F (1986) Co-existence of glicentin and peptide YY in colorectal L-cells in cat and man. An electron microscopic study. Regul Pept 13:283–291
Buffa R, Capella C, Fontana P, Usellini L, Solcia E (1978) Types of endocrine cells in the human colon and rectum. Cell Tissue Res 192:227–240
Cho H-J, Callaghan B, Bron R, Bravo DM, Furness JB (2014a) Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell Tissue Res 356:77–82
Cho H-J, Robinson ES, Rivera LR, McMillan PJ, Testro A, Nikfarjam M, Bravo DM, Furness JB (2014b) Glucagon-like peptide 1 and peptide YY are in separate storage organelles in enteroendocrine cells. Cell Tissue Res 357:63–69
Cho H-J, Kosari S, Hunne B, Callaghan B, Rivera LR, Bravo DM, Furness JB (2015) Differences in hormone localisation patterns of K and L type enteroendocrine cells in the mouse and pig small intestine and colon. Cell Tissue Res 359:693–698
Cooke HJ, Sidhu M, Wang YZ (1997) 5-HT activates neural reflexes regulating secretion in the guinea-pig colon. Neurogastroenterol Motil 9:181–186
Costa M, Furness JB (1984) Somatostatin is present in a subpopulation of noradrenergic nerve fibres supplying the intestine. Neuroscience 13:911–919
Cox HM, Tough IR (2002) Neuropeptide Y, Y1, Y2 and Y4 receptors mediate Y agonist responses in isolated human colon mucosa. Br J Pharmacol 135:1505–1512
Crane JD, Palanivel R, Mottillo EP, Bujak AL, Wang H, Ford RJ, Collins A, Blümer RM, Fullerton MD, Yabut JM (2015) Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med 21:166–172
Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M (2000) Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141:4255–4261
Egerod KL, Engelstoft MS, Grunddal KV et al (2012) A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153:5782–5795
Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M (2002) The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 87:2988–2991
Gribble FM, Reimann F (2015) Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol 78:3.1–3.23
Grider JR, Kuemmerle JF, Jin JG (1996) 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am J Physiol 270:G778–G782
Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CAM, Parker HE, Morley TCE, Yeo GSH, Reimann F, Gribble FM (2012) Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 153:3054–3065
Helander HF, Fändriks L (2012) The enteroendocrine “letter cells” – time for a new nomenclature? Scand J Gastroenterol 47:3–12
Helmstaedter V, Taugner C, Feurle GE, Forssmann WG (1977) Localization of neurotensin-immunoreactive cells in the small intestine of man and various mammals. Histochemistry 3:35–41
Helyes Z, Pintér E, Németh J, Kéri G, Thán M, Oroszi G, Horváth A (2001) Anti-inflammatory effect of synthetic somatostatin analogues in the rat. Br J Pharmacol 134:1571–1579
Heredia DJ, Gershon MD, Koh SD, Corrigan RD, Okamoto T, Smith TK (2013) Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol Lond 591:5939–5957
Hyland NP, Sjöberg F, Tough IR, Herzog H, Cox HM (2003) Functional consequences of neuropeptide Y Y2 receptor knockout and Y2 antagonism in mouse and human colonic tissues. Br J Pharmacol 139:863–871
Kidd M, Modlin IM, Gustafsson BI, Drozdov I, Hauso O, Pfragner R (2008) Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol Gastrointest Liver Physiol 295:260–272
Kovacs TOG, Walsh JH, Maxwell V, Wong HC, Azuma T, Katt E (1989) Gastrin is a major mediator of the gastric phase of acid secretion in dogs: proof by monoclonal antibody neutralization. Gastroenterology 97:1406–1413
Liu M-T, Kuan Y-H, Wang J, Hen R, Gershon MD (2009) 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci 29:9683–9699
Margolis KG, Stevanovic K, Li Z, Yang QM, Oravecz T, Zambrowicz B, Jhaver KG, Diacou A, Gershon MD (2014) Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut 63:928–937
Mortensen K, Christensen LL, Holst JJ, Orskov C (2003) GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept 114:189–196
Patterson M, Murphy KG, le Roux CW, Ghatei MA, Bloom SR (2005) Characterization of ghrelin-like immunoreactivity in human plasma. J Clin Endocrinol Metab 90:2205–2211
Pintér E, Helyes Z, Szolcsányi J (2006) Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol Ther 112:440–456
Polak JM, Pearse AGE, Bloom SR, Buchan AMJ, Rayford PL, Thompson JC (1975) Identification of cholecystokinin-secreting cells. Lancet 22:1016–1018
Rehfeld JF (1978) Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem 253:4022–4030
Rehfeld JF (1998) Accurate measurement of cholecystokinin in plasma. Clin Chem 44:991–1001
Roth KA, Kim S, Gordon JI (1992) Immunocytochemical studies suggest two pathways for enteroendocrine cell differentiation in the colon. Am J Physiol 263:G174–G180
Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E (2006) Colocalization of the α-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol 291:G792–G802
Shin A, Camilleri M, Kolar G, Erwin P, West CP, Murad MH (2014) Systematic review with meta-analysis: highly selective 5-HT4 agonists (prucalopride, velusetrag or naronapride) in chronic constipation. Aliment Pharmacol Ther 39:239–253
Shulkes A, Fletcher DR, Hardy KJ (1983) Organ and plasma metabolism of neurotensin in sheep. Am J Physiol 245:457–462
Sundler F, Håkanson R, Hammer RA, Alumets A, Carraway R, Leeman S, Zimmerman EA (1977) Immunohistochemical localization of neurotensin in endocrine cells of the gut. Cell Tissue Res 197:313–321
Ter Beek WP, Muller ESM, Van Den Berg M, Meijer MJ, Biemond I, Lamers CBHW (2008) Motilin receptor expression in smooth muscle, myenteric plexus, and mucosa of human inflamed and noninflamed intestine. Inflamm Bowel Dis 14:612–619
Walther DJ, Peter J-U, Bashammakh S, Hörtnagl H, Voits M, Fink H, Bader M (2003) Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299:76–76
van der Wielen N, van Avesaat M, de Wit NJW, Vogels JTWE, Troost F, et al. (2014) Cross-species comparison of genes related to nutrient sensingmechanisms expressed along the Intestine. PLoS ONE 9(9):e107531
Yanaihara N, Yanaihara C, Nagai K, Sato H, Shimizu F, Yamaguchi K, Abe K (1980) Motilin-like immunoreactivity in porcine, canine, human and rat tissues. Biomed Res 1:76–83
Acknowledgment
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)
Author information
Authors and Affiliations
Corresponding author
Additional information
Brid Callaghan and John B Furness are equal senior authors
Rights and permissions
About this article
Cite this article
Martins, P., Fakhry, J., de Oliveira, E.C. et al. Analysis of enteroendocrine cell populations in the human colon. Cell Tissue Res 367, 161–168 (2017). https://doi.org/10.1007/s00441-016-2530-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2530-7