Abstract
Free-living amoeba (FLA) research in the Philippines is still in its infancy but has, by far, demonstrated the presence of potentially pathogenic species. Acanthamoeba may cause sight-threatening and central nervous system infections to humans, yet its epidemiologic distribution from local environmental sources is yet to be defined. The present study aimed to provide a baseline epidemiologic distribution of Acanthamoeba spp. in freshwater systems in the Philippines and establish potential pathogenicity of isolates through thermo-tolerance assay. A total of 63 water samples were collected from 13 freshwater systems all over the Philippine archipelago. The low-volume (50 ml) water samples were processed and cultured on non-nutrient agar lawned with Escherichia coli and observed for amoebic growth using light microscopy. Amoebic culture demonstrated 14.28% (9/63) positivity while further molecular testing of culture-positive plates using Acanthamoeba-specific primers demonstrated 100% (9/9) confirmation of Acanthamoeba species. Genotyping of Acanthamoeba isolates revealed T1, T3, T4, T5, T7, T11, and T15 genotypes. Thermo-tolerance assay demonstrated that T5 and T7 genotypes were potentially pathogenic strains. The evidence of environmental distribution of Acanthamoeba spp. in the freshwater systems in the Philippines and thermo-tolerance profile of isolates are significant aspects of amoeba study in public health and calls for initiatives in the dissemination of relevant information and the expansion of knowledge, awareness, and policies on pathogenic waterborne amoeba to mitigate, prevent, detect, and report cases of human infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acanthamoeba spp. are ubiquitous organisms that can cause fatal infections of the central nervous system (CNS) (Visvesvara et al. 2007). Granulomatous amoebic encephalitis (GAE) is a rare and fatal brain infection while Acanthamoeba keratitis (AK) is a sight-threatening infection that has been reported from different parts of the world (Sun Yu et al. 2004; Khan 2005; Martin-Perez et al. 2017; Jercic et al. 2019; Scheid 2015). In addition, case reports of skin infections have been documented (Gullet et al. 1979; Zhang and Pulinthanathu 2016). Several studies have also demonstrated the ability of Acanthamoeba spp. to carry a wide range of endocytobionts (e.g., Legionellae, Mycobacteriae, and viruses) (Scheid 2015; Guimaraes et al. 2016).
Acanthamoeba spp. have been isolated from a diversity of manmade and natural spaces as well as animal host and waste products such as swimming pools (Herrawy et al. 2014), hospital water systems (Cateau et al. 2011), soil (Xuan et al. 2017), freshwater systems (Mahmoudi et al. 2012), bromeliads (Landell et al. 2013), fishes (Dykova et al. 1997), and bat guano (Mulec et al. 2016). Freshwater systems are typical habitats for Acanthamoeba spp. all over the world where lakes and rivers serve as lifelines to human communities and play an important role in the economic, domestic, and recreational needs of humans (Zuo et al. 2016). The Philippines is abundant in inland freshwater systems, the majority of which are sites of aquaculture and abound in anthropogenic activities (Guerrero III 1999; Hagosojos et al. 2020; Milanez et al. 2019). The presence of Acanthamoeba spp., in particular, pathogenic genotypes in freshwater systems, the abundance of anthropogenic activities, and the potential presence of pathogenic endocytobionts make Acanthamoeba spp. a free-living amoeba (FLA) of significant public health importance (Scheid 2014).
Despite the significance of Acanthamoeba spp. to public health, isolation and identification of the same in water sources may not be sufficient to establish pathogenic potential as genotypic variations within genotypes and/or species may exist and can only be defined further through in vitro methods like pathogenicity testing (Siddiqui and Khan 2012). Similarly, subgenus identification of Acanthamoeba spp. may not be enough to establish pathogenicity, because pathogenic and nonpathogenic species exist within the same subgroup (Howe et al. 1997). Thermo-tolerance assay has been widely accepted as an in vitro method to demonstrate the potential pathogenicity of several genera of FLA (Chomicz et al. 2015). To date, there are 20 genotypes of Acanthamoeba that have been reported with only a few demonstrated to be pathogenic to humans (Scheid 2015).
The present study aimed to provide molecular and epidemiological baseline data on the abundance of Acanthamoeba spp. isolated in selected freshwater lakes all over the Republic of the Philippines and define potential human pathogenicity of isolates through thermo-tolerance assay.
Methods
Sampling sites
A total of 63 surface water samples were collected from no more than 30 cm below the water surface from the shorelines of freshwater lakes and a river in 11 different locations in the Philippines namely West Pudoc Lagoon, Paoay Lake, Pantabangan Lake, Bato Lake in Luzon Island; Bito and Danao Lake in Eastern Visayas Islands; and Maiinit, Lake, Sebu Lake, Pulangi Lake, Lanao Lake, and Tagunay River in Mindanao Island and stored in sterile 250-ml polyethylene containers. Sampling sites were selected based on accessibility, established shoreline community, and the presence of fish farms as evidence of anthroponotic activity (Milanez et al. 2020).
Acanthamoeba spp. isolated from previous study
Six previously reported Acanthamoeba spp. collected from two water reservoirs, Ipo and Magat dam (insert name of the sampling sites involved), were included in this study (Milanez et al. 2020) to assess pathogenicity potential through thermo-tolerance assay. Aliquots from these samples were pelleted and cultured in non-nutrient agar (NNA) plates lawned with Escherichia coli and incubated at 30 °C.
Water sample processing, culture, and microscopy
Water samples were transported to the laboratory, transferred to falcon tubes, and pelleted at 3000 rpm for 15 min. The resulting pellets (approximately 1–2 ml) were transferred to NNA plates lawned with E. coli and incubated at 30 °C. Culture was performed in triplicate per water sample to ensure the validity of the examination. Culture plates were observed daily for 14 days for amoebic growth using a light microscope (Nikon Eclipse E100) before being declared negative (Page 1988). In detail, culture plates were observed for the presence of FLA evidenced through observable motile trophozoites and cystic forms. After which, the agar surface was observed to identify the best area of growth, marked, and cut approximately 3 × 3 mm using a sterile scalpel blade. The 3 × 3 mm agar block was transferred upside down onto a new NNA plate lawned with E. coli and were incubated at 30 °C. This step was repeated until a homogenous subculture was obtained (Init et al. 2010).
DNA extraction and molecular analysis
Trophozoites and cysts from positive subculture plates were harvested as described previously by adding approximately 3.0 ml of phosphate-buffered saline (PBS) solution onto the agar surface and scraped to detach cells (Milanez et al. 2017). The suspension was aspirated and transferred into microcentrifuge tubes, and DNA was extracted using Macherey-Nagel DNA extraction kit (NucleoSpin®) following the manufacturer’s protocol. Primer set JDP1 5′-GGCCCAGATCGTTTACCGTGAA-3′ and JDP2 5′-TCTCACAAGCTGCTAGGGAGTCA-3′ was used for PCR amplification with the following cycling conditions: 95 °C for 7-min initial denaturation, 40 cycles of denaturation at 95 °C for 1 min, annealing temperature of 55 °C for 1 min, extension at 72 °C for 2 min, and a final extension of 72 °C for 15 min (Booton et al. 2004). DNA was visualized in 1.5% agarose gel stained with ethidium bromide (5 μl). To identify the Acanthamoeba genotypes, PCR products were sent to a commercial sequencing company (Macrogen, Seoul, South Korea) for further sequencing.
Thermo-tolerance assay testing
Thermo-tolerance assay was performed on all Acanthamoeba-confirmed isolates from the present and previous study based on previously established protocols (Walochnik et al. 2000). In detail, amoebic cysts were harvested from plates and were pelleted. Cysts were counted using a hemocytometer and were brought to a final concentration of 105 cells/ml. One microliter of which was inoculated onto the center of a new NNA plate lawned with E. coli and incubated under varying temperatures (30 °C, 37 °C, and 40 °C). Amoebic growth patterns were observed and documented for 48 h by observing the expanding migration of trophozoites as well as the proliferation rate. The persistence of amoebic growth after 14 days with the number of cysts was used to evaluate the degree of thermo-tolerance of the isolates.
Limitations of study
Physico-chemical and bacteriologic analyses were not performed on water samples, and molecular analysis was only conducted on culture positive water samples in this study.
Results
Culture, microscopy, and molecular results
Culture results demonstrated 14.28% (9/63) of the water samples as positive for amoebic growth. Light microscopy of positive culture plates revealed cystic stages measuring 10 to 15 μm in diameter (Fig. 1a–d) exhibiting irregularly shaped endocyst while trophozoites (Fig. 1e) demonstrated acanthopodia which are morphologic criteria consistent with Acanthamoeba spp..
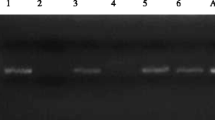
Polymerase chain reaction (PCR) confirmed all nine isolates from culture-positive plates as Acanthamoeba spp. through agarose gel electrophoresis using Acanthamoeba genotype T5 DNA as the positive control (Fig. 2). Sequencing and BLAST percent similarity of DNA revealed Acanthamoeba spp. belonging to genotypes T4, T5, and T15 (Table 1). The DNA sequences of isolates LB3-BW, LDO2, LM13-BW, LM41-SW, LM42-SW, LM43-SW, LM51, LP1-BW, and WP2BW obtained from this study were deposited in the GenBank database and are available under accession numbers MN685221, MN685223, MN685226, MN685227, MN685242, MN685244, MN685268, MN685269, and MN685270, respectively, while the 6 previously isolated Acanthamoeba spp. namely IS1B5, IS4B3, MS45, MS42, IS2B1, and IS1B2 were registered under accession numbers MK886460, MK909919, MK905437, MK910997, MK911021, and MK886514, respectively (Milanez et al. 2020). The overall sampling site prevalence of Acanthamoeba spp. in the present study was reported at 45% (5/11) where West Pudoc Lagoon and Paoay Lake were positive for the target organism in Luzon Island at 50% (2/4), Bito and Danao Lake in Eastern Visayas Islands at 100% (2/2), and Mainit Lake in Mindanao Island at 20% (1/5).
Ethidium bromide–stained agarose gel viewed under UV transilluminator showing band between 400 and 500 bp. PCR reaction was achieved using JDP1 and JDP2 primers. M: 1 kb ladder; C: control well; 1: LB3BW; 2: LDO2; 3: LM13BW; 4: LM41SW; 5: LM42SW; 6: LM43SW; 7: LM51; 8: LP1BW; 9: WP2BW; 10: IS1B5; 11: IS4B3; 12: MS45; and 13: MS42 (note: lanes 1–9 from present study, lanes 10–13 from previous study, IS1B2 and IS2B1 not shown)
Thermo-tolerance results
Thermo-tolerance assay results of the present (n = 9) and previous (n = 6) Acanthamoeba spp. isolates demonstrated 100% (15/15), 93% (14/15), and 47% (7/15) growth at 30, 37, and 40 °C, respectively (Table 1). Five isolates exhibited strong to very strong growth patterns where MS42 and LM13-BW, genotypes T7 and T4, respectively, had very strong growth pattern at 40 °C. In addition, isolates MS45 and IS2B1 belonging to genotypes T11 and T3, respectively, also exhibited growth at 40 °C but were observed to have weaker growth patterns.
Discussion
Acanthamoeba spp. are ubiquitous and can be considered as a part of the natural fauna in environmental freshwater systems. In this study, we present data on water samples collected from 11 significant freshwater systems all over the Philippines plus new data from isolates coming from two major water reservoirs from a previous study (Milanez et al. 2020). Although culture results of Acanthamoeba spp. in the present study (14.28%) were in close agreement to data presented by the neglected, zoonosis, and vector-borne disease research group in Thailand (15.9%) (Thammaratana et al. 2016), it was lower compared with detections in Iran (73% and 76%) (Mahmoudi et al. 2012; Salehi et al. 2019) and in Malaysia (76%) (Mohd Huassain et al. 2019). The current research results, however, provided an expanded knowledge on the molecular and epidemiologic distribution of Acanthamoeba spp. in freshwater systems in the Philippines. Acanthamoeba spp. have previously been reported in Magat and Ipo water reservoir (Milanez et al. 2020) and Buhi Lake (Hagosojos et al. 2020) in Luzon Island while this study further expanded the scope of detecting Acanthamoeba from other freshwater systems in Luzon Island (West Pudoc Lagoon and Paoay Lake), and for the first time in Eastern Visayas Islands (Bito and Danao Lake) and Mindanao Island (Mainit Lake). Relative to freshwater resources, several studies have validated the potential role of fishes as hosts to a variety of FLA, which includes Acanthamoeba (Dykova et al. 1997), and further suggest the capacity of fishes to shed cystic stages through their excrements, as reports of the presence of FLA in fish intestine have been previously published (Laoprasert et al. 2010; Milanez et al. 2017). Freshwater systems that tested positive for the presence of Acanthamoeba spp. in the present study were primary sources of livelihood of the surrounding towns and provinces and abound in anthropogenic activities, which can potentially contribute to and increase the risk of human infections (Declerck et al. 2007; Chang et al. 2010).
Pathogenicity testing of isolates through thermo-tolerance assay demonstrated 47% (7/15) growth at a higher temperature of 40 °C where three isolates of genotypes T4, T5, and T7 exhibited strong to very strong growth. Genotypes T5 and T7 are not well recognized as pathogenic genotypes. Although there have been reports demonstrating the potential pathogenicity of genotype T5 through weak binding capacity to corneal cells in vitro (Khan 2003), a cornea infection in the USA (Leede et al. 2009), and a case of disseminated cutaneous infection (Barete et al. 2007). To date, genotype T5 is still categorized as only potentially pathogenic (Twafeek et al. 2016). Similarly, although perspectives on the pathogenicity of genotype T7 are still not clearly defined, disseminated cutaneous infection has been reported (Gullet et al. 1979) as well as isolation from a keratitis patient in Egypt (Twafeek et al. 2016). Acanthamoeba spp. and its genotypes have demonstrated a somewhat considerable variation in its response to pathogenicity assays, which makes in vitro testing difficult to correlate with actual human-pathogenic capacity (Twafeek et al. 2016). Four isolates in the present study under genotypes T1 and T3, which have been established to cause encephalitis and keratitis, respectively (Khan 2005), thrived in temperatures of 30 °C and 37 °C but demonstrated no growth at 40 °C. The Acanthamoeba genotype T4 isolates in the present study demonstrated consistent thermo-tolerance where very strong growth was observed at 30 to 37 °C and strong to very strong growth observed at 40 °C, which is in agreement with previously published thermo-tolerance results (Castro-Artavia et al. 2017). The existence of pathogenic and non-pathogenic species or strains within the genus Acanthamoeba has been reported (Howe et al. 1997), and it calls for expanded pathogenicity testing using a wider array of assays among all Acanthamoeba species to update each genotypes’ role in human infections. The present study provided further evidence of interspecies variations in terms of potential pathogenicity within Acanthamoeba genotypes through thermo-tolerance assay; it also demonstrates the inability of certain variants of pathogenic genotypes (T1 and T3) to thrive at higher temperatures of 37 °C and 40 °C.
Relative to the presence and biology of Acanthamoeba spp. in freshwater systems, it is important to ponder on the effects of increasing global and water temperatures as well as water crisis all over the world due to climate change (DeNicola et al. 2015; Levy and Patz 2015; Walker 2018). The rising sea levels and salinization of groundwater resources, droughts, and floods may pave the way for the spread of non-tuberculous Mycobacteria, Legionella, Campylobacter, norovirus, and rotavirus to name a few (Walker 2018) as well as contamination of freshwater resources with Acanthamoeba from soil run-off due to increased precipitation events (Xuan et al. 2017). This may lead to the increased interaction between a wide variety of Acanthamoeba and endocytobionts. Similarly, increasing global and water temperatures have contributed to the increase of certain protozoan species even at higher elevations (Bebber et al. 2013). Further, increased salt concentrations in freshwater systems may influence adaptive patterns of osmo-tolerance in FLA, and increased global and water temperatures may also influence changes in thermo-tolerance patterns in amoebic species. Relative to these, great curiosity lies in the osmo-thermo-adaptive capacity of FLA, in particular the pathogenic genotypes, and how these hypothesized evolutionary changes may influence the burden and gravity of amoebic diseases at present and in the future.
Conclusions
The present study describes (a) the first expanded evidence on the molecular and epidemiologic distribution of Acanthamoeba spp. in freshwater systems in the three major Islands of Luzon, Visayas, and Mindanao in the Philippines and (b) the first report and interesting observation of high thermo-tolerance in supposedly non-pathogenic or potentially pathogenic T5 and T7 genotypes suggesting within-genotype and/or within-species variation in thermo-adaptive capabilities. There is a need to further evaluate the role of Acanthamoeba genotypes in relation to human infections using pathogenicity assays or by being able to document actual human cases. Potential osmo-thermo-adaptive changes in Acanthamoeba spp. as a response to rising global and water temperatures due to climate change are expected.
References
Barete S, Combes A, De Jomcheere J, Datry A, Varnous S, Martinez V, Ptacek SG, Caumes E, Capron F, Frances C, Gilbert C, Chosidow O (2007) Fatal disseminated Acanthamoeba lenticulata acanthamebiasis in a heart transplant patient. Emerg Infect Dis 13:736–738. https://doi.org/10.3201/eid1305.061347
Bebber DP, Ramotowski MAT, Gurr SJ (2013) Crop pests and pathogens move polewards in a warming world. Nat Clim Chang 3:985–988
Booton GC, Rogerson A, Bonilla TD, Seal DV, Kelly DJ, Beattie TK, Tomlinson A, Lares-Villa F, Fuerst PA, Byers TJ (2004) Molecular and physiological evaluation of subtropical environmental isolates of Acanthamoeba spp., causal agent of Acanthamoeba keratitis. J Eukaryot Microbiol 51:192–200. https://doi.org/10.1111/j.1550-7408.2004.tb00545.x
Castro-Artavia E, Retana-Moreira L, Lorenzo-Morales J, Abrahams-Sandi E (2017) Potentially pathogenic Acanthamoeba genotype T4 isolated from dental units and emergency combination showers. Mem Inst Oswaldo Cruz 112:817–821. https://doi.org/10.1590/0074-02760170147
Cateau E, Verdon J, Fernandez B, Hechard Y, Rodier MH (2011) Acanthamoeba sp. promotes the survival and growth of Acinetobacter baumanii. FEMS Microbiol Lett 319:19–25. https://doi.org/10.1111/j.1574-6968.2011.02261.x
Chang CW, Wu YC, Ming KW (2010) Evaluation of real-time PCR methods for quantification of Acanthamoeba in anthropogenic water and biofilms. J Appl Microbiol 109:799–807. https://doi.org/10.1111/j.1365-2672.2010.04708.x
Chomicz L, Conn D, Padzik M, Szaflik J, Walochnik J, Zawadzki P, Pawlowski W, Dybicz M (2015) Emerging threats for human health in Poland: pathogenic isolates from drug resistant Acanthamoeba keratitis monitored in terms of their in vitro dynamics and temperature adaptability. Biomed Res Int 2015:231285–231288. https://doi.org/10.1155/2015/231285
Declerck P, Behets J, van Hoef V, Ollevier F (2007) Detection of Legionella spp. and some of their amoeba hosts in floating biofilms from anthropogenic and natural aquatic environments. Water Res 41:3159–3167. https://doi.org/10.1016/j.watres.2007.04.011
DeNicola E, Aburizaiza O, Siddique A, Khwaja H, Carpenter DO (2015) Climate change and water scarcity: the case of Saudi Arabia. Ann Glob Health 81:342–353. https://doi.org/10.1016/j.aogh.2015.08.005
Dykova I, Machackova B, Peckova H (1997) Amoebae isolated from organs of farmed tilapias Oreochromis niloticus. Folia Parasitol 44:81–90
Guerrero RD III (1999) Philippine lakes: status and strategies for sustainable development. Trans Natl Acad Sci Tech Phil 21:278–286
Guimaraes AJ, Gomes KX, Cortines JR, Peralta JM, Peralta RHS (2016) Acanthamoeba spp. as a universal host for pathogenic microorganisms: one bridge from environment to host virulence. Microbiol Res 193:30–38. https://doi.org/10.1016/j.micres.2016.08.001
Gullet J, Mills J, Hadley K, Podemski B, Pitts L, Gelber R (1979) Disseminated granulomatous Acanthamoeba infection presenting as an unusual skin lesion. Am J Med 67:891–896. https://doi.org/10.1016/0002-9343(79)90750-2
Hagosojos B, Masangkay F, Fernandez JB, Lazaro JA, Medroso DE, Olaguera B, Pastores CM, Santos J, Milanez G (2020) Molecular identification of Acanthamoeba sp. in Lake Buhi, Philippines. Ann Parasitol 66:111–114. https://doi.org/10.17420/ap6601.245
Herrawy AA, Bahgat M, Mohammed A, Ashour A, Hikal W (2014) Acanthamoeba species in swimming pools of Cairo, Egypt. Iran J Parasitol 9:194–201
Howe DK, Vodkin MH, Novak RJ, Visvesvara GV, McLaughlin GL (1997) Identification of two genetic markers that distinguish pathogenic and non-pathogenic strains of Acanthamoeba spp. Parasitol Res 83:345–348. https://doi.org/10.1007/s004360050259
Init I, Lau YL, Arin Fadzlun A, Foead AI, Neilson R, Nissapatorn V (2010) Detection of free living amoebae, Acanthamoeba and Naegleria in swimming pools, Malaysia. Trop Biomed 27:566–577
Jercic MI, Aguayo C, Saldarriaga-Cordoba M, Muiňo L, Chenet SM, Lagos J, Osuna A, Fernandez J (2019) Genotypic diversity of Acanthamoeba strains isolated from Chilean patients with Acanthamoeba keratitis. Parasit Vectors 12:58. https://doi.org/10.1186/s13071-019-3302-5
Khan NA (2003) The pathogenesis of Acanthamoeba infections: current status and future implications. Microb Pathog 34:277–285. https://doi.org/10.1016/s0882-4010(03)00061-5
Khan NA (2005) Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev 30:564–595. https://doi.org/10.1111/j.1574-6976.2006.00023.x
Landell MF, Salton J, Caumo K, Broetto L, Rott MB (2013) Isolation and genotyping of free-living environmental isolates of Acanthamoeba spp. from bromeliads in Southern Brazil. Exp Parasitol 134:290–294. https://doi.org/10.1016/j.exppara.2013.03.028
Laoprasert T, Kanchanakhan S, Yagita K, Wada S, Kurata O, Chinabut S, Hatai K (2010) Molecular characterization of an Acanthamoeba sp. isolated from a naturally-infected Oscars Astronotus ocellatus. Aquaculture Sci 58:421–424. https://doi.org/10.11233/aquaculturesci.58.421
Leede DR, Lovieno A, Miller D, Mandal N, Diaz M, Fell J, Fini ME, Alfonso EC (2009) Molecular identification of T4 and T5 genotypes in isolates from Acanthamoeba keratitis patients. J Clin Microbiol 47:1458–1462. https://doi.org/10.1128/JCM.02365-08
Levy BS, Patz JA (2015) Climate change, human rights, and social justice. Ann Glob Health 81:310–322. https://doi.org/10.1016/j.aogh.2015.08.008
Mahmoudi MR, Taghipour N, Eftekhar M, Haghighi A, Karanis P (2012) Isolation of Acanthamoeba species in surface waters of Gilan province-north of Iran. Parasitol Res 110:473–474. https://doi.org/10.1007/s00436-011-2530-1
Martin-Perez T, Criado-Fornelio A, Martinez J, Blanco MA, Fuentes I, Perez-Serrano J (2017) Isolation and molecular characterization of Acanthamoeba from patients with keratitis in Spain. Eur J Protistol 61:244–252. https://doi.org/10.1016/j.ejop.2017.06.009
Milanez G, Masangkay F, Thomas R, Ordona MO, Bernales G, Corpuz VCM, Fortes HSV, Garcia CMS, Nicolas LC, Nissapatorn V (2017) Molecular identification of Vermamoeba vermiformis fom freshwater fish in lake Taal. Exp Parasitol 183:202–206. https://doi.org/10.1016/j.exppara.2017.09.009
Milanez G, Masangkay F, Somsak V, Kotepui M, Tangpong J, Karanis P (2019) Occurrence and the first report of Naegleria australiensis presence in a major lake in the Philippines. J Water Health 17:647–653. https://doi.org/10.2166/wh.2019.034
Milanez G, Masangkay F, Hapan F, Bencito T, Lopez M, Soriano J, Ascaňo A, Lizarondo L, Santiago J, Somsak V, Kotepui M, Tsiami A, Tangpong J, Karanis P (2020) Detection of Acanthamoeba spp. in two major water reservoirs in the Philippines. J Water Health 18:118–126. https://doi.org/10.2166/wh.2020.190
Mohd Huassain RH, Ishak AR, Abdul Ghani MK, Khan NA, Siddiqui R, Anua TS (2019) Occurrence and molecular characterisation of Acanthamoeba isolated from recreation hot springs in Malaysi: evidence of pathogenic potential. J Water Health 17:813–825. https://doi.org/10.2166/wh.2019.214
Mulec J, Dietersdorfer E, Üstüntürk-Onan M, Walochnik J (2016) Acanthamoeba and other free-living amoebae in bat guano, an extreme habitat. Parasitol Res 115:1375–1383. https://doi.org/10.1007/s00436-015-4871-7
Page F (1988) A new key to freshwater and soil Gymnamoebea. Freshwater Biological Association, Ambleside
Salehi M, Niazkar HR, Nasirzadeh A (2019) Isolation and genotyping of Acanthamoeba strains from water sources of Kermanshah, Iran. Ann Parasitol 65:397–402. https://doi.org/10.17420/ap6504.226
Scheid P (2014) Relevance of free-living amoebae as hosts for phylogenetically diverse microorganisms. Parasitol Res 113:2407–2414. https://doi.org/10.1007/s00436-014-3932-7
Scheid P (2015) Free-living amoebae as human pathogens (genus) Acanthamoeba. In: Mehlhorn H (ed) Encyclopedia of parasitology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-27769-6_8.2
Siddiqui R, Khan NA (2012) Biology and pathogenesis of Acanthamoeba. Parasit Vectors 5:6. https://doi.org/10.1186/1756-3305-5-6
Sun Yu H, Hee Kong H, Youl Kim S, Ho Hahn Y, Won Hahn T, Il Chung D (2004) Laboratory investigation of Acanthamoeba lugdunensis from patients with keratitis. Immunol Microbiol 45:1418–1426. https://doi.org/10.1167/iovs.03-0433
Thammaratana T, Laummaunwai P, Boonmars T (2016) Isolation and identification of Acanthamoeba species from natural water sources in the northeastern part of Thailand. Parsitol Res 115:1705–1709. https://doi.org/10.1007/s00436-016-4911-y
Twafeek GM, Biahara SAH, Sarhan RM, Taher EE, Khayyal AE (2016) Genotypic, physiological, and biochemical characterization of potentially pathogenic Acanthamoeba isolated from environment in Cairo, Egypt. Parasitol Res 115:1871–1881. https://doi.org/10.1007/s00436-016-4927-3
Visvesvara GS, Moura H, Schuster FL (2007) Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 50:1–26. https://doi.org/10.1111/j.1574-695X.2007.00232.x
Walker JT (2018) The influence of climate change on waterborne disease and Legionella: a review. Perspect Public Health 138:282–286. https://doi.org/10.1177/1757913918791198
Walochnik J, Obwaller A, Aspöck H (2000) Correlations between morphological, molecular biological, and physiological characteristics in clinical and nonclinical isolates of Acanthamoeba spp. Appl Environ Microbiol 66:4408–4413. https://doi.org/10.1128/aem.66.10.4408-4413.2000
Xuan Y, Shen Y, Ge Y, Yan G, Zheng S (2017) Isolation and identification of Acanthamoeba strain from soil and tap water in Yanji, China. Environ Health Prev Med 22:1–6. https://doi.org/10.1186/s12199-017-0655-2
Zhang B, Pulinthanathu R (2016) Acanthamoeba encephalitis and skin infection in patient with renal transplantation. Am J Clin Pathol 146:279. https://doi.org/10.1093/ajcp/aqw159.050
Zuo Q, Luo Z, Ding X (2016) Harmonious development between socio-economy and river-lake water systems in Xiangyang City, China. Water 8:509. https://doi.org/10.3390/w8110509
Acknowledgments
The authors extend deep gratitude to Dr. Carsten Balczun of the Parasitology Laboratory in the Central Military Hospital in Koblenz, Germany, for his generosity in providing the Acanthamoeba DNA-positive control; The School of Allied Health Sciences, Biomedical Science Department, Walailak University, Department of Biology, and Department of Medical Technology, Far Eastern University-Manila for the technical assistance; laboratory technicians Mr. Romel Solomon, Virgilio Bitagcul, Ma. Lourdes Policarpio, Ariel Lopez, and Ivy Joy Fababier for providing technical support.
Funding
This study was financially supported by Walailak University (Contract No. 23/2562).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Julia Walochnik
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Milanez, G.D., Masangkay, F.R., Scheid, P. et al. Acanthamoeba species isolated from Philippine freshwater systems: epidemiological and molecular aspects. Parasitol Res 119, 3755–3761 (2020). https://doi.org/10.1007/s00436-020-06874-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06874-2