Abstract
Extracranial metastases from glioblastoma (GBM) are uncommon with an estimated incidence of less than 2%. We report two cases of metastatic GBM seen within an 8-week period followed by a literature review. We attempted to identify common factors or a causative mechanism. Factors that predominated among the reviewed cases included male gender, tumor location, and younger age. Causative mechanisms were not apparent. While metastatic disease remains rare, it might be occurring with increasing frequency. This trend might be due to increased diagnosis, better imaging, a more extensive physician workup, or an increase in survival. Metastatic GBM can present and progress quite rapidly, and repeat evaluations of persistent or worsening complaints among GBM patients are warranted. Early diagnosis of metastatic disease spread can help to expedite alleviation of patients’ discomfort, in an already aggressive disease process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extracranial metastases from glioblastoma (GBM) are uncommon with an estimated incidence of less than 2% [1]. Case reports and small series bespeak the rarity of this complication, none of which have reported the identifiable mode for metastatic spread. Most malignant tumors have a propensity for metastases; this rarity is curious for GBM. It might be related to short survivals for GBM patients, issues intrinsic to the central nervous system (CNS) such as the blood brain barrier (BBB) or the lack of lymphatics. We report two cases of metastatic GBM seen within an 8-week period followed by review of literature in attempt to identify common factors, high-risk patients or a causative mechanism.
Case 1
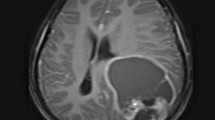
A 72 year-old man with a history of hyperlipidemia and sleep apnea presented after 1 week of left sided weakness, dysarthria, and personality changes. His exam was normal except for a left facial droop with a left upgoing toe. CT and MRI revealed right temporal and right temporal/occipital enhancing mass lesions. A gross total resection of both masses was performed; pathology was consistent with GBM. He was treated with radiation therapy (RT) and temozolomide. Eight months later, he developed focal seizures and repeat MRI showed tumor progression. He was started on bevacizumab and follow-up MRI showed improvement in tumor enhancement. However, the patient progressively developed worsening behavioral and cognitive changes, so carmustine was added to the bevacizumab. Two months later he began to complain of rib/chest, hip and back pain requiring admission for work up. Chest imaging revealed no evidence for pulmonary embolism, but instead destructive osseous lesions with a soft tissue component invading the spinal canal and pulmonary nodules suspicious for metastatic lesions. MRI of his spine revealed multifocal bony lesions to cervical, thoracic, and lumbar spine with some extension into midline paraspinal muscles. Fine needle biopsy of the paraspinal mass revealed pathology similar to his primary GBM (positive for glial fibrillary acidic protein (GFAP)). The patient was transferred to hospice, dying 19 months after his initial diagnosis and about 9 months after his diagnosis of metastasis.
Case 2
A 31 year-old woman with no significant past medical history presented after 2 weeks of headache and 1 week of nausea/vomiting and gait ataxia. On exam she had left facial droop, upward gaze palsy and left greater than right limb ataxia. MRI revealed heterogeneously enhancing mass in the left cerebellum and vermis with obstructive hydrocephalus. MRI of spine was unremarkable with no evidence of leptomeningeal spread. Ventriculostomy was placed and subsequent gross total tumor resection was done; the ventriculostomy was removed after surgery. Pathology was read as GBM. She was started on RT and temozolomide after which she was treated with erlotinib/bevacizumab. Four months after her initial diagnosis, her MRI brain showed progression of the cerebellar lesion and she began experiencing low back pain. MRI of the spine was performed to evaluate for leptomeningeal dissemination, but revealed extensive osseous disease in the cervical, thoracic, lumbar, and sacral spine. She underwent a CT chest/abdomen/pelvis that had no other signs for metastatic disease except for a breast lesion that was biopsied and found to be benign. Biopsy of an osseous lesion was histologically similar to the cerebellar GBM pathology (GFAP positive). She was treated with palliative RT to the spine followed by temozolomide and bevacizumab. Six months after initial diagnosis, she had a bone scan that revealed diffuse progression of her bony disease. The patient died 9 months after her initial diagnosis and 5 months after diagnosis of metastasis.
Materials and methods
We searched PubMed for reports of extracranial metastatic GBM that was confirmed by biopsy or autopsy. Search terms used were extracranial GBM, extraneural GBM, and metastatic GBM. We only used reports from 1960 to 2010 and excluded cases with patients less than 18 years of age or non-English reports. Data collected was as follows: age, gender, location of primary GBM, treatment of initial GBM, local recurrence of initial GBM, location of metastatic disease, time to diagnose metastatic disease, treatment of metastasis, survival from time of diagnosis of metastatic disease, and overall survival (OS).
Results
Seventy-nine cases were found that met our criteria (Table 1) [1–45]. Forty percent (32 patients) of these cases were reported between 1960 and 1980. The median age was 42 (range 18–74). Eighty-five percent of the patients were less than 60 years of age and seventy-seven percent of the patients were men.
We divided initial tumor location into left or right hemisphere and lobe(s) of involvement. Fifty-five percent of patients had right hemisphere involvement compared to 45% on the left. The two most common sites of primary GBM location were right temporal lobe (32%) and left frontal lobe (20%) followed by right parietal lobe, left temporal and right frontal lobe in 14% each.
Forty-four percent of the patients had local tumor recurrence at some point in the course of the disease; of these, 55% had local tumor recurrence prior to the diagnosis of metastatic disease. The median time to diagnosis of metastatic disease was 9.5 months. The diagnosis of metastatic disease was made at autopsy in 6% of the cases.
Organs with metastatic disease included bone, bone marrow, lymph nodes, eyes, lungs, spleen, liver, parotid gland, adrenal gland, subcutaneous tissue, skin, heart, pancreas, diaphragm, kidney, stomach, pelvis, and thyroid. The four most common organs were bone (38%), lymph nodes (37%), lungs (32%), and liver (18%). Most patients had one (62%) or two (28%) organs affected by metastases, then there was a drop-off with three to four metastatic sites (6%) and more than four sites (4%).
The median OS time for patients with metastatic GBM was 13 months (Table 2). Median survival based on the number of organ systems involved was: 14 months for one system, 11 months for two, 14 months for 3–4 and 17 months for >4 systems. The median survival time for patients from the time of their diagnosis of metastatic disease was 5 months (Table 3). In patients with metastasis to one site the median OS from the time of diagnosis of the metastasis was 6 months. It was 5 months for patients with sites or 3–4 sites and 1 month if >4 sites.
Wilcoxon rank-sums were used to evaluate for the effect of GBM treatment type on OS. Total resection compared to subtotal resection of GBM did not significantly affect OS in those with metastatic disease (P = 0.17). RT alone or RT with resection did not affect OS (P = 0.73 and P = 0.26, respectively). However, chemotherapy, either alone or given with RT and resection, was associated with an increase in median OS compared to those who did not receive chemotherapy. The median OS in patients who were treated with chemotherapy alone was approximately 22 months compared to 11 months in those who did not (P = 0.02). The median OS in those who underwent resection, had RT and chemotherapy was 24 months compared to 14 months in those who did not have all three forms of treatment (P = 0.03).
Wilcoxon rank-sums were again used to assess how GBM treatment affected time to diagnosis of metastatic disease. Neither resection alone nor RT alone significantly prolonged the time to diagnosis of metastatic disease. Chemotherapy did show a statistically significantly longer time to metastatic GBM diagnosis (15 months) compared to those who received no chemotherapy (12 months) (P = 0.049). This significance decreased in the presence of resection, RT, or both resection and RT.
Discussion
There has been an increase in the number of reported cases of metastatic GBM in the literature in the time period we reviewed. In 1969, it was reported that 0.44% of patient developed extracranial metastatic GBM [28]; more recently it is thought that the frequency may be as high as 2% [20]. From our literature review, the majority of patients with metastatic GBM were reported after 1980. This trend might be due to increased diagnosis, better imaging, a more extensive physician workup, or an increase in survival. We restricted our review to GBM, but reports of metastases from other gliomas exist [46] and would increase the number of cases reported.
Why the incidence of extracranial metastases is low remains unclear given the aggressive nature of GBM. However, it occurs but who is at risk and why remains unknown. Using our criteria 79 cases were identified over a 50 years period. Given that in the U.S. alone there are 10,000–12,000 cases of GBM per year, the incidence of metastatic GBM is almost certainly far below the reported 2%. The goal of this review was to better assess the common factors among those patients who develop metastatic disease. We are unable to define risk factors for the development of metastatic disease as the number of cases known is only what has been published and this may be an underestimate of cases; we would also need to know all the patients with GBM diagnosed in a given time period to determine “true” risk factors and also incidence.
The median time to diagnosis of metastatic disease was about 10 months. Perhaps the increasing survival times of GBM patients in modern times allows for the development of metastatic disease, explaining the increasing frequency of reported cases in more recent decades; although we would expect even higher numbers. Six percent of the cases reviewed were diagnosed at autopsy, which might have been documented pre-mortem with better diagnostic tools and clinical awareness.
The two most widely accepted modes of spread are hematogenous and lymphatic [16]; in some cases direct extension of tumor is the cause. Hematogenous seeding may occur during surgery or if there is vascular invasion from the tumor. Using the former hypotheses, it would be expected that the greater the resection the more likely the risk but three reported cases had only a biopsy, suggesting vascular invasion may be a feasible explanation [18] [20]. Lymph nodes were among the most frequently metastasized sites. Given the absence of lymphatics in the CNS this is curious, but lymphatic tumor seeding during resection is a possible explanation but we would then expect an earlier occurrence of metastatic disease. Dissemination into the cerebrospinal fluid and then extracranially is another hypothesis, but usually only occurs with ventricular shunts so there is a communication from CSF to extracranial structures (peritoneal cavity). Explanations for the rarity of metastatic GBM include absence of lymphatic vessels in the CNS, the lack of communication between intracranial and extracranial perivascular spaces [20], short survival time of patients, and undiagnosed metastatic disease [18].
Factors that predominated among the reviewed cases included a male sex, tumor location, and younger age at presentation. In general, 60% of GBM cases are in men. However, the proportion of male patients with metastatic GBM was higher than expected with no clear reason. The majority of patients with extra-cranial disease were less than 60 years of age (median 42 years). Age is a well-known prognostic factor, with decreased survival based on age, so increased survival may place some patients at higher risk. Other relevant factors are primary tumor proximity to the ventricular system, whether or not the tumor is multifocal [47], and the functional status of patients at the time of GBM diagnosis. We considered these factors but available data was insufficient for analysis.
The OS times when developing extracranial GBM did not seem to differ from expected survival in patients without metastatic disease. Median OS for patients with GBM is approximately 12–15 months [48], compared to 13 months among the patients reported here with metastatic GBM. Patients with local intracranial tumor recurrence reportedly have a median OS of 7.5 months [49]. Furthermore, according to our data, the median survival time from the time of extracranial disease diagnosis was 5 months. A study looking at median survival from time of intracranial, extraparenchymal spread of GBM (subependymal or subarachnoid space) was found to be 9.25 months [50]. It is difficult to compare these patterns of spread, but the diagnosis of extracranial spread compared to intracranial, extraparenchymal spread seems to carry with it a shorter survival time. However, intracranial, intraparenchymal recurrence seems to carry a poorer prognosis compared to extracranial metastatic disease.
Systemic chemotherapy, regardless of type, was the only treatment that significantly lengthened OS in patients with metastatic GBM. Additionally, it was the only treatment that significantly extended the time to metastatic disease diagnosis. If chemotherapy has a true impact on survival, it is likely a function of local and systemic effects.
Although extracranial GBM does not appear to drastically shorten survival time of patients with GBM, physicians should be aware of its existence. Bone, lymph nodes, and lung were among the most commonly affected organ systems and any symptoms referable to these areas should be evaluated. Bone pain may be commonly mistaken for complications of steroid therapy like avascular necrosis or compression fractures but in our cases, bone pain was the initial manifestation of disease. Likewise, lung metastases causing chest pain or respiratory complaints may be mistaken for pulmonary embolus or pneumonia. Similarly, enlarged lymph nodes should be carefully evaluated for evidence of GBM invasion. The two cases outlined here earlier demonstrated that metastatic disease can present and progress quite rapidly. Repeat or frequent evaluations of persistent, and worsening, complaints among GBM patients are warranted. Early diagnosis of metastatic disease spread can help to expedite alleviation of patients’ discomfort, in an already aggressive disease process.
References
Waite KJ, Wharton SB, Old SE, Burnet NG (1999) Systemic metastases of glioblastoma multiforme. Clinical oncol 11:205–207
Sadik AR, Port R, Garfinkel B, Bravo J (1984) Extracranial metastasis of cerebral glioblastoma multiforme: case report. Neurosurgery 15:549–551
Steinbok P, Dolman CL, Goldie JH (1985) Variation in response to CCNU of glioblastoma multiforme in brain and cervical lymph node. Case report. J Neurosurg 62:918–921. doi:10.3171/jns.1985.62.6.0918
Trattnig S, Schindler E, Ungersbock K, Schmidbauer M, Heimberger K, Hubsch P, Stiglbauer R (1990) Extra-CNS metastases of glioblastoma: CT and MR studies. J Comput Assist Tomogr 14:294–296
Zappia JJ, Wolf GT (1992) Cervical metastatic glioblastoma multiforme. Arch Otolaryngol Head Neck Surg 118:755–756
Wallace CJ, Forsyth PA, Edwards DR (1996) Lymph node metastases from glioblastoma multiforme. AJNR Am J Neuroradiol 17:1929–1931
Greif J, Horovitz M, Marmor S (1998) Pleuropulmonary metastasis from an intracranial glioblastoma. Lung cancer 20:135–137
Beauchesne P, Soler C, Mosnier JF (2000) Diffuse vertebral body metastasis from a glioblastoma multiforme: a technetium-99 m Sestamibi single-photon emission computerized tomography study. J Neurosurg 93:887–890. doi:10.3171/jns.2000.93.5.0887
Yasuhara T, Tamiya T, Meguro T, Ichikawa T, Sato Y, Date I, Nakashima H, Ohmoto T (2003) Glioblastoma with metastasis to the spleen–case report. Neurol Med Chir (Tokyo) 43:452–456
Utsuki S, Tanaka S, Oka H, Iwamoto K, Sagiuchi T, Fujii K (2005) Glioblastoma multiforme metastasis to the axis. Case report. J Neurosurg 102:540–542. doi:10.3171/jns.2005.102.3.0540
Mirzayan MJ, Samii M, Petrich T, Borner AR, Knapp WH, Samii A (2005) Detection of multiple extracranial metastases from glioblastoma multiforme by means of whole-body [18F]FDG-PET. Eur J Nucl Med Mol Imaging 32:853. doi:10.1007/s00259-004-1749-9
Tuominen H, Lohi J, Maiche A, Tormanen J, Baumann P (2005) Mediastinal metastasis of glioblastoma multiforme evolving from anaplastic astrocytoma. J Neurooncol 75:225–226. doi:10.1007/s11060-005-3395-x
Taha M, Ahmad A, Wharton S, Jellinek D (2005) Extra-cranial metastasis of glioblastoma multiforme presenting as acute parotitis. Br J Neurosurg 19:348–351. doi:10.1080/02688690500305506
Rajagopalan V, Kamar FGE, Thayaparan R, Grossbard ML (2005) Bone marrow metastases from glioblastoma multiforme–a case report and review of the literature. J Neurooncol 72:157–161. doi:10.1007/s11060-004-3346-y
Didelot A, Taillandier L, Grignon Y, Vespignani H, Beauchesne P (2006) Concomitant bone marrow metastasis of a glioblastoma multiforme revealed at the diagnosis. Acta Neurochir 148:997–1000. doi:10.1007/s00701-006-0854-x
Zhen L, Yufeng C, Zhenyu S, Lei X (2010) Multiple extracranial metastases from secondary glioblastoma multiforme: a case report and review of the literature. J Neurooncol 97:451–457. doi:10.1007/s11060-009-0044-9
Mentrikoski M, Johnson MD, Korones DN, Scott GA (2008) Glioblastoma multiforme in skin: a report of 2 cases and review of the literature. Am J Dermatopathol 30:381–384. doi:10.1097/DAD.0b013e31817532c4
Kraft M, Lang F, Braunschweig R, Janzer RC (2008) Parotid gland metastasis from glioblastoma multiforme: a case report and review of the literature. Eur Arch Otorhinolaryngol 265:709–711. doi:10.1007/s00405-007-0499-2
Mujic A, Hunn A, Taylor AB, Lowenthal RM (2006) Extracranial metastases of a glioblastoma multiforme to the pleura, small bowel and pancreas. J Clin Neurosci 13:677–681. doi:10.1016/j.jocn.2005.08.016
Piccirilli M, Brunetto GM, Rocchi G, Giangaspero F, Salvati M (2008) Extra central nervous system metastases from cerebral glioblastoma multiforme in elderly patients. Clinico-pathological remarks on our series of seven cases and critical review of the literature. Tumori 94:40–51
Haddon M, Slavin JD, Spencer RP (1989) Multiple bone metastases in a patient with glioblastoma multiforme. Clin Nucl Med 14:13–14
Vural G, Hagmar B, Walaas L (1996) Extracranial metastasis of glioblastoma multiforme diagnosed by fine-needle aspiration: a report of two cases and a review of the literature. Diagn Cytopathol 15:60–65. doi:10.1002/(SICI)1097-0339(199607)15:1<60:AID-DC12>3.0.CO;2-A
Kleinschmidt-Demasters BK (1996) Diffuse bone marrow metastases from glioblastoma multiforme: the role of dural invasion. Hum Pathol 27:197–201
Park CC, Hartmann C, Folkerth R, Loeffler JS, Wen PY, Fine HA, Black PM, Shafman T, Louis DN (2000) Systemic metastasis in glioblastoma may represent the emergence of neoplastic subclones. J Neuropathol Exp Neurol 59:1044–1050
Yung WK, Tepper SJ, Young DF (1983) Diffuse bone marrow metastasis by glioblastoma: premortem diagnosis by peroxidase-antiperoxidase staining for glial fibrillary acidic protein. Ann Neurol 14:581–585. doi:10.1002/ana.410140514
Yokoyama H, Ono H, Mori K, Kishikawa M, Kihara M (1985) Extracranial metastasis of glioblastoma with sarcomatous component. Surg Neurol 24:641–645
Campora RG, Salaverri CO, Ramirez FV, Villadiego MS, Davidson HG (1993) Metastatic glioblastoma multiforme in cervical lymph nodes. Report of a case with diagnosis by fine needle aspiration. Acta cytologica 37:938–942
Smith DR, Hardman JM, Earle KM (1969) Metastasizing neuroectodermal tumors of the central nervous system. J Neurosurg 31:50–58. doi:10.3171/jns.1969.31.1.0050
Anzil AP (1970) Glioblastoma multiforme with extracranial metastases in the absence of previous craniotomy. Case report. J Neurosurg 33:88–94. doi:10.3171/jns.1970.33.1.0088
Dolman CL (1974) Lymph node metastasis as first manifestation of glioblastoma. Case report. J Neurosurg 41:607–609. doi:10.3171/jns.1974.41.5.0607
Ley A, Campillo D, Oliveras C (1961) Extracranial metastasis of glioblastoma multiforme. J Neurosurg 18:313–330. doi:10.3171/jns.1961.18.3.0313
Nigogosyan G, De La Pava S, Pickren JW (1962) Brain tumor with extracranial metastases. Report of two cases. Arch Neurol 6:300–306
Wisiol ES, Handler S, French LA (1962) Extracranial metastases of a glioblastoma multiforme. J Neurosurg 19:186–194. doi:10.3171/jns.1962.19.3.0186
Wakamatsu T, Matsuo T, Kawano S, Teramoto S, Matsumura H (1971) Glioblastoma with extracranial metastasis through ventriculopleural shunt. Case report. J Neurosurg 34:697–701. doi:10.3171/jns.1971.34.5.0697
Hulbanni S, Goodman PA (1976) Glioblastoma multiforme with extraneural metastases in the absence of previous surgery. Cancer 37:1577–1583
Pasquier B, Pasquier D, N’Golet A, Panh MH, Couderc P (1980) Extraneural metastases of astrocytomas and glioblastomas: clinicopathological study of two cases and review of literature. Cancer 45:112–125
Chesnut RM, Abitbol JJ, Chamberlain M, Marshall LF (1993) Vertebral collapse with quadriparesis due to metastatic gliobla multiforme: case report and review of the literature. J Neurooncol 16:135–140
Widjaja A, Mix H, Golkel C, Flemming P, Egensperger R, Holstein A, Rademaker J, Becker H, Hundt M, Wagner S, Manns MP (2000) Uncommon metastasis of a glioblastoma multiforme in liver and spleen. Digestion 61:219–222
Ueda S, Mineta T, Suzuyama K, Furuta M, Shiraishi T, Tabuchi K (2003) Biologic characterization of a secondary glioblastoma with extracranial progression and systemic metastasis. Neuro Oncol 5:14–18
Labitzke HG (1962) Glioblastoma multiforme with remote extracranial metastases. Arch Pathol 73:223–229
Fecteau AH, Penn I, Hanto DW (1998) Peritoneal metastasis of intracranial glioblastoma via a ventriculoperitoneal shunt preventing organ retrieval: case report and review of the literature. Clin Transpl 12:348–350
Datta CK, Weinstein JD, Bland JE, Brager PM, Stewart MA (1998) A case of cervical lymph node metastasis resulting from glioblastoma multiforme. W V Med J 94:276–278
Mihara F, Ikeda M, Rothman MI, Numaguchi Y, Kristt D (1994) Vertebral body metastasis of glioblastoma multiforme with epidural mass formation. Contrast-enhanced MRI study. Clin Imaging 18:386–389
Mousavi M (1980) Bone marrow metastasis from glioblastoma multiforme. J Med Soc N J 77:904–905
Yao YT, Lin WH, Hung CC, Liu KN (1975) A case of glioblastoma multiforme with extracranial metastasis. Taiwan yi xue hui za zhi 74:220–228
Freitas MR, de Muzio SD, Pessoa RC, Stavale JN, Borges LR, Malheiros SM (2009) Diffuse bone marrow metastasis in cerebellar high-grade astrocytoma. A case report. Revista de neurologia 48:242–244
Miliaras G, Tsitsopoulos PP, Markoula S, Kyritsis A, Polyzoidis KS, Malamou-Mitsi V (2009) Multifocal glioblastoma with remote cutaneous metastasis: a case report and review of the literature. Cen Eur Neurosurg 70:39–42. doi:10.1055/s-2008-1080941
Beauchesne P, Bernier V, Carnin C, Taillandier L, Djabri M, Martin L, Michel X, Maire JP, Khalil T, Kerr C, Gorlia T, Stupp R, Pedeux R (2010) Prolonged survival for patients with newly diagnosed, inoperable glioblastoma with 3-times daily ultrafractionated radiation therapy. Neuro Oncol 12:595–602. doi:10.1093/neuonc/noq008
Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, Levin VA, Yung WK (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17:2572–2578
Parsa AT, Wachhorst S, Lamborn KR, Prados MD, McDermott MW, Berger MS, Chang SM (2005) Prognostic significance of intracranial dissemination of glioblastoma multiforme in adults. J Neurosurg 102:622–628. doi:10.3171/jns.2005.102.4.0622
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalokhe, G., Grimm, S.A., Chandler, J.P. et al. Metastatic glioblastoma: case presentations and a review of the literature. J Neurooncol 107, 21–27 (2012). https://doi.org/10.1007/s11060-011-0731-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0731-1