Abstract
We describe morphologically unique Leucocytozoon pterotenuis sp. nov. (Haemosporida, Leucocytozoidae), the first reported leucocytozoid species developing in fusiform host cell found in a Neotropical passeriform bird. The type host of this parasite is the Chestnut-crowned Antpitta (Grallaria ruficapilla, Grallariidae), an elusive native passerine bird whose natural history remains, to a large degree, unexplored. This bird was captured in Palacio forest in the damping zone of Chingaza National Natural Park, Cundinamarca, Colombia, at 2900 m above sea level (asl). Gametocytes of the new species develop both in roundish and fusiform host cells. This parasite is readily morphologically distinguishable from the described Leucocytozoon species because its host cells possess the narrow (needle-like) spindle-shaped processes, which length markedly exceeds their width. Additionally, the host cell nucleus markedly extends into the processes. Phylogenetic relationships were constructed based on a fragment of the mitochondrial cytochrome b gene and the complete mitochondrial genome. Phylogenetic analysis placed the lineage of L. pterotenuis in different positions depending on the length of the sequence analyzed that is likely due to poor sampling of Leucocytozoon species, especially from rare or non-passerine hosts, as well as a paucity of complete mitochondrial sequences of these parasites. Available data indicate that Leucocytozoon parasites are distributed mainly in mountain regions of the Neotropics where unique morphological forms have been recently discovered. To a better knowledge of the diversity of Leucocytozoon spp. and their host–vector–parasite interactions in Neotropical countries, additional deep and intensive samplings are needed, particularly in orders different to Passeriformes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies characterizing biological diversity have gained momentum largely due to the goods and services that ecosystems provide to human communities. There are efforts to measure, assess, and conserve biodiversity that have incorporated parasitic species as a source of information about their hosts. Parasites are a diverse ecological guild that is present in almost all ecosystems. They affect the host behavior, growth, lifespan, and fecundity; moreover, they may provide information about the natural history of the host, trophic relationships in ecosystems in which they occur, their evolution, and distribution patterns (Pérez Ponce de León and García 2001; Poulin et al. 2011).
Currently, approximately 200 species of avian malaria parasites and related hemosporidians have been described based on traditional taxonomy (Valkiūnas 2005). However, it is well known that molecular tools have revealed high hidden lineage diversity (Bensch et al. 2004). Regardless this apparent species richness, limited attention has been given to the characterization and description of new species, particularly in tropical countries.
The Andean mountains have been recognized for their great variety of ecosystems and biodiversity hotspots (Josse et al. 2009). Recent studies in this region have shown new species of avian hemosporidian parasites as well as new ecological host–parasite interactions (Mantilla et al. 2013a, b; Matta et al. 2014a). Considering that these mountains harbor around 2000 avian species with almost 600 of them endemic (Herzog and Kattan 2011), it is expected that such host diversity will correlate with a high parasite diversity. Unfortunately, there are many avian species with limited or no records of their parasites. Among them, we can find those species belonging to the family Grallariidae. Birds of this family are widely distributed from Central to South America, with maximum species diversity in the tropical Andes. Twenty-seven out of 51 species described in the family have been reported in Colombia, and six are categorized as extinction risk (McMullan et al. 2011; IUCN 2014).
Here, we investigate the hemoparasites associated to the Chestnut-crowned Antpitta (Grallaria ruficapilla); this is a ground passerine bird belonging to the family Grallariidae that inhabits the scrublands and understory of humid Neotropical forests (McMullan et al. 2011). This species is widely distributed through the Andes mountains of Colombia, Ecuador, Peru and Venezuela between 1200 to 3600 m above sea level (asl) (BirdLife 2014). Different to other Antpittas, this bird can be easily found in heavily degraded tropical forest (Krabbe and Schulenberg 2004). There is limited information on the host parasite infections where Grallaridae species and its close relatives of the Formicariidae are involved. They have been found infected with Plasmodium, Haemoproteus, microfilaria, and Trypanosoma parasites (Galindo and Sousa 1966; White et al. 1978). Leucocytozoon infections have been detected only by molecular methods in Stripe-headed Antpitta Grallaria andicolus and Grallaria sp. at the Peruvian Andes (Witt and McNew, unpublished data). However, because abortive hemosporidian infections have been reported in birds (Levin et al. 2013), it remains unclear if the reported leucocytozoids complete life cycle or produce gametocytes in Grallaria species. Observation of blood stages is necessary to answer this question. In this study, two different Grallaria species were found infected with Leucocytozoon hemoparasites. In one of them, the Chestnut-crowned Antpitta, a new parasite species was found with gametocytes developing in fusiform host cells, which were reported for the first time in Passeriformes birds in South America. In this study, the morphological description and molecular characterization of Leucocytozoon pterotenuis sp. nov, a parasite of Chestnut-crowned Antpitta, are provided. We discuss phylogenetic relationship and possible host association of this parasite as well as patterns of distribution of Leucocytozoon parasites in the Neotropics.
Materials and methods
Sampling area
Birds were captured using mist nets at the highland at four sites in Colombia: (1) Otun Lagoon, Los Nevados National Natural Park (NNP) from April 2010 to April 2011, (2) Pupiales (Nariño department) in December 2012, (3) Palacio Forest (Chingaza NNP) from February 2012 to February 2014, and (4) Monter Redondo Station (Chingaza NNP) from December 2008 to October 2009 and from June to July of 2012. Otun Lagoon (4° 46′ N, 75° 24′ W) is located at central mountain range in Los Nevados NNP, at 3950 m asl. Landscape is dominated by Páramo ecosystem and Andean forests with an average annual temperature of 6 °C and an annual average rainfall of 1250 mm (Vásquez and Serrano 2009).
Pupiales (0° 54′ N, 77° 39′ W) is located at the south of the Colombian Andes in Nariño department. Sampling was carried out at 3014 m asl, where montane dry and wet forests, as well as Páramo ecosystem, are present; however, the land is used mainly for agricultural activities. In the latter area, the annual media rainfall is 942.85 mm and the average temperature is 12 °C (Cadena et al. 2012). The Chingaza NNP is located in Colombian Oriental mountain range. Palacio forest (4° 41′ N, 73° 50′ W) is an Andean forest situated in the damping zone of the park at 2900 m asl in the lower altitudinal boundary of Páramo ecosystem. The temperature ranged between 12 and 18 °C in this area, but it may be under 0 °C, and the annual average of rainfall is 1900 mm (Vargas-Rios et al. 2004). Monte Redondo Station is located at 3100 m asl in an area covered mainly by Páramo ecosystem, where average temperature ranges between 6 and 7 °C; however, like in Palacio forest, temperature can fall below 0 °C, and the average rainfall in this area can reach 2900 mm (Vargas-Rios et al. 2004). For all localities, except for Pupiales, sampling dates included rainy and dry periods. Birds were identified according to the South American Classification Committee (Remsen et al. 2012).
Sample and blood film examination
Blood samples were obtained by bird brachial vein puncture. From each bird, three thin smears were prepared, air-dried, fixed in absolute methanol for 5 min, and stained with Giemsa (pH 7.2) for 45 min. In addition, three drops of blood (approximately 50 μl) were stored in SET buffer (0.05 M Tris, 0.15 M NaCl, 0.5 M EDTA, pH 8.0) for molecular tests.
Blood films were examined by double-blind microscopic examination, first at ×100 for 10 min and then at ×1000 for 20 min, using a Leica DM750 microscope. Digital images were taken with a Leica EC3 camera; they were processed using the LAS EZ software (Leica Microsystems, Wetzlar, Germany). Morphometric features of parasites were those described by Valkiūnas et al. (2010). At least 100 images of the new parasite species were obtained and studied; measurements were made digitally upon 31 best quality images of gametocytes using ImageJ (Schneider et al. 2012).
Intensity of infection was determined according to Muñoz et al. (1999) by counting the number of parasites in 100 microscopic fields at high magnification (×1000), where red blood cells were forming a single monolayer. Differential counts of white blood cells were done on smears by visualization of 100 white blood cells and counting eosinophils, heterophils, and lymphocytes (Hauptmanova et al. 2002).
DNA extraction, cytochrome b gene, and complete mitochondrial genome amplification and sequencing
The parasite molecular characterization was carried out by using the mitochondrial cytochrome b gene (Escalante et al. 1998). This gene has been widely used in malarial parasites, and there is a substantial database that allows comparing our findings with those reported by others. DNA was extracted only from microscopy-positive blood samples using a standard phenol–chloroform protocol (Sambrook et al. 1989). Due to the limited availability of stored blood samples of the Grallaridae birds, only two samples out of three sampled individuals, one from Chestnut-crowned Antpitta (G. ruficapilla) and one from Tawny Antpitta (Grallaria quitensis) were processed. Molecular detection of Leucocytozoon was made amplifying cytochrome b gene according to the nested PCR protocol recommended by Hellgren et al. (2004). Amplified products were visualized on a 1.5 % agarose gel and purified using differential precipitation with ammonium acetate protocol (Bensch et al. 2000) and sequenced in both senses using a 3730 xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA) through Macrogen (Macrogen Inc.).
In addition to the cytochrome b, the complete mitochondrial genome (mitochondrial DNA (mtDNA)) was also amplified, cloned, and sequenced from both individuals; Chestnut-crowned Antpitta and Tawny Antpitta were infected with different morphospecies of Leucocytozoon. We amplified 5895 base pairs (bp) of the parasite mtDNA using primers forward 5′ GA GGA TTC TCT CCA CAC TTC AAT TCG TAC TTC/reverse 5′ CAG GAA AAT WAT AGA CCG AAC CTT GGA CTC with TaKaRa LA Taq™ Polymerase (TaKaRa Mirus Bio Inc, Shiga, Japan). Details about PCR protocol and cloning are described by Pacheco et al. (2011a, b). Both strands for at least three clones were sequenced using an Applied Biosystems 3730 capillary sequencer. The partial cytochrome b gene and the mtDNA genome sequences were deposited in GenBank accession nos. KM610045, KM610046, KM272250, and KM272251.
Phylogenetic analysis
In order to estimate phylogenetic relationships between the new species and other hemosporidian parasites, two independent alignments were made as follows: one for partial sequences of cytochrome b gene (476 bp) and another for almost complete mtDNA genome (5456 bp excluding gaps). First, an alignment was done using 19 cytochrome b sequences with 476 nucleotides edited and aligned with MEGA5 software (Tamura et al. 2011). Sequences included in the analyses were obtained from GenBank (five sequences) and MalAvi database (six sequences) (Bensch et al. 2009) and generated in this study (eight sequences).
Bayesian methods implemented on MrBayes v3.1.2 (Ronquist and Huelsenbeck 2003) were used to do the phylogenetic reconstructions under the general time-reversible model (GTR+I+Γ), the best of 88 models according to the corrected Akaike information criterion implemented on jModelTest 2.1.1 (Darriba et al. 2012). Phylogeny with nodal support was inferred in MrBayes using two independent runs of 5 × 106 generations, sampled every 100 generations. A majority rule consensus phylogeny was obtained from 25,000 trees after discarding the 25 % of the trees as burn-in period. In addition, a maximum likelihood analysis was conducted using RAxML Black Box through CIPRES portal (Miller et al. 2010). This analysis was carried out applying the same model as for Bayesian inference, using 1000 bootstrap replications. After, the phylogenies were visualized and edited using FigTree v1.3.1 (Rambaut 2006).
Second, the mtDNA genome alignment was made using ClustalX v2.0.12 and Muscle as implemented in SeaView v4.3.5. This alignment included the 18 mitochondrial genomes available in the GenBank for hemosporidians isolated from lizards and birds. The mtDNA alignment was further divided into four categories where each gene (cytochrome oxidase I, cytochrome oxidase III, and cytochrome b) was used as a separate partition plus the non-coding regions (Pacheco et al. 2011a). The phylogenetic relationships were estimated on mtDNA genome alignment by using both the maximum likelihood (ML) method implemented in PhyML v3.0 (Guindon et al. 2010) and Bayesian methods using MrBayes v3.1.2 with the default priors (Ronquist and Huelsenbeck 2003). The reliability of the nodes in the ML tree was assessed by the bootstrap method with 200 pseudo-replications. Bayesian support for the nodes was inferred in MrBayes using 6 × 106 Markov Chain Monte Carlo (MCMC) steps, and after convergence was reached, we discarded 25 % of the sample as “burn-in” periods. In both cases, cytochrome b and mtDNA analyses, convergence is reached when the value of the potential scale reduction factor (PSRF) is between 1.00 and 1.02 and the average standard deviation of the posterior probability is below 0.01 (Ronquist and Huelsenbeck 2003). General time-reversible model (GTR+I+Γ) was also the best fit to these data, as estimated by MEGA v5.0 (Tamura et al. 2011) .
In addition, divergences among specific pairs of Leucocytozoon species were estimated on partial cytochrome b gene and mtDNA genomes using the Kimura two-parameter model of substitution as implemented in MEGA v5.0 (Tamura et al. 2011).
Results
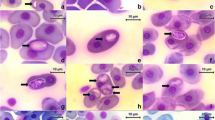
Out of 774 birds sampled from 81 species, 22 families and 9 orders, 36 (4.65 %) were found infected with different Leucocytozoon species diagnosed by microscopic examination of blood film. Three individuals from Grallaridae were captured as follows: one individual of Tawny Antpitta (G. quitensis) in Pupiales, one individual of the same species in Otun Lagoon, and one Chestnut-crowned Antpitta (G. ruficapilla) in the Palacio forest. Two Tawny Antpitta, one from Pupiales and one from Otun Lagoon, were found infected with Leucocytozoon sp. Gametocytes of these birds were seen only in roundish host cells (Fig. 1a–c). The cytoplasm contains small vacuoles; the host cell nucleus had cap-like shape, and it extends less than one half of the circumference of gametocyte (Fig. 1a–c). However, both birds showed low intensity of infection and scarce number of morphological stages, making impossible the species identification. Nevertheless, it was possible to obtain both a cytochrome b lineage and complete mitochondrial DNA from the Antpitta captured in Pupiales (GenBank Accession nos. KM272251, KM610045). On the other hand, the Chestnut-crowned Antpitta captured in the Palacio forest showed an infection with morphologically unique L. pterotenuis sp. nov. (prevalence 0.13 %) (Table 1); its white blood cell counts were 68 % of heterophils, 18 % of eosinophils, and 16 % of lymphocytes. This individual was also found coinfected with microfilaria and Trypanosoma sp.
Leucocytozoon spp. from Grallaria sp. of Colombia. Leucocytozoon sp. found in Tawny Antpitta Grallaria quitensis captured at Otun Lagoon (a, b) and Pupiales (c). Macrogametocytes (d–k) and microgametocyte (l) of Leucocytozoon pterotenuis sp. nov. from the blood of its type vertebrate host, Chestnut-crowned Antpitta Grallaria ruficapilla Antpitta. d–e Gametocytes in roundish host cells. f–l Gametocytes in fusiform host cells. Black arrowheads parasite nucleus, open arrowheads parasite nucleolus, black long arrows vacuoles, open long arrows volutin granules, double-triangle arrows host cell nucleus, asterisk host cell cytoplasm. Giemsa-stained thin blood films. Scale bar = 10 μm
Description
L. (Leucocytozoon) pterotenuis sp. nov.
Macrogametocytes (Fig. 1d–k)
Develop in fusiform (Fig. 1f–k) and roundish (Fig. 1a–c) host cells (Table 2). Gametocytes in roundish host cells are roundish in shapes (Fig. 1d, e). Gametocytes in fusiform host cells vary from oval to ellipsoid in shapes. Parasite nucleus varies from roundish (Fig. 1j) to elongate (Fig. 1g); its position is markedly variable in gametocytes. Nucleolus was visible in 57 % of gametocytes in fusiform host cells. The parasite cytoplasm contains large number of small vacuoles (Fig. 1i, j); volutin granules were seen in 43 % of gametocytes (Fig. 1f, h). The nucleus of fusiform host cell is displaced, is deformed, and lies peripherally like a homogeneous band with ends markedly extending beyond the circumference of gametocytes and not touching the gametocytes (Fig. 1f–k). The cytoplasm of host cells forms two long, narrow spindle-shaped processes, which are of needle-like shape, and their length is markedly greater than widths (Fig. 1f–k). Host cell nucleus markedly extends into the cytoplasmic processes, a distinctive character of this species (Fig. 1f–k). Gametocytes in roundish host cells possess roundish nucleus of variable position; nucleolus was seen in 28 % of the gametocytes. Small vacuoles (Fig. 1d, e) and volutin granules were seen in 43 and 85 % of the gametocytes developing in fusiform and roundish host cells, respectively. The nucleus of roundish host cell is pushed aside, is deformed, and looks like a cap; it extended less than one half of the circumference of gametocyte (Fig. 1d, e). Remnants of the host cell cytoplasm are usually seen around the gametocytes as an envelope of variable form (Fig. 1d, e).
Microgametocytes (Fig. 1l)
General configuration and other features as for macrogametocytes with the usual hemosporidian sexual dimorphic characters. Proportion of microgametocytes and macrogametocytes in the type material is 1:4. Measurements were taken upon one parasitized cell due to marked fragility of the gametocytes during preparation of blood smears, resulting in distorted morphology of the majority observed parasites.
Remarks
L. pterotenuis develops in roundish and fusiform host cells. This parasite can be readily distinguished from other described leucocytozoids due to unique shape of nuclei of its fusiform host cells. Mainly, the nuclei of fusiform host cells assume band-like forms (without thickenings on the ends); their covering between 30 and 55 % of the circumference of the gametocytes possesses markedly narrowed ends and extends into the cytoplasmic processors of host cells. These morphological characters are unique for this parasite.
Gametocytes in fusiform host cells develop in many species of Leucocytozoon which parasitize different orders of birds, but they are rare in passeriform birds. Only three species of leucocytozoids, which inhabit fusiform host cells, parasitize passerines. These are Leucocytozoon balmorali, which parasitizes Grey-headed Bush-shrike (Malaconotus blanchoti), Willow Warbler (Phylloscopus trochilus), Olive Bush-shrike (Malaconotus olivaceus), and Brown-crowned Tchagra (Tchagra australis) and some other bird species (Peirce 1984), Leucocytozoon maccluri which type host is Dark-sided Thrush (Zoothera marginata) (Greiner 1976) and Leucocytozoon hamiltoni which type host is Turkestan Tit (Parus bokharensis) (Valkiunas et al. 2002). None of these parasites possesses band-like host cell nuclei, which extend into the cytoplasmic processers. Due to this character, L. pterotenuis is similar to Leucocytozoon simondi, a specific parasite of birds belonging to the Anseriformes. In the latter parasite, nucleus of fusiform host cell looks like a more or less evident dumbbell-shaped band with clear thickenings at both ends (Valkiūnas 2005). The latter character is not characteristic of the new species.
Taxonomic summary
Type host: the Chestnut-crowned Antpitta, G. ruficapilla (Grallaridae, Passeriformes).
Additional hosts: unknown.
Type locality: Palacio Forest at the damping zone of Chingaza NNP (4° 41′ N, 73° 50′ W, 2950 m asl), Cundinamarca, Colombia.
Type specimens: Hapantotype (accession no. GERPH-07966, intensity of infection is 0.05 %, lineage KM272250 L_GRRUF01, collected by Rocio Hernández, 10 February 2014) was deposited in the biological collection Grupo de Estudio Relación Parásito Hospedero (GERPH) at Universidad Nacional de Colombia, Bogotá, Colombia. Parahapantotypes (accession nos. GERPH-07962, GERPH-07963, GERPH-07964, GERPH-07965, GERPH-07967, GERPH-07968, GERPH-07969, GERPH-07970, and GERPH-07971, other data as for the hapantotype) were deposited in the same collection. Digital images of blood stages of the parasite in the type preparations are available on request from GERPH.
DNA sequences: mitochondrial cytochrome b lineages L_GRRUF01 (GenBank no. KM272250) were detected from the same individual bird, from which hapantotype was originated. The L. pterotenuis complete mtDNA genome sequence was deposited in the GeneBank (KM610046).
Site of infection: blood cells, which origin is unclear.
Prevalence: The prevalence in the type host was 1 of 1 (100 %). In the type locality, 1 of 278 birds captured at Palacio Forest (0.36 %) was infected, as determined by microscopic examination. The overall prevalence (1 of 774 examined birds) was 0.13 % (Table 1).
Etymology: The species name (pterotenuis) was derived from the Latin words tenuis (thin) and ptero (wing); it refers to the slender elongated cytoplasmic processes of host cells of the parasite.
Phylogenetic relationships of parasites
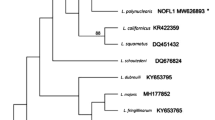
Lineages used to estimate phylogenies based on partial cytochrome b sequences clustered in similar clades in the trees constructed using both phylogenetic methods: Bayesian (Fig. 2a) and ML (Fig. 2b). Nevertheless, it is important to note the low support obtained in many nodes from these analyses. However, in both phylogenetic trees, the cytochrome b sequences of L. pterotenuis did not cluster with other sequences of passerine Leucocytozoon spp. Unlike Leucocytozoon sp. lineages detected in the Peruvian Grallaria species, the Colombian Leucocytozoon sp. found in birds of the same genus has clustered close to Leucocytozoon quynzae, a parasite recently reported in birds of the Trochilidae (Matta et al. 2014a). It is noteworthy that this parasite is located in unsolved clades in both phylogenies.
Bayesian (a) and maximum likelihood (b) phylogenetic hypothesis constructed using 476 bp of cytochrome b sequences of Leucocytozoon sp. from different bird hosts. Lineage of Leucocytozoon pterotenuis sp. nov. is given in bold font. A lineage of Haemoproteus columbae was used as outgroup. GenBank accession numbers or alternative lineage names of sequences from MalAvi are provided before the parasite species names. Bootstrap values and posterior probabilities are shown above the nodes. The branch lengths are drawn proportionally to the amount of change and the scale bars shown substitutions per site
Genetic distance between L. pterotenuis (L_GRRUF_01 GenBank accession no. KM272250) and Leucocytozoon sp. from Tawny Antpitta (GRQUI_01 GenBank accession no. KM272251) estimated on partial cytochrome b sequences was 4.3 %, being the smallest difference reported when compared with another leucocytozoids of passerine birds (Table 3).
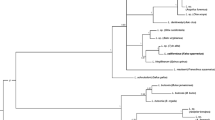
The phylogeny estimated with complete mitochondrial genomes is shown in Fig. 3. In this limited dataset, two well-supported clades of Leucocytozoon species were found sharing a common ancestor. In one clade, L. pterotenuis appears as sister taxa of Leucocytozoon sabrazesi (AB299369) isolated from Malaysian chicken, and the other clade included Leucocytozoon fringillinarum, Leucocytozoon majoris, and L. quynzae. The average genetic divergences using complete mtDNA and the three mitochondrial genes among Leucocytozoon species are given in Table 4.
Phylogenetic hypothesis constructed using complete mitochondrial genomes (5485 bp excluding gaps) of Leucocytozoon spp. The values above branches are posterior probabilities together with bootstrap values (in bold) as a percentage obtained for the maximum likelihood tree (see “Materials and methods” section)
Discussion
The northern portion of the Andean mountain ranges has almost the same number of species of the Amazon basin in an area that is 14-fold smaller (Herzog and Kattan 2011). These levels of species richness and endemicity likely involve a great number of host–parasite relationships that have not been explored. Indeed, in the last 10 years, Leucocytozoon spp. ceased to be considered as exotic parasites in the Neotropical countries, to become common element of resident bird parasitic fauna. However, transmission of leucocytozoids seems to be restricted to mountain areas and is rare on lowlands of the Neotropic (Forrester et al. 2001; Rodríguez et al. 2009; Lotta et al. 2013; Matta et al. 2014a). Leucocytozoids are nearly absent from birds at lowlands of the Neotropics, including the Amazonian basin (White et al. 1978; Belo et al. 2011; Lacorte et al. 2013; Svensson-Coelho et al. 2013). Such markedly spotty occurrence in birds is a unique character of Leucocytozoon spp. distribution of the Neotropics in comparison to other zoographical regions, in which these parasites are present and often prevalent both in lowlands and highlands (Valkiūnas 2005). Experimental research on vectors of leucocytozoids is lacking in the Neotropics but is essential for understanding distribution, epidemiology, and evolution of these parasites, some species of which cause diseases, sometimes even lethal, both in domestic and wild birds (Forrester and Greiner 2008; Santiago-Alarcon et al. 2012).
Antpittas (Grallaridae) are territorial ground passerines, which are of broad altitudinal distribution in Colombia; they have been reported between 300 and 4000 m asl. This genus reaches its highest diversity in Andean mountains above 800 m.; the four endemic species reported for this country are distributed upon 1200 m asl. Few individuals of Grallaridae have been reported as infected by avian hemosporidans (Galindo and Sousa 1966; White et al. 1978). The scarce number of infections of Antpittas might be explained by the ecological and behavioral traits of these birds (Kattan and Beltran 1999); since they are difficult to capture by mist netting. It is noteworthy that 100 % (3 of 3) of individual birds belonging to this genus sampled in Colombia were infected with Leucocytozoon spp. In general hemoparasite infections may be overlooked due to low parasitemias that make difficult to detect parasites using microscopic examination (Valkiūnas et al. 2008). It is noteworthy that the type host was coinfected with other two blood parasites (Trypanosoma and microfilaria) and the proportion of eosinophils was high when compared with previous data of white blood cell counts in birds (Hauptmanova et al. 2002; Gálvez et al. 2010) . It has been reported that coinfections may have synergistic or antagonistic effect on birds (Palinauskas et al. 2011). In this way, the effective detection of this parasite by microscopy could be the result of an increase in the intensity of infection, caused by a synergistic effect of these parasites.
L. pterotenuis is the first parasite described in birds of the Grallaridae; also, this is the first parasite with gametocytes developing in fusiform host cell that is described in Passeriformes in South America. This is an important finding regardless the intensive samplings on the Andean passerine birds as well as in other localities from Neotropical countries (Gabaldon et al. 1974; Gabaldon et al. 1975; Gabaldon and Ulloa 1976; Bennett et al. 1980; Rodríguez et al. 2009; Levin et al. 2012; Lacorte et al. 2013; Marzal et al. 2014; Galen and Witt 2014). The only one Leucocytozoon with fusiform gametocytes reported in South America is Leucocytozoon toddi, which infects Accipitridae (Falconiformes) (Forrester et al. 2001).
A single report of L. pterotenuis in only one avian host does not provide much information about the parasite and its ecology. This parasite is morphologically similar to L. simondi. Unfortunately, the sequence available for L. simondi has poor quality in the region that overlaps with our alignment so we had to exclude it from our phylogenetic analyses. On the other hand, it is worth noting that L. pterotenuis is phylogenetically close to L. sabrazesi (possible synonym of Leucocytozoon macleani according to Valkiūnas 2005), a parasite of the Galliformes birds. Interestingly, the latter parasite also develops gametocytes both in roundish and fusiform host cells, but it has not been reported in the Neotropics so far (Valkiūnas 2005). We could speculate that the presence of fusiform host cells in the type avian host might be a recent host switch from hosts in which fusiform host cells are common. Indeed, it is important to mention that there is sympatric presence of Andean Guan (Penelope montagnii, Cracidae) in Palacio forest; however, we have not sampled this species for parasitology research. Because parasites in new hosts often are highly virulent and even cause mortality (Olias et al. 2011), prevalence of such infections should be low in wildlife populations (Toft and Karter 1990). Determining whether this parasite relates with others found in Cracidae, for example, is simply a speculation at this point. It seems that the putative narrow distribution of L. pterotenuis is better explained by the poor sampling of these rare passerines and many species of non-passerines in Andes (Matta et al. 2014b).
The phylogenetic relationships reconstructed for L. pterotenuis showed different results depending on the length of the sequences used. Reconstructions obtained using only a fragment of the cytochrome b gene placed L. pterotenuis inside unsolved nodes closely related with a parasite from Apodiformes birds. Phylogenies constructed with different methods showed low nodal supports, mainly on the deep nodes. It is associated with the size and nature of this mitochondrial marker. The relatively high rate of evolution of cytochrome b gene makes it ideal to discriminate between closely related species (Escalante et al. 1998; Perkins et al. 2011; Pacheco et al. 2011a); however, the use of a small fragment of the gene may restrict the informative sites for the analysis (Matta et al. 2014a). Additionally, analyses carried out using a barcoding approach on sequences like cytochrome oxidase subunit I or, in our case, cytochrome b require a good baseline traditional taxonomy that allows to discern intraspecific from interspecific genetic divergences (Moritz and Cicero 2004; Valkiūnas et al. 2014). Another factor that could affect the analysis is the use of paralogous genes. Mainly, due to the methodology used to amplify the fragment of cytochrome b, there is a probability to amplify copies inserted on nuclear genome that have evolved independently from target gene (Funk and Omland 2003), leading to a non-reliable result. This is not our case since we also confirmed the cytochrome b fragment with the one derived from the complete (cloned) mtDNA. A more critical problem in the literature is the use of direct sequencing of PCR amplicons since mix infections in avian parasites are common in nature (Pérez-Tris and Bensch 2005; Valkiūnas et al. 2014), as has been reported in mammals (Pacheco et al. 2013).
In the case of primate malarias, it is well known that partial cytochrome b sequences lead to spurious phylogenetic relationships or problems separating species (Pacheco et al. 2013). The interest on avian malaria and related hemosporidians has generated a great number of lineages from a variety of birds; unfortunately many sequences are short and are the result of direct sequencing. Regardless this growing interest, there is still poor knowledge about infections in non-passerine birds and rare passerines as is the case with Antpittas (Dimitrov et al. 2014). The observed poor nodal support as well as the contradictions in the position of L. pterotenuis in different phylogenies might be due to limited sampling of cytochrome b in leucocytozoids developing in fusiform host cells in passerine or non-passerine birds, as well as the length of the sequences used.
Evolutionary relationships based on complete mitochondrial genome shows that L. pterotenuis is more closely related to L. sabrazesi than to other passerine leucocytozooids, supporting the similarity of morphological traits between these two parasites, which both have gametocytes in fusiform host cells (Valkiūnas 2005). In this phylogeny, polytomies seen in the analysis obtained using only cytochrome b fragments were solved with a good nodal support. In this way, an increase of information, preferably from genes of different origins, can improve our interpretation of the evolutionary history of these parasites. It is important to keep in mind that there are only few complete mitochondrial genomes available for Leucocytozoon species. In this way, despite of the new informative sites gained by the inclusion of these genes to the analysis, results obtained with few taxa could be widely criticized by its sensitiveness to homoplasy (Soltis et al. 2004). To obtain a better estimated of the phylogenetic relationships, ideally, these analyses should be constructed using a larger number of informative genes and taxa (Rokas and Carroll 2005).
Most of the surveys addressing avian hemosporidians use mist nets; it is well known that this capture method is biased toward small passerine birds (Valkiūnas et al. 2003). For better estimation of avian hemoparasite, biodiversity is desirable to use different catching methods, for instance, Noose carpet traps (Gosler 2004) and/or Cannon nets and Bow nets (Bennett et al. 1992).
In addition to a morphological description, this study provides the complete mitochondrial genome for the new parasite species, which, eventually coupled with other markers, will allow establishing more accurately phylogenetic relationships among Leucocytozoon species. Additionally, for a better understanding of evolutionary relationships and ecology of these parasites, more studies analyzing vector–host–parasite interactions should be developed.
References
Belo NO, Pinheiro RT, Reis ES, Ricklefs RE, Braga ÉM (2011) Prevalence and lineage diversity of avian haemosporidians from three distinct cerrado habitats in Brazil. PLoS One 6:e17654. doi:10.1371/journal.pone.0017654
Bennett GF, Witt H, White EM (1980) Blood parasites of some Jamaican birds. J Wildl Dis 16:29–38. doi:10.7589/0090-3558-16.1.29
Bennett GF, Montgomerie R, Seutin G (1992) Scarcity of haematozoa in birds breeding on the Arctic Tundra of North America. Condor 94:289–292. doi:10.2307/1368821
Bensch S, Stjernman M, Hasselquist D, Ostman O, Hansson B, Westerdahl H et al (2000) Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc Biol Sci 267:1583–1589. doi:10.1098/rspb.2000.1181
Bensch S, Pérez-Tris J, Waldenström J, Hellgren O (2004) Linkage between nuclear and mitochondrial DNA sequences in avian malaria parasites: multiple cases of cryptic speciation? Evolution 58:1617–1621. doi:10.1111/j.0014-3820.2004.tb01742.x
Bensch S, Hellgren O, Pérez-Tris J (2009) MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9:1353–1358. doi:10.1111/j.1755-0998.2009.02692.x
BirdLife I (2014) Species factsheet: Grallaria ruficapilla. In: Chestnut-crowned Antpitta Grallaria ruficapilla. http://www.birdlife.org/datazone/species/factsheet/22703274. Accessed 10 Apr 2014
Cadena D, Estacio H, Lucero R, Erazo N (2012) Plan de desarrollo “Es tiempo de avanzar”. http://www.pupiales-narino.gov.co/apc-aa-files/33663063366461626161306634386632/plan-de-desarrollo-pupiales-2012-2015.pdf. Accessed 13 Oct 2014
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Meth 9:772–772. doi:10.1038/nmeth.2109
Dimitrov D, Zehtindjiev P, Bensch S, Ilieva M, Iezhova T, Valkiūnas G (2014) Two new species of Haemoproteus Kruse, 1890 (Haemosporida, Haemoproteidae) from European birds, with emphasis on DNA barcoding for detection of haemosporidians in wildlife. Syst Parasitol 87:135–151. doi:10.1007/s11230-013-9464-1
Escalante AA, Freeland DE, Collins WE, Lal AA (1998) The evolution of primate malaria parasites based on the gene encoding cytochrome b from the linear mitochondrial genome. Proc Natl Acad Sci U S A 95:8124–8129. doi:10.1073/pnas.95.14.8124
Forrester DJ, Greiner EC (2008) Leucocytozoonosis. In: Atkinson CT, Thomas NJ, Hunter (eds) Parasitic diseases of wild birds. Blackwell Publishing, Ames, Iowa, pp 54–107
Forrester DJ, Foster GW, Morrison JL (2001) Leucocytozoon toddi and Haemoproteus tinnunculi (Protozoa: Haemosporina) in the Chimango caracara (Milvago chimango) in southern Chile. Mem Inst Oswaldo Cruz. doi:10.1590/S0074-02762001000700024
Funk DJ, Omland KE (2003) Species-level paraphyly and polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annu Rev Ecol Evol Syst 34:397–423. doi:10.1146/annurev.ecolsys.34.011802.132421
Gabaldon A, Ulloa G (1976) Encuesta sobre malaria aviaria en Venezuela: resultados del tercer y último año. Bol Mal Salud Amb 16:107–117
Gabaldon A, Ulloa G, Montcourt A (1974) Encuesta sobre malaria aviaria en Venezuela: resultados del primer año. Bol Mal Salud Amb 14:80–103
Gabaldon A, Ulloa G, Montcourt A (1975) Encuesta sobre malaria aviaria en Venezuela. Resultados del segundo año. Bol Mal Salud Amb 15:73–91
Galen SC, Witt CC (2014) Diverse avian malaria and other haemosporidian parasites in Andean house wrens: evidence for regional co-diversification by host-switching. J Avian Biol 45:374–386. doi:10.1111/jav.00375
Galindo P, Sousa O (1966) Blood parasites of birds from Almirante, Panama with ecological notes on the hosts. Rev Biol Trop 14:27–46
Gálvez CF, Ramírez GF, Osorio JH (2010) Parámetros hematológicos de la mirla Mimus gilvus (paseriformes: mimidae) en cautiverio. Bol Cient Mus Hist Nat Univ Caldas 14:120–128
Gosler A (2004) Birds in the hand. Bird ecology and conservation: a handbook of techniques. Oxford University Press, p 91
Greiner EC (1976) Leucocytozoon maccluri sp. n. (Haemosporida: Leucocytozoidae) from a Thailand Thrush, Zoothera marginata Blyth. J Parasitol 62:545–547. doi:10.2307/3279409
Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi:10.1093/sysbio/syq010
Hauptmanova K, Literak I, Bartova E (2002) Haematology and leucocytozoonosis of Great Tits (Parus major L.) during winter. Acta Vet Brno 71:199–204. doi:10.2754/avb200271020199
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol 90:797–802. doi:10.1645/GE-184R1
Herzog SK, Kattan GA (2011) Patrones de diversidad y endemismo en las aves de los Andes tropicales. In: Herzog SK, Martinez R, Jorgensen PM, Tiessen H (eds) Cambio climatico y biodiversidad en los Andes tropicales. Inter-American Institute for Global Change Research (IAI) and Scientific Committee on Problems of the Environment (SCOPE), Paris, pp 287–305
IUCN (2014) The IUCN Red List of Threatened Species. Version 2014.2. http://www.iucnredlist.org/. Accessed 30 Oct 2014
Josse C, Cuesta F, Navarro G, Barrena V, Cabrera E, Chacón-Moreno E et al (2009) Mapa de Ecosistemas de los Andes del Norte y Centro. Bolivia, Colombia, Ecuador, Perú y Venezuela. Secretaría General de la Comunidad Andina, Programa Regional ECOBONA, CONDESAN-Proyecto Paramo Andino, Programa BioAndes, EcoCiencia, NatureServe, LTA-UNALM, IAvH, ICAE-ULA, CDCUNALM, RUMBOL SRL, Lima
Kattan GH, Beltran JW (1999) Altitudinal distribution, habitat use, and abundance of Grallaria antpittas in the Central Andes of Colombia. Bird Conserv Int 9:271–281. doi:10.1017/S0959270900003452
Krabbe NK, Schulenberg TS (2004) Family Formicariidae (ground antbirds). In: Hoyo J, Elliott A, Christie D (eds) Handbook of the birds of the world (broadbills to tapaculos). Lynx Edicions, Barcelona, pp 682–731
Lacorte GA, Félix GMF, Pinheiro RRB, Chaves AV, Almeida-Neto G, Neves FS et al (2013) Exploring the diversity and distribution of Neotropical avian malaria parasites a molecular survey from Southeast Brazil. PLoS One 8:e57770. doi:10.1371/journal.pone.0057770
Levin II, Valkiūnas G, Iezhova TA, O’Brien SL, Parker PG (2012) Novel Haemoproteus species (Haemosporida: Haemoproteidae) from the swallow-tailed gull (Lariidae), with remarks on the host range of hippoboscid-transmitted avian hemoproteids. J Parasitol 98:847–854. doi:10.1645/GE-3007.1
Levin II, Zwiers P, Deem SL, Geest EA, Higashiguchi JM, Iezhova TA et al (2013) Multiple lineages of avian malaria parasites (Plasmodium) in the Galapagos Islands and evidence for arrival via migratory birds: Plasmodium in galapagos birds. Conserv Biol 27:1366–1377. doi:10.1111/cobi.12127
Lotta IA, Matta NE, Torres RD, Sandino MM, Moncada LI (2013) Leucocytozoon fringillinarum and Leucocytozoon dubreuili in Turdus fuscater from a Colombian páramo ecosystem. J Parasitol 99:359–362. doi:10.1645/GE-3156.1
Mantilla JS, González AD, Valkiūnas G, Moncada LI, Matta NE (2013a) Description and molecular characterization of Plasmodium (Novyella) unalis sp. nov. from the Great Thrush (Turdus fuscater) in highland of Colombia. Parasitol Res 112:4193–4204. doi:10.1007/s00436-013-3611-0
Mantilla JS, Matta NE, Pacheco MA, Escalante AA, González AD, Moncada LI (2013b) Identification of Plasmodium (Haemamoeba) lutzi (Lucena, 1939) from Turdus fuscater (Great Thrush) in Colombia. J Parasitol 99:662–668. doi:10.1645/12-138.1
Marzal A, García-Longoria L, Cárdenas Callirgos JM, Sehgal RN (2014) Invasive avian malaria as an emerging parasitic disease in native birds of Peru. Biol Invasions. doi:10.1007/s10530-014-0718-x
Matta NE, Lotta IA, Valkiūnas G, González AD, Pacheco MA, Escalante AA et al (2014a) Description of Leucocytozoon quynzae sp. nov. (Haemosporida, Leucocytozoidae) from hummingbirds, with remarks on distribution and possible vectors of leucocytozoids in South America. Parasitol Res 113:457–468. doi:10.1007/s00436-013-3675-x
Matta NE, Pacheco MA, Escalante AA, Valkiūnas G, Ayerbe-Quiñones F, Acevedo-Cendales LD (2014b) Description and molecular characterization of Haemoproteus macrovacuolatus n. sp. (Haemosporida, Haemoproteidae), a morphologically unique blood parasite of black-bellied whistling duck (Dendrocygna autumnalis) from South America. Parasitol Res 113:2991–3000. doi:10.1007/s00436-014-3961-2
McMullan M, Quevedo A, Donegan G (2011) Guia de Campo de las Aves de Colombia. Fundación ProAves, Bogotá
Miller M., Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Gateway Computing Environments Workshop (GCE), 2010. IEEE, New Orleans, LA, pp 1–8
Moritz C, Cicero C (2004) DNA barcoding: promise and pitfalls. PLoS Biol 2:e354. doi:10.1371/journal.pbio.0020354
Muñoz E, Ferrer D, Molina R, Adlard RD (1999) Prevalence of haematozoa in birds of prey in Catalonia, north-east Spain. Vet Rec 144:632–636. doi:10.1136/vr.144.23.632
Olias P, Wegelin M, Zenker W, Freter S, Gruber AD, Klopfleisch R (2011) Avian malaria deaths in parrots, Europe. Emerg Infect Dis 17:950–952. doi:10.3201/eid1705.101618
Pacheco MA, Battistuzzi FU, Junge RE, Cornejo OE, Williams CV, Landau I, Rabetafika L, Snounou G, Jones-Engel L, Escalante AA (2011a) Timing the origin of human malarias: the lemur puzzle. BMC Evol Biol 11:299. doi:10.1186/1471-2148-11-299
Pacheco MA, Escalante AA, Garner MM, Bradley GA, Aguilar RF (2011b) Haemosporidian infection in captive masked bobwhite quail (Colinus virginianus ridgwayi), an endangered subspecies of the northern bobwhite quail. Vet Parasitol 182:113–120. doi:10.1016/j.vetpar.2011.06.006
Pacheco M, Cranfield M, Cameron K, Escalante AA (2013) Malarial parasite diversity in chimpanzees: the value of comparative approaches to ascertain the evolution of Plasmodium falciparum antigens. Malar J 12:328. doi:10.1186/1475-2875-12-328
Palinauskas V, Valkiūnas G, Bolshakov CV, Bensch S (2011) Plasmodium relictum (lineage SGS1) and Plasmodium ashfordi (lineage GRW2): the effects of the co-infection on experimentally infected passerine birds. Exp Parasitol 127:527–533. doi:10.1016/j.exppara.2010.10.007
Peirce MA (1984) Haematozoa of Zambian birds. IV. Description of Leucocytozoon balmorali sp. nov. from Malaconotidae. J Nat Hist 18:223–226. doi:10.1080/00222938400770181
Pérez Ponce de León G, García L (2001) Los parásitos en el contexto de la biodivrsidad y la conservación. CONABIO. Biodiversitas 34:11–15
Pérez-Tris J, Bensch S (2005) Diagnosing genetically diverse avian malarial infections using mixed-sequence analysis and TA-cloning. Parasitology 131:15–23
Perkins SL, Martinsen ES, Falk BG (2011) Do molecules matter more than morphology? Promises and pitfalls in parasites. Parasitology 138:1664–1674. doi:10.1017/S0031182011000679
Poulin R, Krasnov BR, Mouillot D, Thieltges DW (2011) The comparative ecology and biogeography of parasites. Phil Trans R Soc B 366:2379–2390. doi:10.1098/rstb.2011.0048
Rambaut A (2006) FigTree Tree Figure Drawing Toll version 1.3.1. Institute of Evolutionary Biology, University of Edinburgh. http://tree.bio.ed.ac.uk/software/figtree/. Accessed 31 Jan 2013
Remsen JV, Cadena CD, Jaramillo A, Nores M, Pacheco JF, Pérez-Emán J et al (2012) A classification of the bird species of South America American Ornithologists’ Union Version 7 December 2012. http://www.museum.lsu.edu/~Remsen/SACCBaseline.html. Accessed 7 Dec 2012
Rodríguez OA, Moya H, Matta NE (2009) Avian blood parasites in the National Natural Park Chingaza: high Andes of Colombia. Hornero 24:1–6
Rokas A, Carroll SB (2005) More genes or more taxa? The relative contribution of gene number and taxon number to phylogenetic accuracy. Mol Biol Evol 22:1337–1344. doi:10.1093/molbev/msi121
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi:10.1093/bioinformatics/btg180
Sambrook J, Fritsch E, Maniatis T (1989) Molecular cloning: a laboratory manual, Second. Cold Spring Harbor Laboratory Press, New York
Santiago-Alarcon D, Palinauskas V, Schaefer HM (2012) Diptera vectors of avian Haemosporidian parasites: untangling parasite life cycles and their taxonomy. Biol Rev Camb Philos Soc 87:928–964. doi:10.1111/j.1469-185X.2012.00234.x
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Meth 9:671–675. doi:10.1038/nmeth.2089
Soltis DE, Albert VA, Savolainen V, Hilu K, Qiu Y-L, Chase MW et al (2004) Genome-scale data, angiosperm relationships, and “ending incongruence”: a cautionary tale in phylogenetics. Trends Plant Sci 9:477–483. doi:10.1016/j.tplants.2004.08.008
Svensson-Coelho M, Blake JG, Loiselle BA, Penrose AS, Parker PG, Ricklefs RE (2013) Diversity, prevalence, and host specificity of avian Plasmodium and Haemoproteus in a western Amazon assemblage. Ornithol Monogr 76:1–47. doi:10.1525/om.2013.76.1.1
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Toft CA, Karter AJ (1990) Parasite-host coevolution. Trends Ecol Evol 5:326–329. doi:10.1016/0169-5347(90)90179-H
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC Press, Boca Raton
Valkiunas G, Iezhova TA, Mironov SV (2002) Leucocytozoon hamiltoni n. sp. (Haemosporida, Leucocytozoidae) from the Bukharan Great Tit Parus bokharensis. J Parasitol 88:577. doi:10.2307/3285453
Valkiūnas G, Salaman P, Iezhova TA (2003) Paucity of hematozoa in Colombian birds. J Wildl Dis 39:445–448. doi:10.7589/0090-3558-39.2.445
Valkiūnas G, Iezhova TA, Križanauskienė A, Palinauskas V, Sehgal RNM, Bensch S (2008) A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J Parasitol 94:1395–1401. doi:10.1645/GE-1570.1
Valkiūnas G, Sehgal RNM, Iezhova TA, Hull AC (2010) Identification of Leucocytozoon toddi group (Haemosporida: Leucocytozoidae), with remarks on the species taxonomy of leucocytozoids. J Parasitol 96:170–177. doi:10.1645/GE-2109.1
Valkiūnas G, Palinauskas V, Ilgūnas M, Bukauskaitė D, Dimitrov D, Bernotienė R et al (2014) Molecular characterization of five widespread avian haemosporidian parasites (Haemosporida), with perspectives on the PCR-based detection of haemosporidians in wildlife. Parasitol Res 113:2251–2263. doi:10.1007/s00436-014-3880-2
Vargas-Rios O, Pedraza P, Universidad Nacional de Colombia (Bogota), Departamento de Biologia (2004) El Parque Nacional Natural Chingaza. Gente Nueva, Bogotá, D.C.
Vásquez VH, Serrano MA (2009) Las áreas naturales protegidas de Colombia. Conservación Internacional-Colombia & Fundación Biocolombia., Bogotá, D.C.
White EM, Greiner EC, Bennett GF, Herman CM (1978) Distribution of the hematozoa of neotropical birds. Rev Biol Trop 26(Suppl 1):43–102
Acknowledgments
Authors would like to thank Chingaza National Natural Park Administrative staff and Grupo de Estudio Relación Parásito Hospedero research group, especially Rocio Hernandez and Rafael Gutierrez, for their field assistance. This work was funded by Colciencias project code 110152128340 contract no. 359–2011 and the Botanical Garden José Celestino Mutis of Bogotá under program of incentives for research Thomas van der Hammen.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lotta, I.A., Gonzalez, A.D., Pacheco, M.A. et al. Leucocytozoon pterotenuis sp. nov. (Haemosporida, Leucocytozoidae): description of the morphologically unique species from the Grallariidae birds, with remarks on the distribution of Leucocytozoon parasites in the Neotropics. Parasitol Res 114, 1031–1044 (2015). https://doi.org/10.1007/s00436-014-4269-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4269-y