Abstract
We describe Leucocytozoon quynzae sp. nov. (Haemosporida, Leucocytozoidae), which is the first Leucocytozoon parasite identified to species level in hummingbirds. It was found in the Amethyst-throated Sunangel (Heliangelus amethysticollis, Trochilidae, Apodiformes) captured in the Palacio Forest, which belongs to the damping zone of Chingaza National Natural Park, Cundinamarca, Colombia, at 2,900 m above sea level where the transmission occurs; the new species were found both in the high Andean forest and Paramo ecosystem. This parasite is described based on the morphology of its blood stages, a fragment of the mitochondrial cytochrome b gene, and the complete mitochondrial genome. Illustrations of blood stages of the new species are given, and the phylogenetic analysis places this lineage in a well-supported clade with other lineages of unidentified to species level leucocytozoids reported in the Trochilidae birds elsewhere. The new species possess gametocytes in roundish host cells; it can be readily distinguished from other similar leucocytozoids, primarily due to (1) a comma-like shape of the host cell nucleus, which extended one half or less of the circumference of the gametocyte and (2) a large number of prominent volutin granules in the cytoplasm. Identical mitochondrial cytochrome b sequence of Leucocytozoon quynzae was found in different hummingbird species at the type locality and also was reported in one passerine bird at the highlands of Peru. Leucocytozoon quynzae is the first leucocytozoid parasite described from South American birds; its transmission occurs both at low temperatures and high elevations. We discuss some patterns of distribution of avian leucocytozoids in South America and the role of Gigantodax spp. (Diptera, Simuliidae) as potential vectors of Leucocytozoon parasites in the Andean Region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leucocytozoon is a diverse genus of avian blood parasites that are widely distributed around the world. In the New World, the highest prevalence and diversity of leucocytozoids have been found in the Nearctic region (Greiner et al. 1975). The prevalence and diversity of Leucocytozoon spp. in the Neotropical region usually are considered to be low (White et al. 1978; Valkiūnas 2005; Forrester and Greiner 2008). However, Forrester et al. (2001) reported high prevalence of Leucocytozoon infection in the Chimango caracara (Milvago chimango) in the mountain region of southern Chile. Additionally, high prevalence of Leucocytozoon was found in Great Trush (Turdus fuscater) in the Páramo ecosystem in Colombia (Rodríguez et al. 2009; Lotta et al. 2013). A positive significant relationship between the prevalence of leucocytozoids and latitude for the parasite lineages was reported by Merino et al. (2008) in Chile; these authors proposed a latitudinal gradient for explanation of distribution of leucocytozoids in South America.
The Andes are the most important mountain range in the region and the longest in the world. This range extends from north to south across Venezuela, Colombia, Ecuador, Peru, Bolivia, Chile and Argentina (Morrone 2001). These mountains include many different ecosystems (estimated to be 133) and are considered as a hotspot of biodiversity with a high level of endemism (Myers et al. 2000; Josse et al. 2009). Avian hemoparasites, however, have not been properly studied in these ecosystems.
Hummingbirds (Trochilidae) are endemic from the New World. This group has 331 recognized species, which are distributed from Alaska to Tierra de Fuego in South America, including the Caribbean islands. These birds are found in a wide variety of environments from sea level until 5,000 m above sea level, including desert scrub forest edges, coastal chaparral and grassland, and even sites where human activity has modified or replaced natural habitats (Williamson 2001; Gutiérrez et al. 2004; McGuire et al. 2008). Out of 162 hummingbird species reported in Colombia, 78 have been reported in the Andes (McMullan et al. 2011). Despite the fact that birds are among the best known groups of organisms in the Andes, there is scarce information on the prevalence, biodiversity and distribution of their hemoparasites.
The first case of Leucocytozoon sp. infection in hummingbirds was reported in one individual of the Amethyst-throated hummingbird Lampornis amethystinus in Mexico (Beltrán 1944). Merino et al. (2008) and Rodriguez et al. (2009) found hummingbirds infected with leucocytozoids in Chile and Colombia, respectively; however, the parasites were not identified to species level in these investigations. Leucocytozoon species remain unidentified in hummingbirds. In this study, we reported a new Leucocytozoon sp. parasite in the Amethyst-throated Sunangel (Heliangelus amethysticollis), which is a non-migrating bird widely distributed in subtropical and tropical moist montane Andean forests of Bolivia, Colombia, Peru, Ecuador and Venezuela. Other habitats of this bird species are tropical high altitudes, humid grasslands and shrublands. The reported altitudinal distribution of this bird is between 1,800 and 3,200 m above sea level; it has been recorded between 2,000 and 3,000 m above sea level in Colombia (McMullan et al. 2011).
The vectors implicated in the transmission of Leucocytozoon spp. around the world are simuliids, with only one species Leucocytozoon (Akiba) caulleryi being transmitted by ceratopogonid flies (Culicoides spp.) (Skidmore 1931; Akiba 1960; Forrester and Greiner 2008; Valkiūnas 2005). According to current knowledge, fauna of the Neotropical simuliids contains 12 genera; two of them are present in Colombia: Simulium and Gigantodax (Adler and Crosskey 2013). Currently, the genus Gigantodax encompasses 65 species distributed from southern USA along the Mesoamerican Mountains, through the Andes to Tierra de Fuego. This genus is one of the largest latitudinally extended taxa in the world; breeding places of the majority of its species are located in small fairly torrential streams (Coscarón and Coscarón-Arias 1995; Adler and Crosskey 2013).
The aims of this study were (1) to provide the morphological descriptions and molecular characterization of a new Leucocytozoon species parasitizing hummingbirds, (2) to discuss its relationship with other species of the genus Leucocytozoon, based on the molecular phylogenies and morphological data, and (3) to discuss our findings in terms of the South American distribution of leucocytozoids and their possible vectors.
Materials and methods
Sampling area
Birds were mist netted in three sites at the highland in the Chingaza National Natural Park (NNP) from April 2002 to May 2003, from December 2008 to October 2009 and from February 2012 to April, 2013. Taxonomic classification of birds is given according to the South American Classification Committee (SACC) (Remsen et al. 2012).
The Chingaza NNP is located in Colombian Oriental mountain range of Cundinamarca and Meta Departments, comprising the complex and rich hydrological landscape of the basins of Negro, Guatiquía and Guavio rivers in the Orinoquia region and Siecha and Tominé rivers from the basin of the Hoya del Magdalena. Despite the fact that the Chingaza NNP has elevations between 1,200 and 4,020 m asl, the great majority of its territory is covered by the Páramo ecosystem (Vargas-Ríos and Pedraza 2004), which is characterized by the following features: (1) an annual median temperature between 0 and 14 °C, (2) an annual median precipitation of 1,900 mm, with the greatest rainfall during June and July, (3) high cloudiness and fog that determine short day insolation (3–5 h) and (4) high atmospheric humidity (80–85 %) (INDERENA 1986).
Samples were collected at three sites: Monteredondo station (4°37′N, 73°43′W, 3,100 m above sea level) and Encenillo Forest (4°36′N to 73°43′W, 3,155 m above sea level). Both sites are covered by Páramo plant communities which are dominated by a homogenous matrix of open vegetation of Espeletia spp. along with a patch of Calamagrostis spp. The open vegetation is interrupted by dense forest fragments of Weinmannia spp., which are frequently seen in orographically protected sites, with several lotic freshwaters running through them (Vargas-Ríos and Pedraza 2004). These two sites have an annual rainfall between 2,345 and 2,918 mm, with the dry season extending from November to March and their rainy seasons occurring from April to October. The third sampling site was Palacio Forest, which is located in the damping zone of Chingaza NNP (4°41′N, 73°50′W, 2,900 m above sea level). This site is an Andean forest (approximately 20 m high), located immediately at the inferior altitudinal limit of the Páramo. The higher stratification is dominated by tree species that give support to many epiphytes species and provide shadow to an understory covered by diverse shrubs and herbs species (Rangel-Ch 2000; Vargas-Ríos and Pedraza 2004). Palacio Forest has an annual rainfall between 1,171 and 2,324 mm, with large periods of fog and cloudiness as well as a high ambient humidity that boost the water influx to the forest (Bruijnzeel 2002).
Sample and blood film examination
In all, 576 birds belonging to 68 species, 19 families and 8 orders were sampled. Three thin blood smears were prepared from each bird using blood obtained by toenail clipping or brachial vein puncture. The smears were air dried, fixed in 100 % methanol for 5 min and stained with Giemsa (pH 7.2) for 45 min.
About 50 μl of whole blood was stored in SET buffer (0.05 M Tris, 0.15 M NaCl, 0.5 M EDTA, pH 8.0) for molecular analysis. Blood samples were kept at room temperature in the field and then maintained at −20 °C in the laboratory.
Blood smears were scanned double-blind using the Leica DM750 microscope, first at low magnification (×100) for 10 min and then at high magnification (×1,000) for 20 min. Digital images were made by the Leica EC3 camera and processed with LAS EZ software (Leica Microsystems, Wetzlar, Germany). Measurements were made digitally from images using ImageJ (Schneider et al. 2012). Morphometric features were those described by Valkiūnas (2005).
Entire positive blood smears with the new species were examined microscopically; approximately 130 images of gametocytes of the new species were prepared and analysed. Intensity of infection was estimated by counting 100 microscopic fields at a magnification of × 1,000 by moving the slide in areas where the blood cells formed a monolayer; each such field contains approximately 100 cells. The intensity of infection was determined by actual counting of the number of parasites per 10,000 erythrocytes (Muñoz et al. 1999).
DNA extraction, cytochrome b gene amplification and sequencing
DNA was extracted, only from positive blood samples diagnosed by microscopy. A standard phenol–chloroform protocol (Sambrook et al. 1989) was used for this purpose. Samples were tested for Leucocytozoon spp. using a nested PCR to amplify the 5′ region of the parasite's cytochrome b gene (478 bp expected product) according to Hellgren et al. (2004). Positive amplifications were detected by running 2 μl of the PCR product on a 1.5 % agarose gel. Amplified products were cleaned using differential precipitation with ammonium acetate protocol (Bensch et al. 2000) and sequenced on the 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA) through Macrogen (Macrogen Inc.). Sequencing was done in both directions using forward and reverse primers.
Amplification and sequencing of complete mitochondrial genome (mtDNA)
In addition to the partial cytochrome b gene, we amplified, cloned and sequenced the complete mitochondrial genome from one individual of Amethyst-throated Sunangel with single infection of the new parasite species. We amplified 5,868 bp of the parasite mtDNA using primers forward 5′ GA GGA TTC TCT CCA CAC TTC AAT TCG TAC TTC/reverse 5′ CAG GAA AAT WAT AGA CCG AAC CTT GGA CTC with TaKaRa LA TaqTM Polymerase (TaKaRa Mirus Bio Inc, Shiga, Japan) as described elsewhere (Pacheco et al. 2011; Mantilla et al. 2013b). Briefly, PCR amplifications were carried out in a 50-μl volume using 20 ng of total genomic DNA. The PCR conditions were as follows: a partial denaturation at 94 °C for 1 min and 30 cycles of 30 s at 94 °C and 7 min at 68 °C, followed by a final extension of 10 min at 72 °C. Following manufactory directions, two independent PCR products (bands of approximately 6 kb) were excised from the gel, purified using QIAquick® Gel extraction kit (Qiagen, GmbH, Hilden, Germany) and cloned in the pGEM®-T Easy Vector systems (Promega, Madison, WI, USA). Both strands for three clones were sequenced using an Applied Biosystems 3730 capillary sequencer. The mtDNA sequence was deposited in GenBank under the accession number KF479480.
Phylogenetic analysis
Independent alignments for partial sequences of cytochrome b gene (478 bp) and almost complete mtDNA genomes (5,485 bp excluding gaps) were made in order to establish the phylogenetic relationship between the new species and other haemosporidian parasites. First, cytochrome b sequences were edited and aligned using MEGA5 software (Tamura et al. 2011). Final alignment was done using 55 cytochrome b sequences with 476 nucleotides as follows: 26 sequences were obtained from MalAvi database (Bensch et al. 2009), 21 were from GenBank and 8 were obtained by our group.
Phylogenetic reconstruction was made using Bayesian inference with MrBayes version 3.1.2 (Ronquist and Huelsenbeck 2003) under the general time-reversible model (GTR + I + Γ). The model was obtained using jModelTest 2.1.1 (Darriba et al. 2012) as the best of 88 models according to the corrected Akaike information criterion.
Two independent runs of 2 × 106 generations were conducted with four chains, sampling every 100 generations. In all, 25 % of the trees were discarded as burn-in period; in total, 15,000 trees were used to construct the majority rule consensus. The phylogeny was visualized and edited using FigTree v1.3.1 (Rambaut 2006). To determine the sequence divergence between the lineages, genetic distances were calculated using a Kimura two-parameter model of substitution, using MEGA v5.05 software (Tamura et al. 2011).
Next, using ClustalX v2.0.12 and Muscle as implemented in SeaView v4.3.5, the new parasite mtDNA sequence was aligned with 14 mitochondrial genomes available in GenBank for other haemosporidians isolated from lizards and birds. This alignment included the only three complete mtDNA genomes currently available for Leucocytozoon species. The phylogenetic relationships were estimated by using Bayesian methods as implemented in MrBayes v3.1.2, as described above (Ronquist and Huelsenbeck 2003). The alignment was divided into four categories where each gene (cytochrome oxidase III, cytochrome oxidase I and cytochrome b) was used as a separate partition plus the non-coding regions (Ronquist and Huelsenbeck 2003). We also used a general time-reversible + gamma model (GTR + Γ), which had the lower number of parameters that best fit the data as estimated by MEGA v5.0 (Tamura et al. 2011). Bayesian support for the nodes was inferred in MrBayes using 4 × 106 Markov chain Monte Carlo (MCMC) steps, and after convergence was reached, we discarded the 50 % of the sample as burn-in. In both cases, convergence is reached when the average standard deviation of the posterior probability is below 0.01 and the value of the potential scale reduction factor (PSRF) is between 1.00 and 1.02 (Ronquist and Huelsenbeck 2003). In order to compare the divergence among a specific pair of Leucocytozoon species, we estimated their genetic divergences on each mtDNA gene and the complete mtDNA genomes using the Kimura two-parameter model as implemented in MEGA v5.0.
Results
Out of 576 sampled bird individuals, 95 were hummingbirds, belonging to 11 species. Leucocytozoon quynzae sp. nov. was found by microscopic examination in the following four individuals belonging to the Trochilidae (Apodiformes): (1) Amethyst-throated Sunangel, Heliangelus amethysticollis, (2) Bronzy Inca, Coeligena helianthea, (3) Mountain Avocetbill, Opisthoprora euryptera and (4) Tyrian Metaltail, Metallura tyrianthina (Table 1). This parasite was found in adult males and females; no evidence of sickness was observed. Additionally, other 481 individual non-trochilid birds were uninfected with the new Leucocytozoon species, as determined by microscopic examination (Table 1).
Description
Leucocytozoon quynzae sp. nov. (Fig. 1, Table 2)
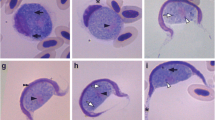
Leucocytozoon quynzae sp. nov. from the blood of its type vertebrate host, the Amethyst-throated Sunangel (Heliangelus amethysticollis). a–g Macrogametocytes. h, i Microgametocytes. Simple long arrows, nuclei of parasites; triangle long arrows, host cell nuclei; short simple arrows, volutin granules; simple arrowheads, vacuoles; triangle simple arrowheads, remnants of the host cell cytoplasm; triangle wide arrowhead, nucleolus. Giemsa-stained thin blood films. Scale bar = 10 μm
Macrogametocytes (Fig. 1a–g): Roundish in form; they develop in roundish host cells. The cytoplasm frequently contains vacuoles of different sizes (Fig. 1e). Prominent roundish volutin granules are present (Fig. 1a, b and f), often in large numbers, a distinctive character of this species. The parasite nucleus is variable both in form and position; the nucleolus is prominent and well seen (Fig. 1b). The nucleus of the host cell extended less or up to one half of the circumference of the gametocyte (Table 2; Fig. 1a–g). The host cell nucleus is displaced, deformed and lies peripherally as a band with a prominent asymmetrical enlargement on one tip; that gives a comma-like shape to the host cell nucleus (Fig. 1a–g), a characteristic readily distinguishable morphological feature of this parasite. The cytoplasm of host cells is invisible in fully grown gametocytes but of variable-shape remnants; however, the cytoplasm sometimes is visible around growing gametocytes (Fig. 1a).
Microgametocytes (Fig. 1h, i): The general configuration and other features are as for macrogametocytes with the usual haemosporidian sexual dimorphic characters. The proportion of microgametocytes and macrogametocytes in the type material is 1:4.
Remarks
Leucocytozoon quynzae develops in roundish host cells. This parasite can be readily distinguished from all described Leucocytozoon species, primarily due to a well-distinguishable comma-like shape of nuclei of its host cells (Fig. 1a–g), a unique feature in avian Leucocytozoon parasites. The presence of numerous prominent volutin granules (Fig. 1a) also helps to distinguish this parasite from many other leucocytozoids developing in a roundish host cell.
Taxonomic summary
Type host: Amethyst-throated Sunangel Heliangelus amethysticollis (Trochilidae, Apodiformes).
Type locality: Palacio Forest (4°41′N, 73°50′W), 2,900 m above sea level, Colombia.
Type specimens: Hapantotype was deposited in the biological collection of Grupo de Estudio Relación Parásito Hospedero (GERPH) at the Department of Biology, Universidad Nacional de Colombia, Bogotá, Colombia (accession nos. GERPH-06253 to GERPH-06255, intensity of parasitemia is 0.31 %, collected by Rafael Gutierrez in Palacio Forest, 19 February 2012, lineage L_ HELIAM01, GenBank KF309188). Digital images of blood stages of the new parasite from the hapantotype are available on request from GERPH.
Additional material: Blood films from Opisthoprora euryptera (three preparations, Mountain Avocetbill, accession nos.GERPH-02261 to GERPH-02263), Metallura tyrianthina (three preparations, Tyrian Metaltail, GERPH-06925 to GERPH-06927) and Coeligena helianthea (one preparation, Blue-throated Starfrontlet, GERPH-07046) were deposited in the collection of GERPH. Digital images of blood stages from these avian hosts are available on request from GERPH.
DNA sequences: Three mitochondrial cytochrome b lineages from different hummingbirds species were obtained as follows: (1) two sequences were identical and correspond to the lineage L_HELIAM01 (GenBank KF309188 from the hapantotype sample and Genbank KF471028 from Metallura tyrianthina, both sequences of 476 bp) and (2) the lineage L_COHEL01 (GenBank KF309189 from Coeligena helianthea). The Leucocytozoon quynzae complete mtDNA genome sequence for Amethyst-throated Sunangel was deposited in GenBank (KF479480).
Site of infection: Blood cells, in which origin is unclear, because gametocytes markedly change morphology of the host cells.
Prevalence: In the type locality, 4 out of 576 birds were infected as determined by microscopic examination (the overall prevalence was 0.69 % in hummingbirds). The prevalence was one of five (20 %) in the type host.
Distribution and additional hosts: Metallura tyrianthina (Tyrian Metaltail) is an additional host at the type locality; it has an identical cytochrome b sequence (GenBank KF471028) as the type host. Additionally, (1) Coeligena helianthea captured in Monterredondo station (GenBank KF309189, Blue-throated Starfrontlet) possesses a lineage with a genetic distance of 0.2 %, and (2) Opisthoprora euryptera (Mountain Avocetbill, captured in Encenillo Forest) was infected with a morphologically similar parasite, but we have no sequence information from this host. In Chile, a hummingbird Sephanoides sephanoides (Green-backed Firecrown) harboured a parasite with the cytochrome b lineage SEPSEP (Genbank EF153659), with a genetic distance of 0.2 % with Leucocytozoon quynzae (Merino et al. 2008); it is likely the same parasite. An identical cytochrome b sequence, as we reported in the type host, was deposited in GenBank (JQ988510); it was reported in a passerine bird, Mecocerculus stictopterus (White-banded Tyrannulet, Tyrannidae) captured at 3,312 m above sea level in the Peruvian Andes, but there is no morphological evidence that this parasite develops to gametocyte stage in this avian host.
Etymology: The species name refers to the word “quynza”, which was used by Muiscas people, a pre-Colombian indigenous group, to name hummingbirds.
Phylogenetic relationships of parasites
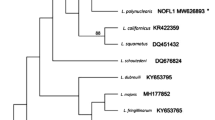
Leucocytozoon quynzae was found in four individuals belonging to four different species of hummingbirds; however, the partial cytochrome b lineages were obtained only from three samples. Sequences obtained from two birds were identical (the lineage L_HELIAM 01 GenBank KF309188 and GenBank KF471028), whilst the lineage L_COHEL01 (GenBank KF309189) had polymorphism in one nucleotide, when compared with L_HELIAM 01 (Fig. 2).
Bayesian phylogeny based on 476 bp of cytochrome b sequences of Leucocytozoon spp. from six different bird orders. Host proveniences of lineages are identified by color in the vertical bar as is indicated in the convention square. Leucocytozoon quynzae sp. nov. lineage is marked with a hummingbird pictogram. Two Haemoproteus lineages and three Plasmodium lineages were used as outgroup. GenBank accession numbers or alternative lineage names of sequences from MalAvi are provided followed by parasite species, hosts and sites where they were found. Values of posterior probabilities below 0.8 are not shown. The branch lengths are drawn proportionally to the amount of change; the scale bars show substitutions per site
The genetic distance analysis showed that Leucocytozoon sp. (lineage SEPSEP01) found in a hummingbird Sephanoides sephanoides in Chile was the closest to Leucocytozoon quynzae, with a genetic distance of 0.2 %; both lineages are grouped in a well-supported clade (Table 3; Fig. 2, clade A).
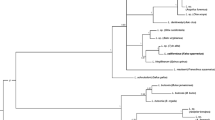
The phylogeny estimated with complete mitochondrial genomes is shown in Fig. 3. As was expected, four Leucocytozoon species cluster together sharing a common ancestor; Leucocytozoon quynzae appears as sister taxa of Leucocytozoon majoris in this limited dataset. The average genetic divergences using complete mtDNA and the three mitochondrial genes among Leucocytozoon quynzae, Leucocytozoon majoris and Leucocytozoon fringilinarum are given in Table 4.
Discussion
The Andean ecosystems are characterized by their biological richness and high levels of endemism (Olson and Dinerstein 1998; Kapelle and Brown 2001). Numerous studies have focused on certain taxonomic groups such as plants (Luna-Vega et al. 1989; Särkinen et al. 2012) and avifauna (Terborgh 1971; Herzog and Kattan 2011), including detailed studies on diversity pattern and taxonomic aspects of hummingbirds (García-Moreno et al. 1999; McGuire et al. 2008). However, in spite of the fact that nearly 25 % of the described hummingbird species of South America are restricted to this region (Herzog and Kattan 2011), little is known about parasites of the trochilids.
Leucocytozoon quynzae is the first described Leucocytozoon species from the Trochilidae birds (Apodiformes) and the first new leucocytozoid parasite, which is described in South American birds. It has distinct morphological features of blood stages and is easy to identify even at light parasitemia. There are few reports of blood parasites in hummingbird (Greiner et al. 1975; White et al. 1979). Interestingly, the prevalence of blood parasites found in hummingbirds during this study is remarkably lower than that reported in other avian species at the same locality (Rodríguez et al. 2009). It remains unclear if the low prevalence of leucytozoids in hummingbirds reflects a true parasitological situation or is a result of possible underestimation of microscopic examination, since light chronic parasitemias are sometimes difficult to detect using this method (Jarvi et al. 2003; Valkiūnas et al. 2006). It is known that birds belonging to some families and orders consistently are reported with low prevalence of haemosporidian parasites (Piersma 1997; Bensch et al. 2012). It can be the case with Trochilidae species. Whereas Leucocytozoon spp. prevalence in such groups of birds could increase if the samples were analysed by PCR, positive PCR parasite amplifications could be the result of detection of abortive haemosporidian infections, when gametocytes are absent from circulation, so the only detection of parasite lineages in birds does not necessarily mean competent infection (Valkiūnas et al. 2009; Levin et al. 2013).
We can only speculate about the factors that could contribute to the low prevalence of parasites in this group of birds. Despite astounding powers of flight of hummingbirds, they spend almost 80 % of their time perched (Williamson 2001); thus, this behaviour allows the contact with potential vectors. The birds’ small body size (in average 8.2–10.4 cm) might be a factor preventing the infection because small organisms can be less attractive to vectors (Anderson and DeFolliart 1961; Valkiūnas 2005). This factor is worth exploring, but we have no sufficient data to test that in the context of this study. Abundance of hosts is another dimension, which can force driving parasite prevalence. Since transmission depends on the rate of host–vector encounters, the abundance and diversity of potential hosts at the study area may produce a dilution effect, which might limit the rate of hummingbird–vector encounters (Keesing et al. 2006; Malmqvist et al. 2004). Finally, if the parasite affects the bird fitness, it is probable that the infection can lead to lethal consequences (González-Acuña et al. 2011). We have no evidence about the pathogenicity of Leucocytozoon quynzae because all sampled infected birds looked healthy and there are no reports indicating the decline of bird populations at our study areas. However, we captured birds with light parasitemia, which usually is relatively benign to birds in comparison to heavy primary infection, which was not assessed during this study. Further studies are needed to understand the relatively low prevalence of haemosporidian parasites in hummingbirds.
The results of this study, using the fragment of the cytochrome b gene, show that Leucocytozoon quynzae lineages are grouped into a well-supported clade with other lineages found in hummingbirds (Fig. 2, clade A). The lineages L_COHEL01 (GenBank KF309189) and L_HELIAM01 (GenBank KF309188) have the same morphological features; so the morphological characters of gametocytes are well correlated with the cytochrome b sequences. The lineage GALLUS06 was genetically most similar to the lineages of clade A, in which the lineage L_HELIAM01 appears, with 4.9 % genetic differences between these two lineages (Table 3; Fig. 2, clade B).
Interestingly, a sequence of Leucocytozoon quynzae, which was reported in the type host, was also found in one Mecocerculus stictopterus in the Peruvian Andes (GenBank JQ988510); however, there is no morphological evidence of the infection in this flycatcher. Similar results, when lineages are shared between hummingbirds and passerine birds, without morphological evidence, are also shown in Fig. 2, clade C. Based only in a fragment of cytochrome b sequences, it seems to contradict data about specificity of leucocytozoids on the level of orders, as was proposed by Valkiūnas (2005) and Forrester and Greiner (2008). However, two facts should be considered: First, parasite detection-based PCR does not distinguish abortive haemosporidian development; thus, reports of lineages need to be supported by microscopic observation of gametocytes if conclusions about specificity are aimed to be achieved (Valkiūnas et al. 2009; Levin et al. 2013). Second, this fragment of the cytochrome b may not have enough informative sites. Indeed, since its introduction in malaria parasite studies (Escalante et al. 1998), cytochrome b has shown several attributes that have made it useful in ecological and evolutionary genetic investigations, but it is still not enough for elucidating complex biogeographic or speciation processes. Nevertheless, this and other mitochondrial genes have been successfully used in many evolutionary biology studies (Valkiūnas et al. 2008; Bensch et al. 2009). It is important to keep in mind that all phylogenies are hypotheses; additional molecular and life history information is needed to better understand the host parasite evolutionary relationships (Martinsen et al. 2008; Palinauskas et al. 2013). Our complete mitochondrial DNA phylogeny shows stronger signals, but the paucity of suitable data from other species does not allow more comprehensive analysis (Fig. 3).
Taking into account results of this study and other molecular data deposited in GenBank about reports of Leucocytozoon spp. from avian hosts in South America, it is seems probable that the positive relationship between latitude and Leucocytozoon spp. prevalence in South America might be oversimplified (Merino et al. 2008). Indeed, additional studies are needed which include more prevalence data across a larger latitudinal transect and also consider altitude in South America before reaching a stronger conclusion. Altitude is an important factor to consider in this region given its landscape and the evidence of presence of an altitudinal gradient in transmission of haemosporidian parasites (LaPointe et al. 2010). Unfortunately, there are still few reports of avian hemoparasite transmission at highlands.
A key ecological result of this study is that Leucocytozoon quynzae was found in non-migrating birds; thus, its transmission certainly occurs at low temperatures (0–14 °C) and as high as 3,155 m above sea level. This is the highest altitude reported for haemosporidian parasite transmission (LaPointe et al. 2010; Mantilla et al. 2013a, b; van Rooyen et al. 2013). Interestingly, the active transmission of Leucocytozoon cheissini was reported at 3,000 m above sea level in Central Tadzhikistan (Valkiūnas 2005). It is probable that transmission at high altitudes is a characteristic feature of avian leucocytozoids throughout the world; that warrants further investigation.
Because all reports of Leucocytozoon spp. in resident birds in South America are restricted to the Andean Region (Forrester et al. 2001; Merino et al. 2008; Rodríguez et al. 2009; this study), the prevalence of these parasites should be driven by vector distribution. Crosskey (1990) and Gryaznov (1984) reported an association between the shapes of claw and the blood feeding behaviour of black flies. Mainly, the species with bifid claw reflect an ornithophilic habitat, and those with subbasal tooth reflect a mammalophilic one. In the Colombian highlands, our group has determined 23 Simuliidae species, 8 of them belonging to the genus Gigantodax and the others to the genus Simulium. All Gigantodax spp. determined have bifid claw, and only one Simulium species had the same feature (data not shown). This fact suggests that species of Gigantodax can be implicated in the Leucocytozoon spp. transmission at our study sites. However, the vectorial competence of Simulium species cannot be discarded.
The recent efforts to explore highland ecosystems, with different bird species and vector communities, have provided new information about the presence of Leucocytozoon spp. in Neotropical resident birds, as well as of their possible vectors. Birds from lowland Neotropical ecosystems seem to lack Leucocytozoon infections (White et al. 1979; Rodríguez and Matta 2001; Braga et al. 2011). The taxonomic resolution is quite important to obtain a reliable estimation of parasite diversity (Poulin and Leung 2011); large areas in the Andes have not yet been surveyed, resulting in incomplete knowledge of the distribution of many species (Herzog and Kattan 2011). Thus, studies in the Andes can contribute to this matter, including the true estimation of the existence of latitudinal gradients for Leucocytozoon parasites and other haemosporidians in South America. The hypothesis about the possible involvement of Gigantodax spp. in transmission of Leucocytozoon parasites is worth testing; these insects have not been reported as leucocytozoids’ vectors so far. This is particularly true because Gigantodax spp. are widespread in the Andes but absent from lowland Neotropical ecosystems (Morrone 2001; Adler and Crosskey 2013). This pattern of distribution of possible vectors coincides with available data about the distribution of Leucocytozoon parasites in South American birds.
References
Adler P, Crosskey R (2013) World blackflies (Diptera: Simuliidae): a comprehensive revision of the taxonomic and geographical inventory, p. 120. http://www.clemson.edu/cafls/biomia/pdfs/blackflyinventory.pdf. Accessed 1 Sep 2013
Akiba K (1960) Studies on the Leucocytozoon found in the chicken, in Japan. II. On the transmission of L. caulleryi by Culicoides arakawae. Nihon Juigaku Zasshi 22:309–317
Anderson JR, DeFolliart GR (1961) Feeding behavior and host preferences of some black flies (Diptera: Simuliidae) in Wisconsin. Ann Entomol Soc Am 54:716–729
Beltrán E (1944) Protozoarios sanguíneos de las aves. Ann Escuela Nac Cienc Biol 3:361–366
Bensch S, Stjernman M, Hasselquist D, Ostman O, Hannson B, Westerdahl H, Pinheiro R (2000) Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc Biol Sci 267:1583–1589
Bensch S, Hellgren O, Pérez-Tris J (2009) MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9:1353–1358
Bensch S, Jönsson J, Copete J (2012) Low prevalence of Haemoproteus infections in Chiffchaffs. Parasitology 139:302–309
Braga ÉM, Silveira P, Belo NO, Valkiūnas G (2011) Recent advances in the study of avian malariae: an overview with an emphasis on the distribution of Plasmodium spp. in Brazil. Mem Inst Oswaldo Cruz 106:3–11
Bruijnzeel L (2002) Hydrology of tropical montane cloud forests: a reassessment. In: Gladwell JS (ed) Proceedings of the second international colloquium on hydrology and water management of the humid tropics, UNESCO, Paris and CATHALAC, Panama City, Panama, pp 353–383
Coscarón S, Coscarón-Arias CL (1995) Distribution of Neotropical Simuliidae (Insecta, Diptera) and its areas of endemism. Rev Acad Colomb Cienc 19:717–732
Crosskey R (1990) The natural history of blackflies. Wiley, Chichester
Darriba D, Taboada G, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772
Escalante AA, Freeland DE, Collins WE, Lal AA (1998) The evolution of primate malaria parasites based on the gene encoding cytochrome b from the linear mitochondrial genome. Proc Natl Acad Sci USA 95:8124–8129
Forrester D, Greiner E (2008) Leucocytozoonosis. In: Atkinson C, Thomas NB (eds) Parasitic diseases of wild birds. Wiley-Blackwell, Ames, pp 54–107
Forrester D, Foster G, Morrison J (2001) Leucocytozoon toddi and Haemoproteus tinnunculi (Protozoa: Haemosporina) in the Chimango Caracara (Milvago chimango) in Southern Chile. Mem Inst Oswaldo Cruz 96:2013–1024
García-Moreno J, Arctander P, Fjeldså J (1999) Strong diversification at the treeline among Metallura hummingbirds. Auk 116:702–711
González-Acuña D, Silva C, Soto M, Mironov S, Moreno L, González-Gómez PL, Badrul H, Kinsella M (2011) Parasites of the Green-backed Firecrown (Sephanoides sephaniodes) in Chile. Rev Mex Biodivers 82:1333–1336
Greiner E, Bennett GE, Coombs R (1975) Distribution of the avian hematozoa of North America. Can J Zool 53:1762–1787
Gryaznov A (1984) Morphological adaptations of bloodsucking blackflies to their hosts. In: Narchuk E, Zlobin V (eds) Diptera (Insecta) of the fauna of the USSR and their significance in ecosystems. Akademiya Nauk SSSR, Leningrad [=St. Petersburg], Russia, pp 31–34
Gutiérrez A, Carrillo E, Rojas S (2004) Guía Ilustrada de los Colibríes de la Reserva Natural Río Ñambí. FPAA, FELCA, ECOTONO, Bogotá
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol 90:797–802
Herzog S, Kattan G (2011) Patterns of diversity and endemism in the birds of the tropical Andes. In: Herzog S, Martínez R, Jørgensen P, Tiessen H (eds) Climate change and biodiversity in the tropical Andes. McArthur Foundation, Inter-American Institute for Global Change Research (IAI) and Scientific Committee on Problems of the Environment (SCOPE), Paris, pp 245–259
INDERENA (1986) Guía del Sistema de Parques Nacionales de Colombia. Ministerio de Agricultura. Instituto Nacional de los Recursos Naturales Renovables y del Ambiente, Bogotá. Colombia
Jarvi SI, Farias MEM, Baker H, Freifeld HB, Baker PE, Van Gelder E, Massey JG, Atkinson CT (2003) Detection of avian malaria (Plasmodium spp.) in native land birds of American Samoa. Conserv Genet 4:629–637. doi:10.1023/A:1025626529806
Josse C, Cuesta F, Navarro G, Barrena V, Cabrera E, Chacón-Moreno E, Ferreira W, Peralvo M, Saito J, Tovar A (2009) Mapa de Ecosistemas de los Andes del Norte y Centro. Bolivia, Colombia, Ecuador, Perú y Venezuela. Secretaría General de la Comunidad Andina, Programa Regional ECOBONA, CONDESAN-Proyecto Paramo Andino, Programa BioAndes, EcoCiencia, NatureServe, LTA-UNALM, IAvH, ICAE-ULA, CDCUNALM, RUMBOL SRL, www.infoandina.org/ecosistemasandinos Lima, Perú
Kapelle K, Brown A (2001) Bosques Nublados del Neotrópico. Instituto Nacional de la Biodiversidad-INBio. Santo Domingo de Heredia, Costa Rica
Keesing F, Holt R, Ostfeld R (2006) Effects of species diversity on disease risk. Ecol Lett 9:485–498
LaPointe DA, Goff ML, Atkinson CT (2010) Thermal constraints to the sporogonic development and altitudinal distribution of avian malaria Plasmodium relictum in Hawaii. J Parasitol 96:318–324
Levin II, Zwiers P, Deem SL, Geest EA, Higashiguchi JM, Iezhova TA, Jiménez-Uzcátegui G, Kim DH, Morton JP, Perlut NG, Renfrew RB, Sari EHR, Valkiūnas G, Parker PG (2013) Multiple lineages of avian malaria parasites (Plasmodium) in the Galapagos Islands and evidence for arrival via migratory birds. Conserv Biol. doi:10.1111/cobi.12127
Lotta IA, Matta NE, Torres RD, Moreno-de Sandino M, Moncada LI (2013) Leucocytozoon fringillinarum and Leucocytozoon dubreuili in Turdus fuscater from a Colombian Paramo Ecosystem. J Parasitol 99:359–362
Luna-Vega I, Almeida-Leñero L, Llorente-Bousquets J (1989) Florística y aspectos fitogeográficos del bosque mesófilo de montaña de las cañadas de Ocuilan, estados de Morelos y México. Ann Inst Biol Univ Nac Auton Mex Bot 59:63–87
Malmqvist B, Strasevicius D, Hellgren O, Adler P, Bensch S (2004) Vertebrate host specificity of wild-caught blackflies revealed by mitochondrial DNA in blood. Proc R Soc B 271:S152–S155
Mantilla JS, González AD, Valkiūnas G, Moncada LI, Matta NE (2013a) Description and molecular characterization of Plasmodium (Novyella) unalis sp. nov. from the Great Thrush (Turdus fuscater) in highland of Colombia. Parasitol Res. doi:10.1007/s00436-013-3611-0
Mantilla JS, Matta NE, Pacheco MA, Escalante AA, Gonzalez AD, Moncada LI (2013b) Identification of Plasmodium (Haemamoeba) lutzi (Lucena, 1939) from Turdus fuscater (Great Thrush) in Colombia. J Parasitol 99:662–668
Martinsen S, Perkins S, Schall J (2008) A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol Phylogenet Evol 47:261–273
McGuire J, Witt C, Remsen J Jr, Dudley R, Altshuler D (2008) A higher-level taxonomy for hummingbirds. J Ornithol 150:155–165
McMullan M, Quevedo A, Donegan T (2011) Guia de Campo de las Aves de Colombia. Fundación ProAves, Bogotá
Merino S, Moreno J, Vásquez R, Martínez J, Sánchez-Monsalvez I, Estádes C, Ippi S, Sabat P, Rozzi R, McGehee R (2008) Haematozoa in forest birds from southern Chile: latitudinal gradients in prevalence and parasite lineage richness. Austral Ecol 33:329–340
Morrone J (2001) Biogeografía de América Latina y el Caribe. M&T-Manuales & Tesis, SEA, Zaragoza
Muñoz E, Ferrer D, Molina RRA (1999) Prevalence of haematozoa in birds of prey in Catalonia, northeast Spain. Vet Rec 144:623–636
Myers N, Mittermeier R, Mittermeier C, da Fonseca G, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Olson D, Dinerstein E (1998) The Global 200: a representation approach to conserving the Earth's most biologically valuable ecoregions. Conserv Biol 12:502–515
Pacheco MA, Battistuzzi FU, Junge RE, Cornejo OE, Williams CV, Landau I, Rabetafika L, Snounou G, Jones-Engel L, Escalante AA (2011) Timing the origin of human malarias: the lemur puzzle. BMC Evol Biol 11:299
Palinauskas V, Križanauskienė A, Iezhova T, Bolshakov C, Jönsson J, Bensch S, Valkiūnas G (2013) A new method for isolation of purified genomic DNA from haemosporidian parasites inhabiting nucleated red blood cells. Exp Parasitol 133:275–280
Piersma T (1997) Do global patterns of habitat use and migration strategies co-evolve with relative investments in immunocompetence due to spatial variation in parasite pressure? Oikos 80:623–631
Poulin R, Leung T (2011) Latitudinal gradient in the taxonomic composition of parasite communities. J Helminthol 85:228–233
Rambaut A (2006) FigTree: tree figure drawing too, version1.3.1, in: Institute of Evolutionary Biology, U.o.E. (Ed.), http://tree.bio.ed.ac.uk/software/figtree/. Accessed 31 Jan 2013
Rangel-Ch J (2000) La región paramuna y franja aledaña en Colombia. In: Rangel-Ch J (ed) Colombia diversidad biótica III: La región de vida paramuna. Universidad Nacional de Colombia. Editorial Unibiblos, Bogotá DC. Colombia, pp 1–23
Remsen J, Cadena C, Jaramillo A, Nores M, Pacheco J, Pérez-Emán J, Robbins M, Stiles F, Stotz D, Zimmer K (2012) A classification of the bird species of South America. American Ornithologists’ Union, Version 7 December 2012, http://www.museum.lsu.edu/~Remsen/SACCBaseline.html. Accessed 7 Dec 2012
Rodríguez OA, Matta NE (2001) Blood parasites in some birds from eastern plains of Colombia. Mem Inst Oswaldo Cruz 96:1173–1176
Rodríguez OA, Moya H, Matta NE (2009) Avian blood parasites in the Chingaza National Natural Park: high Andes of Colombia. Hornero (B Aires) (en linea) 24:1–6
Ronquist F, Huelsenbeck J (2003) MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Sambrook J, Fritsch E, Maniatis T (1989) Molecular cloning: A laboratory manual, 2nd edn. Cold Spring Harbour Laboratory, Cold Spring Harbor
Särkinen T, Pennington R, Lavin M, Simon M, Hughes C (2012) Evolutionary islands in the Andes: persistence and isolation explain high endemism in Andean dry tropical forests. J Biogeogr 39:884–900
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Skidmore LV (1931) Leucocytozoon smithi infection in turkeys and its transmission by Simulium occidentale Townsend. J Parasitol 18:130
Tamura K, Peterson D, Peterson N, Stecher G, Nei MSK (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Terborgh J (1971) Distribution on environmental gradients: theory and a preliminary interpretation of distributional patterns in the avifauna of the Cordillera Vilcabamba, Peru. Ecology 52:23–40
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC, Boca Raton
Valkiūnas G, Bensch S, Iezhova TA, Križanauskienė A, Hellgren O, Bolshakov CV (2006) Nested cytochrome b polymerase chain reaction diagnostics underestimate mixed infections of avian blood haemosporidian parasites: microscopy is still essential. J Parasitol 92:418–422
Valkiūnas G, Zehtindjiev P, Dimitrov D, Križanauskienė A, Iezhova TA, Bensch S (2008) Polymerase chain reaction-based identification of Plasmodium (Huffia) elongatum, with remarks on species identity of haemosporidian lineages deposited in GenBank. Parasitol Res 102:1185–1193
Valkiūnas G, Iezhova T, Loiseau C, Thomas S, Sehgal R (2009) Nested cytochrome b polymerase chain reaction diagnostics detect sporozoites of hemosporidian parasites in peripheral blood of naturally infected birds. J Parasitol 95:1512–1525
van Rooyen J, Lalubin F, Glaizot O, Christe P (2013) Altitudinal variation in haemosporidian parasite distribution in great tit populations. Parasite & Vectors 6:139. doi:10.1186/1756-3305-6-139
Vargas-Ríos O, Pedraza P (2004) El Parque Nacional Natural Chingaza. Editorial Gente Nueva, Bogotá
White E, Greiner EE, Bennet GF, Herman CM (1978) Distribution of the hematozoa of Neotropical birds. Rev Biol Trop 26:43–102
White E, Bennett GN, Williams NA (1979) Avian Haemoproteidae. 11. The haemoproteids of the hummingbird family Trochilidae. Can J Zool 57:908–913
Williamson S (2001) Hummingbirds of North America. Peterson Field Guide. Houghton Mifflin Company, New York
Acknowledgments
Fieldwork was done under the permission of Unidad Administrativa Especial del Sistema de Parques Nacionales Naturales UAESPNN y el Ministerio de ambiente vivienda y desarrollo territorial. This work was supported by Colciencias code 110152128340 contract no. 359-2011. The funding source had no role on the study design, data analysis or publication submission. This research was done in accordance with Universidad Nacional de Colombia's Institutional Animal Care, and ethical Committee of Veterinary Medicine Faculty. We thank Dr. Tatjana Iezhova for technical assistance during preparation of the illustration plate. We are grateful to the students belonging to Grupo “Estudio Relación Parásito Hospedero”, for their assistance with sample collection, and to the staff of Chingaza National Natural Park.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matta, N.E., Lotta, I.A., Valkiūnas, G. et al. Description of Leucocytozoon quynzae sp. nov. (Haemosporida, Leucocytozoidae) from hummingbirds, with remarks on distribution and possible vectors of leucocytozoids in South America. Parasitol Res 113, 457–468 (2014). https://doi.org/10.1007/s00436-013-3675-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3675-x