Abstract
Standard serum creatinine (S–Cr) levels in healthy children fluctuate with age and sex. However, it is unclear if this fluctuation in S–Cr levels is present for children with Down syndrome (DS) who show atypical growth rate. Therefore, we aimed to establish specific reference S–Cr levels for DS and compare them with the prevailing standard levels. We retrospectively reviewed 984 children with DS aged 3 months to 18 years who visited our medical center. Patients with diseases affecting S–Cr levels were excluded. We calculated the reference S–Cr levels according to sex, age, and length/height using medical records. A total of 3765 examinations of 568 children with DS were registered for this study. Ages and S–Cr levels were examined for boys (y = 0.032x + 0.20; r = 0.868, P < 0.0001), and girls (y = 0.024x + 0.23; r = 0.835, P < 0.0001). S–Cr levels in children aged >9 years were significantly higher in boys than in girls. The 430 children with DS aged 2–8 years were examined 1867 times. Height and S–Cr levels showed a significantly strong positive correlation (r = 0.670, P < 0.001) with regression equation y = 0.37x. The quintic equations calculated with S–Cr levels and length/height for boys (336 children, 2043 tests, r = 0.887) and girls (232 children, 1722 tests, r = 0.805) werey = − 6.132x5 + 32.78x4 − 67.86x3 + 68.31x2 − 33.14x + 6.41, and y = 0.09542x5 + 1.295x4 − 6.401x3 + 10.35x2 − 6.746x + 1.772. All calculated results varied from the standard levels for healthy children.
Conclusion: This study established reference S–Cr levels and quintic equations specific for children with DS. These reference levels would be potentially useful in evaluating S–Cr levels and renal function in this population.
What is Known: •Standard serum creatinine levels vary with age and sex to reflect muscle mass. •Reference serum creatinine levels specific to children with Down syndrome who show growth rates different from those of healthy children have not been established. | |
What is New: •Serum creatinine levels in children with Down syndrome showed different trajectories for sex, age, and length/height when compared with the standard levels for healthy children. •This report on specific reference serum creatinine levels for children with Down syndrome is useful in the assessment of renal function in these children. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The standard serum creatinine (S–Cr) levels currently used in pediatric are based on calculations obtained for healthy children [1,2,3]. Because endogenous S–Cr production is associated with muscle mass, it is considerably influenced by growth-related factors such as age and sex. However, it is unclear whether standard S–Cr levels can be appropriately applied to children with DS, who are generally known to have different growth rates [4, 5]. DS is the most frequent numerical chromosomal abnormality among the various malformation syndromes, with a frequency of 1:700–1:800 offspring. Trisomy 21 is most commonly caused by non-disjunction and accounts for 95–96% of cases [6, 7]. In addition, mosaic trisomy 21 is known to occur in 1–2% of cases and translocation trisomy 21 in 4–5% of cases [7, 8]. Much is known about their associated conditions, such as congenital heart disease (CHD) and thyroid dysfunction [9, 10]. In contrast, few reports have been published on S–Cr levels, which are indicators of renal function, and no specific reference levels have been established. With increased renal diseases and improved life prognosis in recent years, the need to evaluate renal function in DS has increased [11]. However, the general formula for estimated glomerular filtration rate, which is derived from standard S–Cr levels for healthy children, cannot be applied for children with DS [12]. When applying the standard S–Cr levels obtained in different populations depending on the presence or absence of disease, the results can be prone to errors [13]. As drug doses and diagnostic imaging modalities using contrast media depend on renal function [14, 15], having an accurate yet practical method of monitoring renal function is crucial to protect the kidneys [16]. These problems must be resolved because they not only hinder proper treatment of the child but also obscure future prospects, including renal replacement therapy, and cause anxiety for the family. Therefore, a specific set of reference levels for DS is required. The present study was conducted to address the need to establish reference S–Cr levels specific to children with DS.
Materials and methods
Study population
We retrospectively reviewed the data of children with DS aged between 3 months and 18 years who underwent blood tests during regular outpatient visits at Saitama Children’s Medical Center in Saitama, Japan, between 2003 and 2020. Those who were hospitalized or had emergency visits were excluded from the study. Length was measured in the supine position for children aged <2 years and height in the upright position for children aged >2 years. The length/height of the patients on the closest date and time within 3 months before or after the blood test were recorded. All specimens were collected for clinical purposes only and measured using the enzymatic method at our center promptly following blood collection. The S–Cr levels were determined by an enzymatic method using an automatic biochemistry analyzer (Labospect 008; Hitachi, Tokyo, Japan). Commercially available kits of enzyme used Accuras-auto CRE (Shino-test Corporation, Tokyo, Japan) until 2010, L type WAKO•M (Wako Pure Chemical Industries, Ltd., Tokyo, Japan) from 2010 to 2014, and Cygnus-auto CRE (Shino-test Corporation, Tokyo, Japan) from 2014 to the final observation point. DS was diagnosed using the G-banding method, and mosaic trisomy 21 was identified in an analysis with 20–30 cells.
Exclusion criteria
Children with diseases or symptoms affecting the S–Cr levels were excluded, as the purpose of this study was to determine the S–Cr levels of the general population of children with DS. These include kidney diseases that change the glomerular filtration rate, conditions that change the S–Cr levels, and diseases that change the muscle mass. The following are the examples of these diseases: chronic glomerulonephritis (CGN), congenital anomalies of the kidney and urinary tract (CAKUT) detected using abdominal ultrasonography, vesicoureteral reflux (VUR), infectious and inflammatory diseases, dehydration, muscle disease, neurological disease, malignant tumors, hypertension, severe CHD, liver or pancreatic diseases, and combinations of DS and other malformative syndromes. VUR was diagnosed using voiding cystourethrography in children with recurrent urinary tract infections. Children who were given anticonvulsants by pediatric neurologists were diagnosed with neurological disease. Severe CHD was defined as cases that have been treated with, for example, catheter treatments and intracardiac repair or considered to have treatment indications by pediatric cardiologists or cardiac surgeons. This included patients with treatment indications but who had not actually undergone surgery because their families not opting for it. Specifically, these treatment indications included patent ductus arteriosus (PDA), atrial septal defects (ASDs) or patent foramen ovale (PFO), ventricular septal defects (VSDs), atrioventricular septal defects (AVSDs), tetralogy of Fallot (TOF), and double-outlet right ventricle (DORV).
Statistical analysis
Reference S–Cr levels were established according to sex and age. Linear regression, multinomial regression, and Pearson’s product–moment analyses for serum creatinine levels according to sex, age, and length/height were conducted using R software (version 3.6.1) and MATLAB R2020b (MathWorks, Inc., Cambridge, MA, USA). First, the regression equation of S–Cr levels for age was calculated for each sex. Next, the regression equation of S–Cr levels for height (m) at the age with no sex-related difference was calculated. Finally, the S–Cr levels for length/height (m) in each sex were regressed by quintic equations considering the growth rate. As repeated tests in a single patient could represent a source of bias or a disproportionately larger amount of data from younger children, we analyzed data from the final examination only, which were not biased to younger ages, for validity using the quintic equation calculated in this study with P30 (the percentage of each S–Cr level within 30% of the quintic equation calculated in this study), mean error (ME), mean absolute error (MAE), and root mean square error (RMSE). In all analyses, P < 0.01 was considered to be of statistical significance.
Ethical approval
The study was approved by the local ethics committee for human research (protocol number 2020-04-025). All procedures involving human participants were conducted according to the ethical standards of the institutional and/or national research committee and within the guidelines of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Results
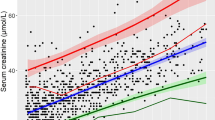
A total of 984 children with DS were examined at our children’s medical center during the study period. Of these children, 416 had the following complications warranting exclusion: 27 kidney diseases (Table 1), 36 neuromuscular diseases, and 380 severe CHD. Duplicates were also counted separately, but the distribution of severe CHD cases was as follows: 147 PDA, 108 ASD or PFO, 162 VSD, 91 AVSD, 23 TOF, 8 DORV, and 6 unknown. A final total of 568 children with DS (336 boys and 232 girls) were included in the study. Among the children were 111 with mild CHD (29 PDA, 70 ASD or PFO, 37 VSD, 4 AVSD, 1 TOF, 2 Ebstein’s anomaly, and 1 coarctation of the aorta; including duplicates) who did not require treatment. Of these children, 93.8% had been diagnosed with standard trisomy 21 in their chromosomes; 1.9%, mosaic trisomy 21; and 4.2%, the translocation trisomy 21. A total of 3765 blood tests (median 5 tests/child; interquartile range 2–11 tests/child) were performed, including 2043 tests in 336 boys and 1722 tests in 232 girls. The relations between age and S–Cr level tabulated according to sex are shown in Table 2. The linear regression by sex was y = 0.032x + 0.20 for boys and y = 0.024x + 0.23 for girls. Because a significant difference was observed between boy and girl children older than 9 years, the relationship between S–Cr level and height was shown for children aged 2–8 years. A significant positive correlation was observed between S–Cr level and height in 430 children aged 2–8 years who had been examined 1867 times (r = 0.670, P < 0.001). The regression equation was y = 0.44x − 0.07 and passed through the origin at y = 0.37x. In all subjects, the relationships between length/height and S–Cr level were determined by polynomial regression analysis for boys and girls separately, and reference S–Cr levels were expressed as a quintic equation of length/height. The regression equations were y = − 6.132x5 + 32.78x4 − 67.86x3 + 68.31x2 − 33.14x + 6.41 and y = 0.09542x5 + 1.295x4 − 6.401x3 + 10.35x2 − 6.746x + 1.772 (Fig. 1). The final test for each patient was extracted and evaluated for the quintic equations obtained in this study, which were P30 (boys, 91.4%; girls, 90.5%), ME (boys, 0.0063 ± 0.0870; girls, 0.0132 ± 0.0735), MAE (boys, 0.0631; girls, 0.0562), and RMSE (boys, 0.0871; girls, 0.0744).
Scatter plots and reference serum creatinine (S–Cr) levels in boys (a) and girls (b) with Down syndrome (DS), added standard S–Cr levels in healthy children for comparison. The reference S–Cr (mg/dL) levels drawn with a thick line is represented by a quintic equation that shows the correlation between S–Cr levels and length/height in boys and girls with DS aged between 3 months and 18 years. The significant correlation between S–Cr level and length/height was determined using a polynomial regression analysis. Reference S–Cr levels were compared with the standard S–Cr levels from healthy children of the same ethnic groups, expressed by a quintic equation and drawn with a dotted line [3]
Discussion
This is the first report establishing reference S–Cr levels for children with DS, which vary from the standard levels for healthy children both in age and length/height. In general, kidney disease in DS is considered less frequent than other complications [11, 17, 18]. However, although many CAKUT are asymptomatic, they pose a risk of renal failure and urinary tract infection [19]. The exact frequency of glomerular disease is unclear, but the incidence of CAKUT is known to be approximately 0.1–4% [20,21,22,23]. It is present at the same incidence rate in this study, and the reference S–Cr level is necessary to accurately evaluate them, as shown in Table 1. In contrast, several children with DS have CHD, so whether they should be included in marking the reference S–Cr values is controversial. A previous study has reported that the incidence of renal failure after surgery in children with DS was not significantly different from that in children without DS [24]. In addition, even children who have undergone surgery requiring cardiopulmonary bypass showed no effect on renal function 5 years after the surgery [25]. On the other hand, cyanotic CHD that requires surgery is a risk factor for chronic kidney disease [26]. Whether the possible causes of these are technical issues or changes over time remains ambiguous. Therefore, this study ruled out severe CHD that required surgery to remove factors that have a secondary effect on S–Cr levels.
Schwartz et al. [1] reported standard S–Cr levels based on age for the first time in 1976, which were y = 0.025x + 0.35 for boys and y = 0.018x + 0.37 for girls, in a study with 1398 children. In addition, Savory et al. [2] evaluated 2110 children and showed significant differences between boys and girls aged >15 years. These show distinctly different slopes and changes by age from those derived in the present study. One factor that can be identified is the differences in measurement methods [27, 28], but this does not affect the slope of the straight line obtained. Another factor is that the cohorts compared are of different ethnic groups [29, 30], and both need to be aligned to solve this problem. A study from China with 4765 children aged 0–18 years from the same Asian race as subjects showed a significant difference in S–Cr level between boys and girls except for those aged 7–9 years [31]. The S–Cr levels of 1594 children aged 3–18 years from Iran were y = 0.03x + 0.5 for boys and y = 0.02x + 0.5 for girls [32]. However, these studies also showed results different from our results. According to a study with the same ethnic group as in our study, the regression equation of the S–Cr levels for height at the age without a sex-related difference was y = 0.34x − 0.044 (r = 0.670, P < 0.001), and the equation passing through the origin was y = 0.30x in 1151 children [3]. The formulas for children with DS obtained in this study showed different slopes. In addition, as shown in Fig. 1, the S–Cr levels based on length/height were different in children with DS, even in the quintic equation that accounted for the growth of children at all ages. The abovementioned findings show that the transition of the S–Cr level in children with DS is different from the standard levels. The study results might have originated from the difference in growth rates or activity for age. On the other hand, S–Cr level fluctuates with time, meaning that it changes with changes in demographic indicators [33]. This also applies to children with DS, as their own growth rates are also changing due to advances in medical care and nutritional status [4]. Therefore, it is important to continue the evaluation including their changes over time.
This study had the following some limitations: it was retrospective in nature and conducted in a single center, and selection and ascertainment biases were possible because the subjects were not a general population. However, as our center had a specialized outpatient clinic for children with DS, we were satisfied with the number of patients considered. In addition, as these outpatients underwent regular follow-up concerning development at our center, our study population included many children with no complications or comorbidities; therefore, a little risk of bias existed. In addition, the inclusion of mosaic trisomy 21 in the study population is also considered a limitation, but the genotype ratio in this study showed the same tendency as the general ratio. Thus, we believe that the patients included in this study represent the general population of children with DS. However, it is desirable that reference levels be planned for a prospective study and created on the basis of many facilities and regions. More future studies are needed because the S–Cr levels of children with DS are different from the standard level for healthy children. Another limitation was that we could not exclude the possibility that the test may have been conducted frequently in certain patients due to high S–Cr levels. However, we excluded patients with diseases affecting S–Cr levels. Even assuming that children with high S–Cr levels were tested frequently, the assumption was based on general standard S–Cr levels that were inappropriate for children with DS. Therefore, the patients in this study were considered to represent the general population of children with DS. In addition, as this study involved a time-based evaluation of length/height and age, it should have had little impact on the results, such as increased bias in test results at specific times. Based on P30, ME, MAE, and RMSE, the quintic equation was considered to be valid and reliable. This study presents reference S–Cr levels for children with DS, which appear to be useful but should be validated for all ages. The reference levels may change depending on the proportion of diseases and advances in medical care, so further consideration of fluctuation over time to evaluate the reference levels is warranted.
The reference S–Cr levels for age and regression equation for length/height obtained in this study showed a strong correlation, which can be applied for children with DS. We suggest using these reference levels to assess S–Cr levels in children with DS.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable
Abbreviations
- ASD:
-
Atrial septal defect
- AVSD:
-
Atrioventricular septal defect
- CAKUT:
-
Congenital anomalies of the kidney and urinary tract
- CGN:
-
Chronic glomerulonephritis
- CHD:
-
Congenital heart disease
- DORV:
-
Double-outlet right ventricle
- DS:
-
Down syndrome
- MAE:
-
Mean absolute error
- ME:
-
Mean error
- PDA:
-
Patent ductus arteriosus
- PFO:
-
Patent foramen ovale
- RMSE:
-
Root mean square error
- S–Cr:
-
Serum creatinine
- TOF:
-
Tetralogy of Fallot
- VSD:
-
Ventricular septal defect
- VUR:
-
Vesicoureteral reflux
References
Schwartz GJ, Haycock GB, Spitzer A (1976) Plasma creatinine and urea concentration in children: normal values for age and sex. J Pediatr 88:828–830
Savory DJ (1990) Reference ranges for serum creatinine in infants, children and adolescents. Ann Clin Biochem 27(Pt 2):99–101
Uemura O, Honda M, Matsuyama T, Ishikura K, Hataya H, Yata N, Nagai T, Ikezumi Y, Fujita N, Ito S, Iijima K, Kitagawa T (2011) Age, gender, and body length effects on reference serum creatinine levels determined by an enzymatic method in Japanese children: a multicenter study. Clin Exp Nephrol 15:694–699
Zemel BS, Pipan M, Stallings VA, Hall W, Schadt K, Freedman DS, Thorpe P (2015) Growth charts for children with Down syndrome in the United States. Pediatrics 136:e1204–e1211
Hatch-Stein JA, Zemel BS, Prasad D, Kalkwarf HJ, Pipan M, Magge SN, Kelly A (2016) Body composition and BMI growth charts in children with Down syndrome. Pediatrics 138:e20160541
Hook EB, Cross PK (1982) Paternal age and Down’s syndrome genotypes diagnosed prenatally: no association in New York state data. Hum Genet 62:167–174
Mutton D, Alberman E, Hook EB (1996) Cytogenetic and epidemiological findings in Down syndrome, England and Wales 1989 to 1993. National Down Syndrome Cytogenetic Register and the Association of Clinical Cytogeneticists. J Med Genet 33:387–394
Papavassiliou P, Charalsawadi C, Rafferty K, Jackson-Cook C (2015) Mosaicism for trisomy 21: a review. Am J Med Genet A 167A:26–39
Bull MJ, Committee on G (2011) Health supervision for children with Down syndrome. Pediatrics 128:393–406
Capone GT, Chicoine B, Bulova P, Stephens M, Hart S, Crissman B, Videlefsky A, Myers K, Roizen N, Esbensen A, Peterson M, Santoro S, Woodward J, Martin B, Smith D, Down Syndrome Medical Interest Group D-USAAHCW (2018) Co-occurring medical conditions in adults with Down syndrome: a systematic review toward the development of health care guidelines. Am J Med Genet A 176:116–133
Malaga S, Pardo R, Malaga I, Orejas G, Fernandez-Toral J (2005) Renal involvement in Down syndrome. Pediatr Nephrol 20:614–617
Nishino T, Endo S, Miyano H, Umeda C, Tomii Y, Watanabe Y, Nakagawa M, Kakegawa D, Fujinaga S (2020) Is the eGFR formula useful for evaluating the renal function of Down syndrome? Pediatr Int. https://doi.org/10.1111/ped.14539
Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med 354:2473–2483
Daschner M (2005) Drug dosage in children with reduced renal function. Pediatr Nephrol 20:1675–1686
Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, Almen T, Aspelin P, Bellin MF, Clement O, Heinz-Peer G, Contrast Media Safety Committee of European Society of Urogenital R (2011) Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 21:2527–2541
Schwartz GJ, Work DF (2009) Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 4:1832–1843
Subrahmanyam AB, Mehta AV (1995) Renal anomalies in Down syndrome. Pediatr Nephrol 9:253–254
Kallen B, Mastroiacovo P, Robert E (1996) Major congenital malformations in Down syndrome. Am J Med Genet 65:160–166
Niamien-Attai C, Bacchetta J, Ranchin B, Sanlaville D, Cochat P (2017) Renal abnormalities in Down syndrome: A review. Arch Pediatr 24:1013–1018
Stoll C, Dott B, Alembik Y, Roth MP (2015) Associated congenital anomalies among cases with Down syndrome. Eur J Med Genet 58:674–680
Rankin J, Tennant PW, Bythell M, Pearce MS (2012) Predictors of survival in children born with Down syndrome: a registry-based study. Pediatrics 129:e1373–e1381
Morris JK, Garne E, Wellesley D, Addor MC, Arriola L, Barisic I, Beres J, Bianchi F, Budd J, Dias CM, Gatt M, Klungsoyr K, Khoshnood B, Latos-Bielenska A, Mullaney C, Nelen V, Neville AJ, O'Mahony M, Queisser-Luft A, Randrianaivo H, Rankin J, Rissmann A, Rounding C, Sipek A, Stoianova S, Tucker D, de Walle H, Yevtushok L, Loane M, Dolk H (2014) Major congenital anomalies in babies born with Down syndrome: a EUROCAT population-based registry study. Am J Med Genet A 164A:2979–2986
Kupferman JC, Druschel CM, Kupchik GS (2009) Increased prevalence of renal and urinary tract anomalies in children with Down syndrome. Pediatrics 124:e615–e621
Toth R, Szanto P, Prodan Z, Lex DJ, Sapi E, Szatmari A, Gal J, Szanto T, Szekely A (2013) Down syndrome and postoperative complications after paediatric cardiac surgery: a propensity-matched analysis. Interact Cardiovasc Thorac Surg 17:691–697
Greenberg JH, Zappitelli M, Devarajan P, Thiessen-Philbrook HR, Krawczeski C, Li S, Garg AX, Coca S, Parikh CR, Consortium T-A (2016) Kidney outcomes 5 years after pediatric cardiac surgery: the TRIBE-AKI study. JAMA Pediatr 170:1071–1078
Huynh L, Rodriguez-Lopez S, Benisty K, Dancea A, Garros D, Hessey E, Joffe A, Joffe R, Mackie A, Palijan A, Paun A, Pizzi M, Zappitelli M, Morgan C (2020) Follow-up after neonatal heart disease repair: watch out for chronic kidney disease and hypertension! Pediatr Nephrol 35:2137–2145
Beyer C (1993) Creatine measurement in serum and urine with an automated enzymatic method. Clin Chem 39:1613–1619
Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M, Eckfeldt JH, National Kidney Disease Education Program Laboratory Working G (2006) Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 52:5–18
Agamah ES, Webber LS, Lawrence M, Wattigney W, Berenson GS (1990) Serum creatinine and its relation to cardiovascular disease risk variables in children and young adults from a biracial community. The Bogalusa Heart Study. J Lab Clin Med 116:327–334
Groesbeck D, Kottgen A, Parekh R, Selvin E, Schwartz GJ, Coresh J, Furth S (2008) Age, gender, and race effects on cystatin C levels in US adolescents. Clin J Am Soc Nephrol 3:1777–1785
Liu C, Wen J, Xiang J, Ouyang X, Yang Y, Lu W, Wang J, Huang J, Min X (2019) Age- and sex-specific reference intervals for the serum cystatin C/creatinine ratio in healthy children (0-18 years old). J Int Med Res 47:3151–3159
Ghasemi A, Azimzadeh I, Afghan M, Momenan AA, Bagheripour F, Azizi F (2015) Pediatric reference values for serum creatinine and estimated glomerular filtration rate in Iranians: Tehran Lipid and Glucose Study. Arch Iran Med 18:753–759
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470
Acknowledgements
We would like to thank Dr. Uemura for his kind advice.
Funding
There is no funding source.
Author information
Authors and Affiliations
Contributions
Tomohiko Nishino mainly drafted the manuscript and performed the statistical analysis; Tomohiko Nishino, Shota Endo, Hiroki Miyano, Yoichi Takemasa, Masahito Saito, Chisato Umeda, and Yuji Tomii contributed to the conception and design of this study; Yoshitaka Watanabe, Mayu Nakagawa, and Daisuke Kakegawa critically reviewed the manuscript; Shuichiro Fujinaga supervised the whole study process. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the local ethics committee for human research (protocol number 2020-04-025). All procedures involving human participants were conducted according to the ethical standards of the institutional and/or national research committee and within the guidelines of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The requirement for informed consent was waived by the institutional review board due to the retrospective nature of the study.
Consent for publication
In consideration of human rights and ethics, this research was conducted under the review of the ethics committee of the medical center, and the study plan outline was presented on our center’s website, where patients and parents could ask questions about the study and opt-out from the use of their data.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Gregorio Paolo Milani
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishino, T., Endo, S., Miyano, H. et al. Reference serum creatinine levels according to sex, age, and height in children with Down syndrome. Eur J Pediatr 180, 2977–2983 (2021). https://doi.org/10.1007/s00431-021-04078-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-021-04078-z