Abstract
Acanthamoeba are free-living protozoa present ubiquitously in numerous environmental reservoirs that exist as an actively feeding trophozoite or a dormant cyst stage. The pathogenic Acanthamoeba are known to cause Acanthamoeba keratitis (AK) and granulomatous amoebic encephalitis (GAE). Despite their omnipresence, the number of infections is quite low. The reason behind this low frequency of Acanthamoeba infections could be the existence of many non-pathogenic strains or a successful host immune response to these infections. Studies in the past have proposed a few physiological parameters for the differentiation of pathogenic and non-pathogenic strains. Additionally, in vivo experiments are known to play an essential role in understanding the virulence of parasites, immunological aspects, and disease pathogenesis. The thermotolerance (30 °C, 37 °C, and 40 °C) and osmotolerance (0.5 M, 1 M, and 1.5 M) tests were performed on 43 Acanthamoeba isolates from patients with keratitis (n = 22), encephalitis (n = 5), and water samples (n = 16). In addition, the genotype of 10 Acanthamoeba isolates (keratitis (n = 2), encephalitis (n = 2), water (n = 6)) was determined and were then evaluated for pathogenicity on mouse model by inducing Acanthamoeba keratitis and amoebic encephalitis. The results of the thermotolerance and osmotolerance assays categorized 29/43 (67.4%) isolates as pathogenic, 8 as low pathogenic (18.6%), and the remaining 6 (13.9%) as non-pathogenic. The 10 Acanthamoeba isolates were categorized as T11 (5 isolates), T5 (2 isolates), T4 (2 isolates), and T10 (1 isolate) genotypes. Out of 10 Acanthamoeba isolates, 9 were successful in establishing AK, amoebic encephalitis, or both in the mice model, and a single isolate was found non-pathogenic. Two isolates from water samples were non-pathogenic in the physiological tests but successfully established Acanthamoeba infection in the mice model. The results of the physiological assays and in vivo experiments were analogous for 7 isolates while 1 isolate from the water was low pathogenic in the physiological assays but failed to produce pathogenicity during in vivo experiments. The physiological parameters are not very dependable to test the pathogenic potential of Acanthamoeba isolates, and thus results must always be validated by in vivo experiments. There is no infallible approach for determining the potential pathogenicity of environmental isolates of Acanthamoeba because several parameters regulate the pathogenic potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acanthamoeba, a genus of single-celled protozoa, encompasses a diverse group of microorganisms that thrive in various aquatic and terrestrial environments. These organisms exhibit intricate biological characteristics and play significant roles in ecosystems yet they also pose a potential threat to human health (Garcia et al. 2013; Ong et al. 2017). Upon successful invasion of the human host, the pathogenic isolates are responsible for causing serious illness of the central nervous system (granulomatous amoebic encephalitis-GAE), eye (Acanthamoeba keratitis-AK), and rarely cutaneous or disseminated disease (Siddiqui and Khan 2012; Khurana and Sharma 2020; Lorenzo-Morales et al. 2015). GAE is mainly documented in immunocompromised patients and is associated with high mortality (da Rocha-Azevedo et al. 2009). AK is mainly reported among individuals wearing contact lenses, or those sustaining injuries to the eyes, and loss of vision is a common outcome (Dodangeh et al. 2018; Khan 2009; Megha et al. 2021). Also, various serological surveys have indicated the presence of Acanthamoeba-specific serum antibodies in about 90% of the healthy adult population but infections are relatively rare which may be attributed to an effective host response or the presence of non-pathogenic strains (Alizadeh et al. 2001; Niederkorn 2021). Hence it is crucial to study the factors and triggers related to pathogenicity and thus it is essential to differentiate reliably between pathogenic and non-pathogenic isolates. Many studies have suggested physiological parameters for determining pathogenicity, but they are not very precise and reliable (Dodangeh et al. 2018; Khan and Tareen 2003). With this background, we have attempted to check the reliability of thermo-tolerance, osmotolerance assays, and in vivo studies for determining the pathogenic potential of Acanthamoeba isolates.
Materials & Methods
Sampling, and confirmation of Acanthamoeba, and maintenance of Acanthamoeba
The study included 43 isolates obtained from patients with keratitis (n = 22), encephalitis (n = 5), and water samples (n = 16). The isolates CHA1 to CHA27, CHA29, and CHA30 have been published previously (Khurana et al. 2015; Megha et al. 2018, Megha et al. 2021; Megha et al. 2023). The isolates CHA32-CHA46 were recorded for the first time in the present study, however, genotyping was not performed for all the isolates. The keratitis and encephalitis isolates were obtained from clinical specimens (corneal scraping, cerebrospinal fluid, brain biopsy) and inanimate samples (contact lens and lens solution) of patients who had presented to the Postgraduate Institute of Medical Education and Research, Chandigarh, India. The water samples were obtained from different locations in Chandigarh, India, and are being maintained in the Department of Medical Parasitology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. The water sample was centrifuged, and the pellet was inoculated onto the non-nutrient agar (NNA) plate having a lawn of Escherichia coli, and the growth of Acanthamoeba was monitored until seven days.
Physiological parameters for discriminating pathogenic and non-pathogenic Acanthamoeba
The temperature and osmolarity tolerance assays were performed on 43 isolates. For the thermo-tolerance assay, an agar block containing Acanthamoeba cysts was sliced, kept on the surface of a freshly prepared 1.5% non-nutrient agar (NNA) agar plate having a lawn of Escherichia coli, and incubated in triplicates at three different temperatures; 30 °C, 37 °C, and 40 °C. For the osmolarity tolerance assay, an agar block containing Acanthamoeba cysts was sliced and kept on the surface of freshly prepared 1.5% NNA agar plates having different mannitol (D-Mannitol, HiMedia Pvt. Ltd., New Delhi, India) concentrations (0.5 M, 1 M, and 1.5 M) and having a lawn of E. coli that were incubated at 30 °C. The growth in the plates was monitored at 24, 48, and 72 h for their ability to grow. The limit of expansion was scored based on the distance reached by the trophozoites and cysts from the center of the plate as described previously (Khan et al. 2001; Mohd Hussain et al. 2022a; Possamai et al. 2018). The strains showing maximum growth (+ + +) at the high temperature (40 °C) and mannitol concentration (1.5 M) were designated pathogenic whereas the non-pathogenic strains were the ones incapable of proliferating at high temperature (40 °C) and mannitol concentration (1.5 M). The strains exhibiting scanty growth (+) at high temperature (40 °C) and mannitol concentration (1.5 M) were designated low-pathogenic (Possamai et al. 2018).
Animal model for the differentiation of pathogenic and non-pathogenic Acanthamoeba
We evaluated 10 Acanthamoeba isolates (2 isolates each of keratitis, encephalitis, and 6 isolates from water) in the mouse model for the establishment of keratitis and encephalitis. A total of 120 male Balb/c mice were used in the study as detailed below. All the animal experimentation was done according to the guidelines of the Committee for Control and Supervision of Experiments on Animals and recommendations of the Institutional Scientific Advisory Committee. All the necessary approvals were taken from the Institutional Ethics Committee (Ref. No. 95/93/IAEC/658).
Maintenance of Acanthamoeba isolates
All the Acanthamoeba isolates were inoculated on non-nutrient agar (NNA) having a lawn of inactivated E. coli and incubated at 37 °C for 72 h. The trophozoites were scraped, and suspended in sterile phosphate-buffered saline (PBS); centrifuged at a low speed and the pellet was washed with 0.9% isotonic NaCl, then the pellet was resuspended in PBS and the cell count was adjusted to103 trophozoites/ml (Sharma et al. 2021).
Acanthamoeba keratitis
The pathogenic potential of ten Acanthamoeba isolates was evaluated using a keratitis model on 40 mice (4 mice per isolate). The right eyes of all the mice were used for infection, and the left eyes were used as control. The mice were given 0.1 ml of an anaesthetic cocktail per 20 g of body weight (ketamine 60 mg/kg + xylazine 5 mg/kg), and 0.5% proparacaine ophthalmic solution was also used to achieve corneal anaesthesia. The eyes were traumatized using a surgical blade no. 15 three times vertically and horizontally under a dissection microscope. Small lenses made of Parafilm that had been preincubated with Acanthamoeba were then applied to the abraded corneal surface. The eyelids were sewn by performing tarsorrhaphy using 6–0 (0.7 metric) vicryl sutures as described previously (Sharma et al. 2021). The mice were graded on the fifth-day post-infection based on the clinical features of mild to severe corneal cloudiness & presence of corneal infiltrate. The mice presenting with clinical features were euthanized on day seven, and the eyes were removed for culture, stored in PBS for Acanthamoeba-specific PCR (Schroeder et al. 2001), and stored in formalin for histopathological examination.
Amoebic encephalitis
The amoebic encephalitis mouse model was performed on 80 Balb/c mice (8 mice per isolate including 5 mice for infection and 3 as controls) with 10 Acanthamoeba isolates. Amoebic encephalitis was established with slight modifications in the protocol previously described (Mirjalali et al. 2013; Omaña-Molina et al. 2017). Briefly, the mice were immune-suppressed with 2 doses of cyclophosphamide (EndoxanTM-N, Cadila Healthcare (Zydus), Ahmedabad, India) injected intra-peritoneally at a dosage of 100 mg/kg on alternate days. The immune-suppressed mice were anesthetized with 0.1 ml of anesthetic cocktail (ketamine 60 mg/kg + xylazine 5 mg/kg) for an easy intranasal instillation of 103 Acanthamoeba trophozoites. The mice were monitored daily for signs of encephalitis like in-coordination, ruffled hair, and lethargy for 21 days. The mice presenting with severe clinical features were sacrificed earlier and those healthy were sacrificed at the end of the time-point (21 days). The brain, lung, liver, and spleen were removed from euthanized mice and used for culture, kept in PBS for PCR (Schroeder et al. 2001), and stored in formalin for histopathologic examination.
Sequencing for genotype determination
The genotyping for isolates CHA5, CHA20, CHA24, and CHA 27 had been performed previously and the accession numbers are provided in Table 2. The purified amplified products of the remaining six isolates were sequenced using the Big-Dye 3.1 Terminator sequencing kit (Applied Biosystems, Foster City, CA, USA) on an ABI 3130 Genetic Analyzer automated sequencer following the manufacturer's instructions (Applied Biosystems, Foster City, CA, USA). The obtained raw sequences were compared with GenBank by using Blast (http://blast.ncbi.nlm.nih.gov/Blast.cgi) for determining the genotype and the sequence similarity.
Results
The thermo-tolerance and osmo-tolerance assays were performed on 43 Acanthamoeba isolates including 22 isolates from AK, 5 from GAE patients, and 16 from water samples. All the isolates were previously inoculated on an NNA medium and confirmed by PCR assay for their confirmation as Acanthamoeba species. Twenty-nine out of 43 isolates (67.4%) showed pathogenic potential based on thermo-tolerance and osmo-tolerance assays, 8 were low pathogenic (18.6%), and the remaining 6 (13.9%) isolates were not able to tolerate high temperature and osmolarity conditions and thus were designated non-pathogenic (Table 1). In addition to the physiological assays, 10 isolates were sequenced for determining the specific genotype and were subsequently employed in AK and amoebic encephalitis animal models each to assess the pathogenic potential of the Acanthamoeba isolates. In the present study, ten Acanthamoeba sequences that belonged to T4, T5, T10, and T11 genotypes were detected upon comparing the raw data to the NCBI database. The genotype T10 was detected in one clinical isolate and T5 was found in two isolates from water. Furthermore, five isolates were similar to the T11 genotype and one isolate each from a keratitis patient and water was detected as the T4 genotype (Table 2). The “Identity to reference accession number” in Table 2 represents the accession number to which our isolate showed the highest similarity.
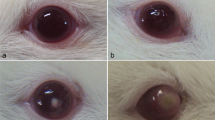
Forty mice were used to investigate the pathogenic potential of 10 isolates to cause keratitis, and the clinical assessment was carried out 5 days after infection. The 2 AK isolates, 2 encephalitis, and 5 environmental isolates successfully established AK in the mice model (Table 3). The successful establishment of AK in the mice eye was confirmed clinically by the presence of characteristic corneal infiltrate and corneal cloudiness. In addition, the inoculation of corneal scraping demonstrated Acanthamoeba trophozoites and cysts on culture (Fig. 1) and was used for the successful amplification of the Acanthamoeba by PCR (Fig. 2). The clinical and histopathological examination of the mouse cornea further validated AK in the mouse model (Fig. 3). The three confirmatory tests corroborated with the clinical evaluation of the mouse eye. In addition to the AK model, we have assessed the pathogenic potential of 10 Acanthamoeba isolates in an amoebic encephalitis mice model. Out of 10 isolates, the 2 GAE, and 1 environmental isolate successfully established an amoebic encephalitis model that was confirmed using culture (Fig. 1), PCR (Fig. 2), and histopathology of the brain, lung, liver, and spleen (Fig. 4) from the infected mice. Based on the results of physiological parameters and in vivo experiments, the 4 clinical isolates were designated pathogenic. In addition, 4 isolates from the water had the potential of inducing keratitis alone, and 1 isolate was capable of inducing keratitis and encephalitis in the animal model. The result of the physiological assays for these 5 isolates was quite variable that designated 1 isolate as pathogenic; 2 isolates each as low pathogenic and non-pathogenic (Table 3). Additionally, 1 isolate was found non-pathogenic in the in vivo experimentation and it was designated as low pathogenic by the physiological assays.
Histopathological examination of hematoxylin and eosin stained sections of brain, lung, liver, and spleen of mouse infected with amoebic encephalitis. a: Brain section displaying hypoxia in the form of red shrunken neurons; b: Alveolar spaces in the lung studded with neutrophils & macrophages, congestion and nectrotizing pneumonia can be seen; c: Sinusoidal congestion and portal vein dilation observed in liver; d: Spleen section displaying mild hyperplasia (10X magnification)
Discussion
Acanthamoeba species are ubiquitous in nature; however, cases of AK and GAE have been relatively rare (Lorenzo-Morales et al. 2006; Rezaeian et al. 2008; Possamai et al. 2018). Many different parameters in the past have helped in demarcating pathogenic isolates from non-pathogenic Acanthamoeba. Studies have used molecular and protease markers, in vivo models, and physiological parameters to assess the pathogenic potential of Acanthamoeba isolates (Howe et al. 1997; Khan et al. 2000; Khan et al. 2001; Khan et al. 2002; Garate et al. 2006; Castro-Artavia et al. 2017). Additionally, comparisons between osmotolerance, thermotolerance, and Acanthamoeba genotypes have been reported previously (Kahraman & Polat 2022). The virulence of an isolate depends on its capability to adapt & survive in the mammalian host and the isolates taken from environmental sources should be evaluated by animal experimentation for their pathogenicity (Khan 2006; Landell et al. 2013; Kahraman & Polat 2022). In addition, studies in the past have shown that the pathogenic potential of free-living amoebae, including those from the genus Acanthamoeba, may be examined in vitro using known cell lines or primary and secondary human cell cultures. Additionally, using them under controlled conditions reduces the individual and species-specific variability of the experimental animals, enhances the reproducibility of the experiment, and provides excellent data on both the pathogenicity characteristics of these protozoa and the defensive response of the host (Koehsler et al. 2009; Martín-Navarro et al. 2010; Mohd Hussain et al. 2022b; Walochnik et al. 2000). The pathogenic Acanthamoeba is reported to withstand high temperatures and osmolarity and also show high levels of heat shock proteins (Pérez-Serrano et al. 2000; Podlipaeva et al. 2006; Landell et al. 2013; Kahraman & Polat 2022). In the present study, we have used physiological parameters of thermo-tolerance and osmo-tolerance along with results from in vivo study to ascertain the pathogenicity of isolates. The verification of isolates based on the results of the animal model has helped in providing an appropriate confirmation. Previous reports have assessed the pathogenicity based on different physiological parameters and cytopathogenicity that suggested a weak correlation between the clinical origin of isolate and physiological assays (Nagyova et al. 2010; Siddiqui and Khan 2012; Possamai et al. 2018), but they did not perform an animal model based test to confirm their results from in vitro experiments.
The physiological criteria in the current study misclassified 4 clinical isolates either as low or non-pathogenic. Three out of 27 clinical isolates were detected as low pathogenic and 1 was non-pathogenic. Among 16 isolates from water sources, physiological assays suggested 6 isolates as pathogenic and the remaining 10 were either low pathogenic or non-pathogenic (Table 1). Additionally, based on genotyping, the 10 clinical and environmental isolates included for the in vivo experimentation were categorized into genotypes T4, T5, T10, and T11 (Table 2). Studies in the recent past have demonstrated the presence of T4, T5, and T11 genotypes in samples from water, soil, and dust (Behnia et al. 2017; Milanez et al. 2020; Paknejad et al. 2020; Salehi et al. 2022). Likewise, different Acanthamoeba genotypes have been associated with clinical cases of keratitis and encephalitis with T4 being the most common genotype among all (Esboei et al. 2020; Hajialilo et al. 2016; Kalra et al. 2020; Megha et al. 2018). The fact that we did not genotype every isolate makes it unjust to say which genotype is the most prevalent.
The ten isolates used for in vivo experiments included environmental and clinical isolates each of keratitis and encephalitis. The keratitis isolates (CHA 5 and CHA 20) were obtained from the corneal scrapings of AK patients. At the time of presentation, the size of the ulcers was 1 × 1 mm and 05 × 0.5 mm respectively and the visual acuity was 6/6. The patients were prescribed Polyhexamethylene Biguanide (PHMB 0.02%) half hourly for one week, then hourly for one week and then gradually tapered according to the response. The clinical outcomes in both cases were healed corneal opacities. On the other hand, the two encephalitis isolates belonged to an immunocompetent individual (CHA24) and an individual under treatment for acute myeloid leukaemia (CHA27). The diagnosis of GAE was made postmortem in the case of CHA24 and antemortem for CHA27 isolates.
Based on the results of in vivo mouse model, 5 environmental isolates were found to establish keratitis, amoebic encephalitis, or both, and 1 was found non-pathogenic (Fig. 1–4). We could not conduct in vivo experiments on a larger set of Acanthamoeba isolates from water due to certain constraints in procuring a sufficient number of animals. The results of the physiological assays and in vivo experiments were analogous for 7 isolates. In addition to the establishment of AK and amoebic encephalitis by the respective clinical isolates, we observed the GAE isolate establish keratitis as well. Our results are different from a previous report in which the isolate from the keratitis patient was not showing positive results in the keratitis mouse model. However, there was a direct relationship between their physiological results and animal experiments (Mirjalali et al. 2013). The long-term axenic cultivation of the strain and certain host factors might have played a role that led to this discordance. The growth at high osmolarity has been correlated with the propensity to counter high osmotic pressure, a situation Acanthamoeba face while acting as parasites of the cornea (Siddiqui and Khan 2012). Additionally, the capacity of thermo-tolerance is directly proportional to the ability of Acanthamoeba to produce cellular damage (Walochnik et al. 2000). Even if these two characteristics provide amoebae with an adaptative advantage while parasitizing a host, the mere presence or absence of these characteristics is not enough to define the amoeba as pathogenic. For instance, different species of Acanthamoeba may be thermotolerant and osmotolerant but they are not always pathogenic (De Jonckheere 1980; Schuster and Visvesvara 2004). In addition, the pathogenic potential of environmental isolates of Acanthamoeba cannot be determined with absolute certainty because other factors, such as the genetic makeup of the protozoan, epigenetic factors, and the biological characteristics of the experimental models (animal, tissue, or cellular) must also be taken into account. In our data, none of the pathogenic isolates from the physiological assay was found non-pathogenic in the mouse model. However, this could be explained by the less number of isolates included in our study for in vivo experimentation. Nevertheless, physiological properties are not sufficient to ascertain whether a given isolate is always pathogenic. There are other aspects like the ability to invade the host, protease profile, and encystment rate which play a role in the pathogenesis and might act as an indicator of pathogenicity.
Data availability
All the necessary data has been added to the main manuscript file.
References
Alizadeh H, Apte S, El-Agha MS, Li L, Hurt M, Howard K, Cavanagh HD, McCulley JP, Niederkorn JY (2001) Tear IgA and serum IgG antibodies against Acanthamoeba in patients with Acanthamoeba keratitis. Cornea 20(6):622–627
Behnia M, Hatam-Nahavandi K, Hajialilo E, Niyyati M, Tarighi F, Akram AB, Salimi M, Rezaeian M (2017) Occurrence of Acanthamoeba genotypes in wastewater samples in Tehran. Iran Iranian J Parasitol 12(4):516
Castro-Artavia E, Retana-Moreira L, Lorenzo-Morales J, Abrahams-Sandí E (2017) Potentially pathogenic Acanthamoeba genotype T4 isolated from dental units and emergency combination showers. Mem Inst Oswaldo Cruz 112(12):817–821
da Rocha-Azevedo B, Tanowitz HB, Marciano-Cabral F (2009) Diagnosis of infections caused by pathogenic free-living amoebae. Interdisciplinary Perspect Infect Dis 1–14. https://doi.org/10.1155/2009/251406
De Jonckheere JF (1980) Growth characteristics, cytopathic effect in cell culture, and virulence in mice of 36 type strains belonging to 19 different Acanthamoeba spp. Appl Environ Microbiol 39(4):681–685
Dodangeh S, Kialashaki E, Daryani A, Sharif M, Sarvi S, Moghaddam YD, Hosseini SA (2018) Isolation and molecular identification of Acanthamoeba spp. from hot springs in Mazandaran province, northern Iran. J Water Health 16(5):807–13
Esboei BR, Fakhar M, Saberi R, Barati M, Moslemi M, Hassannia H, Dadimoghadam Y, Jalallou N (2020) Genotyping and phylogenic study of Acanthamoeba isolates from human keratitis and swimming pool water samples in Iran. Parasite Epidemiol Control 11:e00164
Garate M, Marchant J, Cubillos I, Cao Z, Khan NA, Panjwani N (2006) In vitro pathogenicity of Acanthamoeba is associated with the expression of the mannose-binding protein. Invest Ophthalmol vis Sci 47(3):1056–1062
Garcia A, Goñi P, Cieloszyk J, Fernandez MT, Calvo-Beguería L, Rubio E, Fillat MF, Peleato ML, Clavel A (2013) Identification of free-living amoebae and amoeba-associated bacteria from reservoirs and water treatment plants by molecular techniques. Environ Sci Technol 47(7):3132–3140
Hajialilo E, Behnia M, Tarighi F, Niyyati M, Rezaeian M (2016) Isolation and genotyping of Acanthamoeba strains (T4, T9, and T11) from amoebic keratitis patients in Iran. Parasitol Res 115:3147–3151
Howe DK, Vodkin MH, Novak RJ, Visvesvara G, McLaughlin GL (1997) Identification of two genetic markers that distinguish pathogenic and nonpathogenic strains of Acanthamoeba spp. Parasitol Res 83(4):345–348
Kahraman M, Polat ZA (2022) Are thermotolerant and osmotolerant characteristics of acanthamoeba species an indicator of pathogenicity? Res Square 1–14. https://doi.org/10.21203/rs.3.rs-1724089/v1
Kalra SK, Sharma P, Shyam K, Tejan N, Ghoshal U (2020) Acanthamoeba and its pathogenic role in granulomatous amebic encephalitis. Exp Parasitol 208:107788
Khan N (2006) Acanthamoeba: Biology and Increasing Importance in Human Health. FEMS Microbiol Rev 30(4):564–59. https://doi.org/10.1111/j.1574-6976.2006.00023.x
Khan NA (2009) Acanthamoeba. British Library Cataloguing-in-Publication Data, Biology and pathogenesis England
Khan NA, Tareen NK (2003) Genotypic, phenotypic, biochemical, physiological and pathogenicity-based categorisation of Acanthamoeba strains. Folia Parasitol 50(2):97–104
Khan NA, Jarroll EL, Panjwani N, Cao Z, Paget TA (2000) Proteases as Markers for Differentiation of Pathogenic and Nonpathogenic Species of Acanthamoeba. J Clin Microbiol 38(8):2858–2861
Khan NA, Jarroll EL, Paget TA (2001) Acanthamoeba can be differentiated by the polymerase chain reaction and simple plating assays. Curr Microbiol 43(3):204–208
Khan NA, Jarroll EL, Paget TA (2002) Molecular and physiological differentiation between pathogenic and nonpathogenic Acanthamoeba. Curr Microbiol 45(3):197–202
Khurana S, Sharma M (2020) Parasitic keratitis–An under-reported entity. Tropical Parasitology 10(1):12
Khurana S, Biswal M, Kaur H, Malhotra P, Arora P, Megha K, Taneja N, Sehgal R (2015) Free living amoebae in water sources of critical units in a tertiary care hospital in India. Ind J Med Microbiol 33(3):343–348
Koehsler M, Leitsch D, Duchêne M, Nagl M, Walochnik J (2009) Acanthamoeba castellanii: growth on human cell layers reactivates attenuated properties after prolonged axenic culture. FEMS Microbiol Lett 299(2):121–127
Landell MF, Salton J, Caumo K, Broetto L, Rott MB (2013) Isolation and genotyping of free living environmental isolates of Acanthamoeba spp. from bromeliads in Southern Brazil. Experimental Parasitology 134(3):290–294
Lorenzo-Morales J, Ortega-Rivas A, Martínez E, Khoubbane M, Artigas P, Periago MV, Foronda P, Abreu-Acosta N, Valladares B, Mas-Coma S (2006) Acanthamoeba isolates belonging to T1, T2, T3, T4 and T7 genotypes from environmental freshwater samples in the Nile Delta region. Egypt Acta Tropica 100:63–69
Lorenzo-Morales J, Khan NA, Walochnik J (2015) An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite 22:10. https://doi.org/10.1051/parasite/2015010
Martín-Navarro CM, Lorenzo-Morales J, Machín RP, López-Arencibia A, Valladares B, Piñero JE (2010) Acanthamoeba spp: in vitro effects of clinical isolates on murine macrophages, osteosarcoma and HeLa cells. Experimental Parasitology 126(1):85–8
Megha K, Sehgal R, Khurana S (2018) Genotyping of Acanthamoeba spp. isolated from patients with granulomatous amoebic encephalitis. The Indian J Med Res 148(4):456
Megha K, Sharma M, Gupta A, Sehgal R, Khurana S (2021) Microbiological diagnosis of Acanthamoebic keratitis: experience from tertiary care center of North India. Diagn Microbiol Infect Dis 100(2):115339
Megha K, Sharma M, Gupta A, Sehgal R, Khurana S (2023) Sub-Genotyping of Acanthamoeba T4 Complex: Experience from North India. Parasitologia 3(1):69–78
Milanez GD, Masangkay FR, Scheid P, Dionisio JD, Somsak V, Kotepui M, Tangpong J, Karanis P (2020) Acanthamoeba species isolated from Philippine freshwater systems: epidemiological and molecular aspects. Parasitol Res 119:3755–3761
Mirjalali H, Niyyati M, Abedkhojasteh H, Babaei Z, Sharifdini M, Rezaeian M (2013) Pathogenic assays of Acanthamoeba belonging to the T4 genotype. Iran J Parasitol 8(4):530
Mohd Hussain RH, Abdul Ghani MK, Khan NA, Siddiqui R, Anuar TS (2022a) Acanthamoeba species isolated from marine water in Malaysia exhibit distinct genotypes and variable physiological properties. J Water Health 20(1):54–67
Mohd Hussain RH, Abdul Ghani MK, Khan NA, Siddiqui R, Aazmi S, Halim H, Anuar TS (2022b) In vitro cytopathogenic activities of acanthamoeba T3 and T4 genotypes on heLa cell monolayer. Pathogens 11(12):1474
Nagyová V, Nagy A, Janeček Š, Timko J (2010) Morphological, physiological, molecular and phylogenetic characterization of new environmental isolates of Acanthamoeba spp from the region of Bratislava. Slovakia. Biologia 65(1):81–91
Niederkorn JY (2021) The biology of Acanthamoeba keratitis. Exp Eye Res 202:108365
Omaña-Molina M, Hernandez-Martinez D, Sanchez-Rocha R, Cardenas-Lemus U, Salinas-Lara C, Mendez-Cruz AR, Colin-Barenque L, Aley-Medina P, Espinosa-Villanueva J, Moreno-Fierros L, Lorenzo-Morales J (2017) In vivo CNS infection model of Acanthamoeba genotype T4: the early stages of infection lack presence of host inflammatory response and are a slow and contact-dependent process. Parasitol Res 116(2):725–733
Ong TY, Khan NA, Siddiqui R (2017) Brain-eating amoebae: Predilection sites in the brain and disease outcome. J Clin Microbiol 55(7):1989–1997
Paknejad N, Hajialilo E, Saraei M, Javadi A (2020) Isolation and identification of Acanthamoeba genotypes and Naegleria spp from the water samples of public swimming pools in Qazvin. Iran J Water Health 18(2):244–51
Perez-Serrano J, Martı́nez J, Perez B, Bernadina WE, Rodrı́guez-Caabeiro F (2000) In vitro shock response to different stressors in free living and pathogenic Acanthamoeba. Int J Parasitol 30(7):829–835
Podlipaeva IuI, Shmakov LA, Gilichinskiı DA, Gudkov AV (2006) Heat shock protein of HSP70 family revealed in some contemporary freshwater Amoebae and in Acanthamoeba sp. from cysts isolated from permafrost samples. Tsitologiia 48(8):691–694
Possamai CO, Loss AC, Costa AO, Falqueto A, Furst C (2018) Acanthamoeba of three morphological groups and distinct genotypes exhibit variable and weakly inter-related physiological properties. Parasitol Res 117(5):1389–1400
Rezaeian M, Niyyati M, Farnia S, Motevalii-Haghi A (2008) Isolation of Acanthamoeba spp. from different environmental sources. Iran J Parasitol 3:44–47
Salehi M, Spotin A, Hajizadeh F, Soleimani F, Shokri A (2022) Molecular characterization of Acanthamoeba spp from different sources in Gonabad, Razavi Khorasan. Iran Gene Reports 27:101573
Schroeder JM, Booton GC, Hay J, Niszl IA, Seal DV, Markus MB et al (2001) Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of Acanthamoebae from humans with keratitis and from sewage sludge. J Clin Microbiol 39:1903–1911
Schuster FL, Visvesvara GS (2004) Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol 34(9):1001–1027
Sharma C, Thakur A, Bhatia A, Gupta A, Khurana S (2021) Acanthamoeba keratitis in a mouse model using a novel approach. Indian J Med Microbiol 39(4):523–527
Siddiqui R, Khan NA (2012) Biology and pathogenesis of Acanthamoeba. Parasit Vectors 5(1):6
Walochnik J, Obwaller A, Aspöck H (2000) Correlations between morphological, molecular biological, and physiological characteristics in clinical and nonclinical isolates of Acanthamoeba spp. Appl Environ Microbiol 66(10):4408–4413
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
The study conception, funding acquisition, review and editing, project administration, supervision was done by Sumeeta Khurana. Chayan Sharma performed the experimental work and data analyses. Chayan Sharma and Kirti Megha prepared the figures and formal analysis. Anchal Thakur and Amit Gupta performed the clinical evaluation of the mouse model. Alka Bhatia performed the histopathological analysis. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Institutional Review Board Statement: The work was duly approved by the Institutional Ethics Committee vide NK/5283/PhD/213 dated 28–03-2019 and the Institutional Animal Ethics Committee vide 95/93/IAEC/658 dated 13–10-2018.
Consent to participate
Not applicable.
Conflicts of Interest/ Competing Interests
The authors declare no conflict of interest.
Additional information
Section Editor: Sutherland Maciver
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, C., Khurana, S., Megha, K. et al. Assessment of pathogenic potential of Acanthamoeba isolates by in vitro and in vivo tests. Parasitol Res 122, 2109–2118 (2023). https://doi.org/10.1007/s00436-023-07910-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-023-07910-7