Abstract
Patterns of the rockcod Notothenia coriiceps infection with helminths were analysed to understand the dynamics of parasite communities in this Antarctic fish and to test their stability over time. The study was performed using helminth samples collected from 183 N. coriiceps in 2014–2015 and 2020–2021 in the vicinity of the Ukrainian Antarctic station (UAS) “Akademik Vernadsky”, Galindez Island, Argentine Islands, West Antarctica. Overall, 25 helminth taxonomical categories (nine trematodes, four cestodes, five nematodes, and seven acanthocephalans) were subjected to analysis. A direct comparison of the helminth population characteristics showed that nine species significantly changed their infection parameters during the 6 years between the samples. Seven of them (Pseudoterranova sp., Contracaecum sp., Ascarophis nototheniae, monolocular metacestodes, bilocular metacestodes, Metacanthocephalus rennicki, and Diphyllobothrium sp.) were found to have a significant impact on the differences between helminth infracommunities in 2014–2015 and 2020–2021. Most studied patterns of helminth component community appeared to show a stable tendency, and observed fluctuations were close to the steady trend. Slight but significant changes in the infection patterns observed in this study might have been caused by changes in the populations of intermediate, paratenic, and definitive hosts of helminths (marine invertebrates, mammals, and birds), which participate in helminth transmission in Antarctic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ecological changes caused by global warming and anthropogenic influences can be observed in terrestrial and marine ecosystems on a global scale (Walther et al. 2002; Hoegh-Guldberg and Bruno 2010; Doney et al. 2012; Chen 2021). In the polar regions of the Arctic and Antarctic, these changes are especially pronounced and are apparent as rapid reductions in ice cover and the ozone layer and in changes of the biodiversity of marine and terrestrial ecosystems (Laws et al. 1992; Clarke and Harris 2003; Convey and Peck 2019; Post et al. 2019). A high level of endemicity in marine fishes and invertebrates was recently reported in Antarctica. Between 50 and 97% of Southern Ocean species from various taxonomic groups, including sponges, polychaetes, amphipods, molluscs, isopods, pantopods, and notothenioid fish, are endemic (De Broyer et al. 2014). Thus, it is reasonable to consider Antarctica as a model region for investigating global ecological and evolutionary processes (Clarke et al. 2007; Barnes and Peck 2008; Klimpel et al. 2017).
Parasitic organisms from various taxonomic groups are known as some of the most sensitive indicators of the state of ecosystems, especially in the marine environment (Mouritsen and Poulin 2002; Hudson et al. 2006; Poulin 2006; Poulin and Mouritsen 2006), which is associated with the complex life cycles of separate groups of parasites, including various invertebrate and vertebrate animals as definitive, intermediate, and paratenic hosts (Poulin 2011). Because of the complexity of host-parasite systems, changes in the composition of parasite communities reflect the changes in the ecosystem state even more rapidly than they could be documented by monitoring geological or oceanographic parameters (Poulin and Mouritsen 2006; Poulin 2011; Kvach and Kuzmina 2020). Most Antarctic animals such as birds and mammals are strictly protected (Shirihai 2008), while the populations of most teleost fishes in the Southern Ocean are still abundant and, consequently, are available for extensive parasitological examinations.
The fish fauna in the Southern Ocean around Antarctica is dominated by the perciform suborder Notothenioidei, which comprises up to 90% of the fish biomass and about 77% of fish species diversity (Near 2009; Near et al. 2012). Notothenioidei is uniquely adapted to the cold environment and is endemic to the Antarctic and sub-Antarctic regions (Eastman 1991; Near 2009). Notothenioidei is a food source for various mammals and birds in the Antarctic ecosystem food chains (La Mesa et al. 2004) and is involved in the complex life cycles of different groups of parasites of predatory fish, fish-eating birds, and marine mammals as their intermediate and/or paratenic hosts (Palm et al. 1998; Rocka 2006). Therefore, the parasite fauna of this group of bony fish has high species diversity in all ecoregions of the Southern Ocean (Oguz et al. 2015; Klimpel et al. 2017; Münster et al. 2017; Kuzmina et al. 2020, 2021a, b, 2022a, b). Several notothenioid fish species, such as Antarctic black rockcod, Notothenia coriiceps Richardson, 1844, are promising objects for long-term monitoring studies of parasite communities because they are abundant in different parts of the Southern Ocean including West Antarctica and during the last decades did not demonstrate steady population dynamics (Barrera-Oro et al. 2000; Barrera-Oro and Marschoff 2007; Near 2009). Moreover, in the coastal water area of the Ukrainian Antarctic station (UAS) “Akademik Vernadsky”, N. coriiceps compose 76–90% of fishes in ichthyological catches (Manilo 2006; Veselskyy and Khoetskyy 2018). Such N. coriiceps is a good model organism to study the dynamics of helminth populations in the West Antarctic marine ecosystems (Kvach and Kuzmina 2020).
The present study was performed as a part of the project exploring helminth fauna of Antarctic fish. N. coriiceps appeared to be the most convenient fish host species for testing within the project’s scope due to the large samples collected in 2014–2015 and 2020–2021 demonstrating its rich helminth fauna. The similarity analysis of helminth infracommunities (Kuzmina et al. 2022c) revealed that although the species richness of helminths was similar in the samples from 2014–2015 to 2020–2021, the helminth abundance in the infracommunities was significantly higher in the sample collected in 2020–2021 compared to that collected in 2014–2015 due to higher intensity of rockcod infection with larval stages of nematodes (Pseudoterranova sp., Contracaecum sp.), diphyllobothriid metacestodes, and the acanthocephalans Corynosoma spp.
This study aimed to characterise the patterns of helminth community of N. coriiceps and test how stable helminth communities were over a middle-time scale by comparison the community patterns and infection parameters of separate helminth species in 2014–2015 and 2020–2021.
Materials and methods
Fish sampling
One hundred eighty-three specimens of black rockcod, Notothenia coriiceps, were collected from April 2014 to January 2015 and from February 2020 to January 2021 in the vicinity of the Ukrainian Antarctic station (UAS) “Akademik Vernadsky”, Galindez Island, Argentine Islands Archipelago (65°15′ S, 64°16′ W). The fishes were caught using a fishing rod off the shore at depths of 10–30 m. All fish collected were immediately transported to the laboratory, measured, dissected, and examined using standard parasitological techniques (see Zdzitowiecki and Laskowski 2004; Weber and Govett 2009). For each fish, information on the total length was collected and analysed. Sampled fish were processed on the same day they were caught; precautions were taken to meticulously label vials with specimens to prevent confusion about the parasites between fish specimens.
Parasite collection and identification
Parasites were collected manually from the skin, body cavity, stomach, intestine, liver, and mesentery. In total, 30,951 individual specimens of 25 taxonomic categories (nine trematodes, four cestodes, five nematodes, and seven acanthocephalans) were collected and analysed (see Kuzmina et al. 2022c for details). All collected parasites were washed in saline and fixed in 70% ethanol. Acanthocephalans were kept in tap water for 30 min to 3 h for proboscis evagination prior to their fixation in ethanol. Parasite identification based on morphological characters was performed at the Institute of Zoology in Kyiv, Ukraine, using dissecting and compound microscopes. Due to complications of the excystation technique, all encysted stages of Corynosoma spp. collected in 2014–2015 were identified only to the genus level. In the present analysis, we combined all specimens of Corynosoma spp. Therefore, we use the term “taxonomic categories” in the results, referring to the identified helminth species and the taxa, including several species. All helminth specimens were deposited in the parasitological collections of the I. I. Schmalhausen Institute of Zoology in Kyiv, Ukraine.
Meteorological data
All meteorological data (air temperature, water temperature, and water salinity) used in our analysis were obtained from the Meteorological Archive of the State Institution National Antarctic Scientific Centre of Ukraine (NASC of Ukraine at http://meteodata.uac.gov.ua/).
Data analysis
We used the suggested terminology (Bush et al. 1997) for describing parasite communities. The parasitological dataset analysed in this study was the same as that reported by Kuzmina et al. (2022a,c). We analysed the dataset at the levels of component community and infracommunity using relevant approaches to determine whether infection patterns in black rockcod differed between the 2014–2015 and 2020–2021 seasons. Most analyses were performed in R 4.2.0 (R core Team 2022). We used the package tidyverse (Wickham et al. 2019) for data manipulation and visualisation.
For each helminth species, we calculated the mean intensity and prevalence of infection. Confidence intervals (95%) for infection prevalence were calculated using Sterne’s method with the function epi.prev from the package epiR (Stevenson et al. 2018; Rózsa et al. 2000). Statistical differences in infection prevalence and mean intensity of each species in the two samples were estimated using the unconditional exact test and the bootstrap t-test, respectively, in the Quantitative Parasitology 3.0 software (Rózsa et al. 2000).
To estimate the probability of whether each species is common or rare, we used a fuzzy clustering algorithm implemented in the FuzzyQ package (Balbuena et al. 2021). It simultaneously evaluates the dissimilarities in occupancy and abundance among species in a community and applies fuzzy clustering to allocate them into two clusters of rare and common species.
To reveal direct and indirect associations between helminth species of the community, we visualised a multispecies generalised linear model by a copula graphical model using the ecoCopula package (Popovic et al. 2019). Before that, we fitted the negative binomial regression models, using the year as a predictor and the matrix with raw data on intensity as a response. Also, we used the same models for testing whether the year significantly drives community composition and which species contribute significantly to that. The models were fitted with the function manyglm from the package mvabund (Wang et al. 2012). To visualise the similarity across infracommunities, we used nonmetric multidimensional scaling (nMDS). For this, we utilised the function metaMDS from the package vegan (Oksanen et al. 2022) to visualise community composition based on the Bray–Curtis dissimilarities matrix of untransformed abundance data. Then, we used the functions iNEXT and ggiNEXT from the package iNEXT (Hsieh et al. 2016) to plot rarefaction curves. Furthermore, we statistically compared these curves with the function EcoTest.sample from the package rareNMtests (Cayuela et al. 2015). To fit the model and plot nMDS, we excluded the helminth species that infected less than ten hosts from the dataset.
Additionally, to test whether fish body length affects the intensity of infection, we fitted a negative binomial generalised linear mixed model (GLMM). In the model, we included the intensity of infection as a response, fish body length as a predictor, and the years as a random factor. We tested the fitted model for overdispersion and singularity. We used the packages lme4 (Bates et al. 2015) and performance (Lüdecke et al. 2021) to fit and test the model.
We used the Student’s t-test to reveal differences in the means between seasons for the climate variables (air temperature, water temperature, and salinity of water) and fish body length. Before this test, we checked the normality of the samples with the Shapiro–Wilk test.
Results
Comparison of climate variables
Analysis of meteorological data revealed a significant difference in the mean air and water temperature and water salinity between 2014–2015 and 2020–2021. The mean air temperature (Fig. 1A) in 2014–2015 was -2.6 °C and ranged from -19.8 °C in August to +5.1 °C in May. In 2020–2021, the mean air temperature was -2 °C and ranged from -13.3 °C in August to +4.5 °C in May. The mean water temperature (Fig. 1B) in 2014 was -1 °C and ranged from -2.1 °C in May to +2.8 °C in February. In 2020–2021, the mean water temperature was -0.8 °C and ranged from -1.9 °C in May to +2.4 °C in February. The mean water salinity (Fig. 1C) in 2014–2015 was 33.7‰ and ranged from 29.1‰ in July to 35‰ in August. In 2020–2021, the mean water salinity was 32.2‰ and ranged from 24.5‰ in July to 34‰ in August.
The t-test comparison showed a significant difference in the mean water temperature (t = − 2.2, p = 0.03), salinity (t = 16.17, p = 1.9e−50), and air temperature (t = − 2.38, p = 0.018) between 2014–2015 and 2020–2021.
Analysis of helminth component community patterns
The copula graphical model (Fig. 2) revealed 11 positive and one negative association between taxonomic categories in the community. Bilocular metacestodes and the nematode Pseudoterranova sp. had the largest number of associations. One negative association was observed between the acanthocephalan Metacanthocephalus rennicki and bilocular metacestodes, probably indicating a mutual competitive relationship.
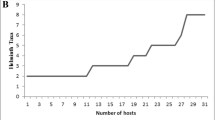
A comparison of the rarefaction curves showed a high similarity between 2014–2015 and 2020–2021 (Fig. 3). The observed species richness was almost identical, and the estimated species richness was slightly higher for 2020–2021; confidence intervals for each year overlapped for observed and estimated species richness. The result of the EcoTest for comparing rarefaction curves was insignificant (p = 0.465) (Fig. 3). Hence, the unrejected null hypothesis suggests that the parasite communities did not differ between 2014–2015 and 2020–2021 in the species richness.
In a comparison (Fig. 4, Table 1), the helminth community in 2014–2015 included almost the same number (24) of helminth taxonomic categories as the community in 2020–2021 (23). In 2014–2015, 17 helminth taxonomic categories were rare, and seven were common. Caudotestis kerguelensis and Macvicaria microtestis were unique for 2014–2015 and Echinorhynchus petrotschenkoi was unique for 2020–2021. Meanwhile, in 2020–2021, 13 helminth taxonomic categories were rare and ten were common. Three helminth taxonomic categories changed from rare to common between 2014–2015 and 2020–2021: the nematode Contracaecum sp., bilocular metacestode, and trematode E. oatesi. However, the change in E. oatesi’s status is doubtful as the negative silhouette width value of the species suggests a poor fit for the cluster of common species in 2020–2021.
Twenty-two helminth taxonomic categories were present in both samples. Most of them (16) had similar values of infection prevalence in 2014–2015 and 2020–2021 (Table 1). Moreover, the prevalence of black rockcod infection with the nematodes Pseudoterranova sp. and D. fraseri, the trematodes E. oatesi, L. garrardi, N. antarctica, and D. johnstoni, and the acanthocephalans M. campbelli and A. megarhynchus was statistically not different (p = 1) in 2014–2015 and 2020–2021. Significant changes in the prevalence of infection were found for six helminth taxonomic categories. The occurrence of Contracaecum sp. and bilocular metacestodes in N. coriiceps increased significantly in 2020–2021 compared to 2014–2015. The prevalence of infection confidence intervals in these two species did not overlap (Fig. 5), which demonstrated the strict differences between samples. The infection prevalence in 2020–2021 was significantly lower than in 2014–2015 for the nematode A. nototheniae, monolocular metacestodes, and the acanthocephalans M. rennicki and M. dalmori. Despite the confidence intervals of the infection prevalence of these species slightly overlapping in the two samples (Fig. 5), the differences were statistically significant (p < 0.05).
The intensity of black rockcod infection significantly increased in 2020–2021 compared to 2014–2015 in three helminth taxonomic categories parasitising N. coriiceps in immature (larval) stages: Pseudoterranova sp., bilocular metacestodes, and Corynosoma spp. In Diphyllobothrium sp., the increase in the intensity of infection was evident, though not statistically significant (p = 0.09) (Table 1). In two acanthocephalan species, M. rennicki and M. johnstoni, the intensity of infection was significantly lower in 2020–2021 than in 2014–2015.
Comparison helminth infracommunity patterns
Nonmetric multidimensional scaling (Fig. 6) did not detect any apparent clustering of the helminth infracommunities related to the year’s factor. On the other hand, the multivariate analysis showed that the factor year did have a significant multiplicative effect (p = 0.0009) on the mean abundance in the infracommunities. The following helminth taxonomical categories were affected by the factor year: Pseudoterranova sp. (p = 0.001), Contracaecum sp. (p = 0.042), Ascarophis nototheniae (p = 0.034), Diphyllobothrium sp. (p = 0.022), monolocular metacestode (p = 0.022), bilocular metacestode (p = 0.001), and Metacanthocephalus rennicki (p = 0.017).
Influence of fish body size on fish infection
The mean total body length of the fish (Fig. 1D) in 2014–2015 was 32.2 cm and ranged from 21.5 to 44.5 cm; the mean fish total body length in 2020–2021 was 31.4 cm and ranged from 18.5 to 47.7 cm. The difference between means was statistically insignificant (t = 0.88, p = 0.379). There was a significant positive effect of fish body size on the intensity of infection (coefficient = 0.037, z-value = 6.038, p = 1.6e−09) (Fig. 7).
Discussion
The present analysis of the time scale changes in helminth communities in N. coriiceps demonstrates that community structure patterns almost did not change during the 6-year term, although the climate variables differed. The analysis of meteorological data in the UAS water area revealed a slight but significant increase in water and air temperature and a decrease in water salinity, which is consistent with the results of long-term observations in various regions of West Antarctica (Vaughan et al. 2003; Turner et al. 2014; Gutt et al. 2015). However, the range of these changes was apparently insufficient to significantly impact the helminth community patterns. Most community patterns did not change between 2014–2015 and 2020–2021; however, few statistical changes are observed on different community levels. The differences in the species compositions of communities are very slight. On the other hand, the essential differences in component community patterns were caused by the differences in prevalence and intensity of N. coriiceps infection by separate helminth species.
The direct comparison of prevalence and intensity of the helminth taxonomic categories between the samples collected in 2014–2015 and 2020–2021 showed that nine of these categories significantly changed the infection parameters during the 6 years between them (see Table 1). According to comparison with model approach, only six species (Pseudoterranova sp., Contracaecum sp., A. nototheniae, monolocular metacestodes, bilocular metacestodes, Metacanthocephalus rennicki) and Diphyllobothrium sp. were also found to have a significant impact on the differences between helminth infracommunities in 2014–2015 and 2020–2021. Not all of those taxonomical categories were classified in the cluster of “common” species (see Fig. 4). Namely, A. nototheniae and monolocular metacestode are in a “rare” category cluster; the status of bilocular metacestodes and Contracaecum sp. is unstable. It can be supposed that the changes are not significant because they did not affect the majority of the “core” species in the community.
Three taxonomic categories, the acanthocephalan M. rennicki, bilocular metacestodes, and the nematode Pseudoterranova sp., are central nodes for species associations (Fig. 2), indicating their essential role in the formation of the community patterns. In the two latter species, the infection intensity increased significantly in 2020–2021 compared with 2014–2015, while both infection prevalence and intensity decreased in M. rennicki. In our opinion, these changes may indicate a particular direction in changes in the helminth community of the studied N. coriiceps population. Further studies on the transmission peculiarities of the species in the marine ecosystem near Galindez Island may reveal the exact reasons for such changes. A similar increase in infection parameters of larval helminths in Parachaenichthys charcoti were reported previously from the same area (Kuzmina et al. 2021b) as well as in other Antarctic fish species (Rokicki et al. 2009; Kuhn et al. 2018; Muñoz and Cartes 2020); while in other cases, researchers observed a decrease in the abundance of larval stages of acanthocephalans or some ascaroid nematodes (Laskowski et al. 2012; Rokicki et al. 2009). Presumably, increasing infection rates of various fishes with helminth larvae are caused by the increase in the abundance and density of populations of their definitive hosts, which are marine mammals and fish-eating birds in Antarctica.
The associations between helminth taxonomical categories revealed in our study using the copula graphical model, in our opinion, are related to the biology and routes of transmission of these individual parasite species. The high intensity of associations of two acanthocephalan species M. rennicki (MREN) and M. johnstoni (MJOH) or trematodes G. bowersi (GBOW) and E. oatesi (EOAT) is undoubtedly due to the similarity of their transmission routes using the same species of intermediate and/or paratenic hosts (Rocka 2006; Faltynkova et al. 2017, 2022). Presumably, the high intensity of association between trematodes and bilocular metacestodes is also related to their transmission using the same intermediate/paratenic hosts (Rocka 2017).
Three taxonomically distant helminth species, the nematode Pseudoterranova sp. (PSEU), cestode Diphyllobothrium sp. (DIPH), and acanthocephalans Corynosoma spp. (CORY), have the same definitive hosts, namely the Antarctic seals (Rocka 2004, 2006, 2017; Laskowski and Zdzitowiecki 2017). For polymorphic acanthocephalans (Order Polymorphida) such as Corynosoma spp., crustaceans of the order Amphipoda are known as intermediate hosts in Antarctic waters (Hoberg 1986; Zdzitowiecki 2001; Zdzitowiecki and Presler 2001). Intermediate hosts of diphyllobothriid cestodes and anisakid nematodes in Antarctic waters remain unknown (Rocka 2003, 2017). We suppose that the solid associations of these helminth species revealed in our study may suggest that the same species of Amphipoda serve as the intermediate hosts for all these helminths, which promotes simultaneous fish infection. An increase in the populations of Weddell seals, Leptonychotes weddellii, and other pinnipeds observed in the waters of Galindez Island in recent decades (Dykyy and Peklo 2012) increased helminth infection of teleost fishes with these helminths (Kuzmina et al. 2022b).
The negative association between the acanthocephalan Metacanthocephalus rennicki (MREN) and bilocular metacestodes (MBIL) may indicate a mutual competitive relationship. However, direct competition is hardly possible in the two species with different niches (site of infection) within the host. Presumably, factors other than direct competition might cause a negative association between these species. Investigation of those factors may be the subject of a separate study.
The positive correlation between fish size and intensity of infection found in our study is somewhat expected and is reasonable as bigger fish provide a more resource-rich ecological niche for helminths (Rocka 2006, 2017; Klimpel et al. 2017). Also, it was documented for different Antarctic fish species in previous research (Hoogester and White 1981; Palm et al. 1998; Zdzitowiecki and Laskowski 2004; Münster et al. 2017; Muñoz and Rebolledo 2018; Muñoz and Cartes 2020; Kuzmina et al. 2020; Alt et al. 2022) that bigger individuals, which are usually older than smaller ones, accumulate a larger number of larval stages of helminths compared to smaller specimens.
Thus, the results of our study show that despite the presence of small but significant changes in the climatic factors (mean air and water temperature and water salinity) between 2014–2015 and 2020–2021, there were no significant differences in helminth of N. coriiceps component community patterns between these years. On the other hand, we believe that some differences in infection patterns revealed in the present study may be associated with the influence of biotic factors that affect the helminths’ transmission such as changes in the populations of intermediate, paratenic, and definitive hosts of helminths (marine invertebrates, mammals, and birds) in Antarctic ecosystems.
Data availability
The original dataset is available in Supplement 1 at: https://doi.org/10.1007/s00436-023-07785-8.
References
Alt KG, Cunze S, Kochmann J, Klimpel S (2022) Parasites of three closely related Antarctic fish species (Teleostei: Nototheniinae) from Elephant Island. Acta Parasitol 67:218–232. https://doi.org/10.1007/s11686-021-00455-8
Balbuena JA, Monlleó-Borrull C, Llopis-Belenguer C, Blasco-Costa I, Sarabeev VL, Morand S (2021) Fuzzy quantification of common and rare species in ecological communities (FuzzyQ). Methods Ecol Evol 12:1070–1079. https://doi.org/10.1111/2041-210X.13588
Barnes DKA, Peck LS (2008) Vulnerability of Antarctic shelf biodiversity to predicted regional warming. Clim Res 37:149–163. https://doi.org/10.3354/cr00760
Barrera-Oro ER, Marschoff ER, Casaux RJ (2000) Trends in relative abundance of fjord Notothenia rossii, Gobionotothen gibberifrons and Notothenia coriiceps at Potter Cove, South Shetland Islands, after commercial fishing in the area. CCAMLR Sci 7:43–52
Barrera-Oro ER, Marschoff E (2007) Information on the status of fjord Notothenia rossii, Gobionotothen gibberifrons and Notothenia coriiceps in the lower South Shetland Islands, derived from the 2000–2006 monitoring program at Potter Cove. CCAMLR Sci 14:83–87
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583. https://doi.org/10.2307/3284227
Cayuela L, Gotelli NJ, Colwell RK (2015) Ecological and biogeographic null hypotheses for comparing rarefaction curves. Ecol Monogr 85:437–455. https://doi.org/10.1890/14-1261.1
Chen D (2021) Impact of climate change on sensitive marine and extreme terrestrial ecosystems: recent progresses and future challenges. Ambio 50:1141–1144. https://doi.org/10.1007/s13280-020-01446-1
Clarke A, Harris CM (2003) Polar marine ecosystems: major threats and future change. Environ Conserv 30:1–25. https://doi.org/10.1017/S0376892903000018
Clarke A, Johnston NM, Murphy EJ, Rogers AD (2007) Introduction: Antarctic ecology from genes to ecosystems: the impact of climate change and the importance of scale. PHILOS T R SOC B 362:5–9. https://doi.org/10.1098/rstb.2006.1943
Convey P, Peck LS (2019) Antarctic environmental change and biological responses. Sci Adv 5:eaaz0888. https://doi.org/10.1126/sciadv.aaz0888
De Broyer C, Koubbi P, d’Acoz AV, Danis B, David B, Grant S, Julian GU, Christoph HE, Hosie G, Huetmann F, Alexandra POST, Ropert-Coudert Y (eds.) (2014) Biogeographic atlas of the Southern Ocean. Scientific Committee on Antarctic Research, Cambridge, 1–498
Doney SC, Ruckelshaus M, Duffy JE, Barry JP, Chan F, English CA, Galindo HM, Grebmeier JM, Hollowed AB, Knowlton N, Polovina J, Rabalais NN, Sydeman WJ, Talley LD (2012) Climate change impacts on marine ecosystems. Ann Rev Mar Sci 4:11–37. https://doi.org/10.1146/annurev-marine-041911-111611
Dykyy IV, Peklo AM (2012) Seals of the Argentine Islands (Antarctica). Zbirnyk Prats Zoologichnogo Muzeju 43:104–116 (in Russian)
Eastman JT (1991) Evolution and diversification of Antarctic notothenioid fishes. Am Zool 31:93–109. http://www.jstor.org/stable/3883462
Faltýnková A, Georgieva S, Kostadinova A, Bray RA (2017). Biodiversity and evolution of digeneans of fishes in the Southern Ocean In: Klimpel S, Kuhn T, Mehlhorn H (ed) Biodiversity and evolution of parasitic life in the Southern Ocean, Parasitology Research Monographs. Springer Nature, Cham, 9 pp. 49–75. https://doi.org/10.1007/978-3-319-46343-8_5
Gutt J, Bertler N, Bracegirdl TJ, Buschmann A, Comiso J, Hosie G, Isla E, Schloss IR, Smith CR, Tournadre J, Xavier JC (2015) The Southern Ocean ecosystem under multiple climate change stresses–an integrated circumpolar assessment. Glob Chang Bio Bioenergy 21:1434–1453. https://doi.org/10.1111/gcb.12794
Hoberg EP (1986) Aspects of ecology and biogeography of Acanthocephala in Antarctic seabirds. Ann Parasitol Hum Comp 61:199–214. https://doi.org/10.1051/parasite/1986612199
Hoegh-Guldberg O, Bruno J (2010) The impact of climate change on the world’s marine ecosystems. Science 328(5985):1523–1528. https://doi.org/10.1126/science.1189930
Hoogester JN, White MG (1981) Notes on parasite infestation of inshore fish at Signy Island, South Orkney Islands. BAS Bulletins 54:23–31
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456. https://doi.org/10.1111/2041-210X.12613
Hudson PJ, Dobson AP, Lafferty KD (2006) Is a healthy ecosystem one that is rich in parasites? Trends Ecol Evol 21:381–385. https://doi.org/10.1016/j.tree.2006.04.007
Klimpel S, Kuhn T, Mehlhorn H (2017) Biodiversity and evolution of parasitic life in the Southern Ocean. Parasitology Research Monographs vol 9. Springer Nature, Cham:13–31. https://doi.org/10.1007/978-3-319-46343-8
Kuhn T, Zizka VMA, Münster J, Klapper R, Mattiucci S, Kochmann J, Klimpel S (2018) Lighten up the dark: metazoan parasites as indicators for the ecology of Antarctic crocodile icefish (Channichthyidae) from the north-west Antarctic Peninsula. PeerJ 6:e4638. https://doi.org/10.7717/peerj.4638
Kuzmina TA, Salganskij OO, Lisitsyna OI, Korol EM (2020) Helminths of Antarctic rockcod Notothenia coriiceps (Perciformes, Nototheniidae) from the Akademik Vernadsky station area (Argentine Islands, West Antarctica): new data on the parasite community. Zoodiversity 54:99–110. https://doi.org/10.15407/zoo2020.02.099
Kuzmina T, Dykyy I, Salganskij O, Lisitsyna O, Korol E, Kuzmin Y (2021) Helminth diversity in teleost fishes from the area of the Ukrainian Antarctic station “Akademik Vernadsky”, Argentine Islands, West Antarctica. Zoodiversity 55:251–264. https://doi.org/10.15407/zoo2021.03.251
Kuzmina T, Salganskij O, Dykyy I, Lisitsyna O, Korol E, Faltynkova A, Kuzmin Y (2021b) Helminths of the Antarctic dragonfish, Parachaenichthys charcoti (Perciformes, Notothenioidei, Bathydraconidae) studied near Galindez Island (Argentine Islands, West Antarctica). Acta Parasitol 66(4):1424–1430. https://doi.org/10.1007/s11686-021-00417-0
Kuzmina T, Vishnyakova K, Lisitsyna O, Korol E, Kuzmin Y (2022) Helminth diversity in teleost fishes from the South Orkney Islands region, West Antarctica. Zoodiversity 56:135–152. https://doi.org/10.15407/zoo2022.02.135
Kuzmina T, Laskowski Z, Salganskij O, Zdzitowiecki K, Lisitsyna O, Kuzmin Y (2022b) Helminth assemblages of the Antarctic black rockcod, Notothenia coriiceps (Actinopterygii: Nototheniidae) in coastal waters near Galindez Island (Argentine Islands, West Antarctic): temporal changes in the endoparasite community. Acta Parasitol 67. https://doi.org/10.1007/s11686-021-00448-7
Kuzmina T, Kuzmin Y, Salganskij O, Lisitsyna O, Korol E (2022) Analysis of the helminth community of the Antarctic Black Rockcod, Notothenia coriiceps (Actinopterygii: Nototheniidae) studied near Galindez Island, West Antarctica, in 2014–2015 and 2020–2021. Ukr Ant J 20:85–95. https://doi.org/10.33275/1727-7485.1.2022.691
Kvach Y, Kuzmina T (2020) Parasitological research in Antarctica: review of the issues and future prospects. Ukrainian Antarctic Journal 1:102–110. https://doi.org/10.33275/1727-7485.1.2020.383 (in Ukrainian)
La Mesa M, Eastman JT, Vacchi M (2004) The role of notothenioid fish in the food web of the Ross Sea shelf waters: a review. Polar Biol 27:321–338. https://doi.org/10.1007/s00300-004-0599-z
Laskowski Z, Korczak-Abshire M, Zdzitowiecki K (2012) Changes in acanthocephalan infection of the Antarctic fish Notothenia coriiceps in Admiralty Bay, King George Island, over 29 years. Pol Polar Res 33:99–108. https://doi.org/10.2478/v10183−012−0005−4
Laskowski Z, Zdzitowiecki K (2017) Acanthocephalans in Sub-Antarctic and Antarctic. In: Klimpel S, Kuhn T, Mehlhorn H (eds.) Biodiversity and evolution of parasitic life in the Southern Ocean. Parasitology Research Monographs. vol. 9. Springer, Cham, 141–182. https://doi.org/10.1007/978-3-319-46343-8_8
Laws RM, Weller G, Stonehouse B, Robin GDeQ, Behrendt JC, Barker PF, Drewry DJ, Wynn-Williams DD, Walton DWH, Basson M (1992) Antarctica and environmental change: closing remarks [and discussion]. Phil Trans Biol Sci. 338(1285):329–334. http://www.jstor.org/stable/55727
Lüdecke D, Ben-Shachar MS, Patil I, Waggoner P, Makowski D (2021) performance: An R package for assessment, comparison and testing of statistical models. J Open Source Softw 6:3139. https://doi.org/10.21105/joss.03139
Manilo LG (2006) Ichthyofauna and morphobiological characteristics of mass fish species of coastal waters of Argentine Islands (Antarctica). Zbirnyk Prats Zoologichnogo Muzeju 38:5–22 (in Ukrainian)
Mouritsen KN, Poulin R (2002) Parasitism, climate oscillations and the structure of natural communities. Oikos 97:462–468. https://doi.org/10.1034/j.1600-0706.2002.970318.x
Muñoz G, Cartes FD (2020) Endoparasitic diversity from the Southern Ocean: is it really low in Antarctic fish? J Helminthol 94:1–10. https://doi.org/10.1017/S0022149X20000590
Muñoz G, Rebolledo M (2018) Comparison of the parasite community of two notothens, Notothenia rossii and N. coriiceps (Pisces: Nototheniidae), from King George Island, Antarctica. J Helminthol:1–6. https://doi.org/10.1017/S0022149X18000858
Münster J, Kochmann J, Grigat J, Klimpel S, Kuhn T (2017) Parasite fauna of the Antarctic dragonfish Parachaenichthys charcoti (Perciformes: Bathydraconidae) and closely related Bathydraconidae from the Antarctic Peninsula. Southern Ocean Parasites Vectors 10:235. https://doi.org/10.1186/s13071-017-2176-7
Near TJ (2009) Notothenioid fishes (Notothenioidei). In: Hedges SB, Kumar S (eds) The time tree of life. Oxford University Press, Oxford, pp 339–343
Near TJ, Dornburg A, Kuhn KL, Eastman JT, Pennington JN, Patarnello T, Zane L, Fernández DA, Jones CD (2012) Ancient climate change, antifreeze, and the evolutionary diversification of Antarctic fishes. Proc Natl Acad Sci USA 109:3434–3439. https://doi.org/10.1073/pnas.1115169109
Oğuz MC, Tepe Y, Belk MC, Heckmann RA, Aslan B, Gürgen M, Bray RA, Akgül Ü (2015) Metazoan parasites of Antarctic fishes. Turkiye Parazitol Derg 39:174–178. https://doi.org/10.5152/tpd.2015.3661
Oksanen J, Simpson GL, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Solymos P, Stevens MHH, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, Caceres MD, Durand S, Evangelista HBA, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill MO, Lahti L, McGlinn D, Ouellette M-H, Cunha ER, Smith T, Stier A, Braak CJFT, Weedon J (2022) Vegan: community ecology package. https://CRAN.R-project.org/package=vegan
Palm HW, Reimann N, Spindler M, Plötz J (1998) The role of the rock cod Notothenia coriiceps in the life cycle of Antarctic parasites. Polar Biol 19:399–406. https://doi.org/10.1007/s003000050265
Popovic GC, Warton DI, Thomson FJ, Hui FKC, Moles AT (2019) Untangling direct species associations from indirect mediator species effects with graphical models. Methods Ecol Evol 10:1571–1583. https://doi.org/10.1111/2041-210X.13247
Post E, Alley RB, Christensen TR, Macias-Fauria M, Forbes BC, Gooseff MN, Iler A, Kerby JT, Laidre KL, Mann ME, Olofsson J, Stroeve JC, Ulmer F, Virginia RA, Wang M (2019) The polar regions in a 2°C warmer world. Sci Adv 5:eaaw9883. https://doi.org/10.1126/sciadv.aaw9883
Poulin R (2006) Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parasitology 132:143–151. https://doi.org/10.1017/S0031182005008693
Poulin R (2011) Evolutionary ecology of parasites. Princeton University Press. https://doi.org/10.1515/9781400840809
Poulin R, Mouritsen KN (2006) Climate change, parasitism and the structure of intertidal ecosystems. J Helminthol 80:183–191. https://doi.org/10.1079/JOH2006341
R core Team (2022) R: a language and environment for statistical computing. A language and environment for statistical computing. R foundation for statistical computing'. Vienna, Austria. Available at: https://www.r-project.org/
Rocka A (2003) Cestodes of the Antarctic fishes. Pol Polar Res 24:261–276
Rocka A (2004) Nematodes of the Antarctic fishes. Pol Polar Res 25:135–152
Rocka A (2006) Helminths of Antarctic fishes: life cycle biology, specificity and geographical distribution. Acta Parasitol 51:26–35. https://doi.org/10.2478/s11686-006-0003-y
Rocka A (2017) Cestodes and nematodes of Antarctic fishes and birds. In: Klimpel S, Kuhn T, Mehlhorn H (eds) Biodiversity and evolution of parasitic life in the Southern Ocean. Parasitology Research Monographs. Springer, Cham, pp. 77–107. https://doi.org/10.1007/978-3-319-46343-8_6
Rokicki J, Rodjuk G, Zdzitowiecki K, Laskowski Z (2009) Larval ascaridoid nematodes (Anisakidae) in fish from the South Shetland Islands (Southern Ocean). Pol Polar Res 30:49–58
Rózsa L, Reiczigel J, Majoros G (2000) Quantifying parasites in samples of hosts. J Parasitol 86:228–232. https://doi.org/10.1645/0022-3395(2000)086[0228:QPISOH]2.0.CO;2
Shirihai H (2008) A complete guide to Antarctic wildlife. Princeton University Press
Stevenson M, Nunes T, Heuer C, Marshall J, Sanchez J, Thornton R, Reiczigel J, Robison-Cox J, Sebastiani P, Solymos P, Yoshida K, Jones G, Pirikahu S, Firestone S, Kyle R, Popp J, Jay M (2018). epiR: tools for the analysis of epidemiological data. R package version 0.9–96.
Turner J, Barrand N, Bracegirdle T, Convey P, Hodgson D, Jarvis M, Jenkins A, Marshall G, Meredith MP, Roscoe H, Shanklin J, French J, Goosse H, Guglielmin M, Gutt J, Jacobs S, Kennicutt MC II, Masson-Delmotte V, Mayewski P, Navarro F, Robinson S, Scambos T, Sparrow M, Summerhayes C, Speer K, Klepikov A (2014) Antarctic climate change and the environment: an update. Polar Rec 50:237–259. https://doi.org/10.1017/S0032247413000296
Vaughan DG, Marshall GJ, Connolley WM, Parkinson C, Mulvaney R, Hodgson DA, King JC, Pudsey CJ, Turner J (2003) Recent rapid regional climate warming on the Antarctic Peninsula. Clim Change 60:243–274. https://doi.org/10.1023/A:1026021217991
Veselskyy MV, Khoetskyy PB (2018) Ichthyofauna of the waters of the archipelago of the Argentine Islands (Ukrainian Antarctic expedition 2015–2016). Naukovi Zapysky Ternopilskogo Natsionalnogo Pedagigichnogo Universytety, Seria Biologia 1:36–43 (in Ukrainian)
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJ, Fromentin JM, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416(6879):389–395. https://doi.org/10.1038/416389a
Wang Y, Naumann U, Wright ST, Warton DI (2012) mvabund– an R package for model-based analysis of multivariate abundance data. Methods Ecol Evol 3:471–474. https://doi.org/10.1111/j.2041-210X.2012.00190.x
Weber EP, Govett P (2009) Parasitology and necropsy of fish. Compend Contin Educ Vet 31(2):E12
Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, Grolemund G, Hayes A, Henry L, Hester J, Kuhn M, Pedersen TL, Miller E, Bache SM, Müller K, Ooms J, Robinson D, Seidel DP, Spinu V, Takahashi K, Vaughan D, Wilke C, Woo K, Yutani H (2019) Welcome to the tidyverse. J Open Source Softw 4:1686. https://doi.org/10.21105/joss.01686
Zdzitowiecki K (2001) Acanthocephala occurring in intermediate hosts, amphipods, in Admiralty Bay (South Shetland Islands, Antarctica). Acta Parasitol 46:202–207
Zdzitowiecki K, Laskowski Z (2004) Helminths of an Antractic fish, Notothenia coriiceps, from the Vernadsky Station (Western Antarctic) in comparison with Admirality Bay (South Shetland Islands). Helminthologia 41:201–207
Zdzitowiecki K, Presler P (2001) Occurrence of Acanthocephala in intermediate hosts, Amphipoda, in Admiralty Bay, South Shetland Islands, Antarctica. Pol Polar Res 22:205–212
Funding
This study was partially supported by the National Research Foundation of Ukraine (Project Number 2020.02/0074) and the National Antarctic Scientific Center of the Ministry of Education and Science of Ukraine (Projects H/03–2021).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualisation: Yaroslav Y. Syrota, Yuriy I. Kuzmin, and Tetiana A. Kuzmina. Material collection and processing were performed by Oleksander O. Salganskiy and Ihor V. Dykyy; helminth identifications and data processing were performed by Tetiana A. Kuzmina, Olga I. Lisitsyna, Eleonora M. Korol, Louis H. du Preez, and Ivanna G. Dmytrieva. Data analysis: Yaroslav Y. Syrota, Yuriy I. Kuzmin, and Tetiana A. Kuzmina. The first draft of the manuscript was written by Yaroslav Y. Syrota, Yuriy I. Kuzmin, and Tetiana A. Kuzmina, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Robin Flynn
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Syrota, Y.Y., Kuzmin, Y.I., Lisitsyna, O.I. et al. Infection patterns of helminth community in black rockcod Notothenia coriiceps in West Antarctica over a 6-year term. Parasitol Res 122, 853–865 (2023). https://doi.org/10.1007/s00436-023-07785-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-023-07785-8