Abstract
Determination of plasma creatinine (Pcr) should be associated to an estimation of glomerular filtration rate (eGFR). Pottel et al. established a height-independent equation, eGFR = 107.3/(Pcr/Q) where Q is the median of Pcr (Pottel–Belgium). The aims were to 1) determine a local height-independent equation (Pottel–Lyon), 2) evaluate the performance of these equations compared to the Schwartz 2009 and Schwartz–Lyon equations, and 3) evaluate the height-independent equations in laboratory routine. Therefore, 1) all first pediatric Pcr determination (December 2009–June 2011) were collected, and median of Pcr was determined for each 1-year age interval (Q-Lyon), 2) GFR was measured (mGFR) in 359 children (438 measures) and compared to eGFR, and 3) all first Pcr determination (January 2012–June 2013) were used to calculate eGFR with the Pottel–Lyon and the Pottel–Belgium equations. Pcr was determined by an IDMS-standardized enzymatic assay. In the population with a mGFR, the Pottel–Lyon and the Schwartz–Lyon showed the best performance (bias, P10 and P30). However, the performance in identifying patients with a mGFR < 75 mL/min/1.73 m2 was similar for all the studied equations.
Conclusion: The performance of the height-independent and dependent equations to identify mild renal dysfunction is similar. The height-independent Pottel equation could be proposed as an excellent screening tool for kidney disease when height information is not available.
“What is Known:” |
• Determination of plasma creatinine in children is rarely associated to an estimation of glomerular filtration rate due to the lack of height information. |
• Pottel et al. developed a height-independent equation (eGFR = 107.3/(Pcr/Q) where Q is the median of Pcr for each age class. |
“What is New:” |
• The performance of the height-independent (Pottel) or height-dependent (Schwartz) equations is similar to identify renal dysfunction (GFR < 75 mL/min/1.73 m 2 ) in children. |
• The height-independent Pottel equation could be an excellent screening tool for kidney disease in a general pediatric laboratory when height information is not available. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a general consensus that screening for chronic kidney disease (CKD) in adults is of great importance, and according to the 2012 Clinical Practice Guidelines of the National Kidney Foundation (Kidney Disease Improving Global Outcomes; KDIGO) [17, 27], determination of plasma creatinine (Pcr) should be associated to an estimation of glomerular filtration rate (eGFR) with a GFR estimating equation based on characteristics of the patient, that is sex, age, ethnic origin. In the pediatric population, the last KDIGO recommends the use of the Schwartz 2009 formula to estimate GFR in children. However, this GFR predicting equation requires height information, which is normally not available in clinical laboratory databases. Therefore, Pcr determination is usually not associated to an eGFR which leads to miss many diagnosis of mild or moderate CKD. Pottel et al. [14, 20] developed a height-independent equation based on the determination of median Pcr for each age class. The concept of this formula can be applied in every laboratory with a large data base (i.e., large laboratory pediatric hospital) leading to an equation adapted to the laboratory and/or to the local population characteristics (independent of ethnicity). As the original equation was developed in a European population with an IDMS-traceable Pcr determination (traceable to an isotope dilution mass spectrometry reference measurement procedure), the published coefficients could, from a theoretical point of view, be used in every European laboratory if standardized Pcr method is used. Pottel et al. claimed that this equation can be used as a screening tool for a possible underlying kidney problem in a general population of children [20]. Therefore, the aims of this study were to estimate 1) the feasibility of determining a local height-independent equation based on results of the laboratory hospital; 2) the performance in estimating GFR and in identifying patients with a CKD (i.e., GFR <75 or <60 mL/min/1.73 m2) of the locally adapted (Pottel–Lyon), the original height-independent equation (Pottel–Belgium), and the height-dependent (Pottel–L–Belgium) eGFR formulas compared to the internationally recommended Schwartz 2009 equation [24] and the locally adapted Schwartz–Lyon equation [9] (both height-dependent) in a population of children undergoing a reference GFR measurement in our department; 3) the practicability of using the height-independent GFR estimating equation in children either with the original (Pottel–Belgium) or the locally adapted (Pottel–Lyon) height-independent equation in laboratory routine.
Materials and methods
Clinical laboratory data set for determination of the adapted Pottel–Lyon equation
The databases were obtained from the laboratory of the pediatric hospital. All patient data were recorded in an anonymous manner such that subjects could not be identified directly or through identifiers linked to the subjects. The study was therefore exempted from institutional review board. All Pcr value determined in children aged between 1 and 18 years (either in outpatients or hospitalized ones) from December 2009 to June 2011 were collected. Only the first test result for each patient was used for further analysis. Also, data below the detection limit (12 μmol/L) and outliers (determined by Grubbs test for outliers) were not taken into account. This reduced the total amount of data from n = 80,668 to n = 12,219. The children were then further divided in subgroups according to sex and age (age in years is calculated as (day of test − day of birth)/365.25). Median Pcr values together with lower and upper limits may be calculated using nonparametric statistics (Pct 2.5 and Pct 97.5 are considered lower and upper limits). This allowed to determine the Q-Lyon value which is the median of healthy children at a specific age in our laboratory.
Population of patients with a measured GFR
To evaluate the performance of GFR estimating equations, we used a data set on 359 consecutive children (438 measurements) referred to our center between January 2010 and July 2013 to undergo a GFR measurement (inulin or iohexol clearance) as a routine procedure for renal evaluation because of suspected underlying kidney pathology. A written informed consent was obtained from all patients or their families prior to obtaining the measured GFR (mGFR). The consent form contained information referring to the procedure itself as well as the later use of the information in research. According to the French Law, concerning the use of a database without direct identification of patients, it was not necessary to obtain an ethical approval (law 2006–450, April 19th 2004; Commission nationale de l’informatique et des libertés-CNIL). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Weight, height, as well as standard deviation of size for age (SDS) according to French pediatric growth charts [25], body mass index (BMI), and BMI Z-score were recorded. Weight categories were defined according to World Health Organization (WHO) cutoff points for BMI (BMI Z-score < −2: underweight patients, BMI Z-score between −2 and +2: normal weight, and BMI Z-score > +2: obese patients). Size categories were defined by a height < −2 SDS (undersized), between −2 and +2 SDS (normal size for age), and > +2SDS oversized for age. All patients had concomitant determinations of Pcr in our hospital laboratory allowing a GFR estimation using the five formulas (Table 1).
Testing data set for determination of eGFR with the Pottel equation in the general population
We determined eGFR with the height-independent GFR predicting equation either with the previously determined Q-Lyon value (Pottel–Lyon) or with the original Pottel formula (Pottel–Belgium). All the first determinations of Pcr performed in children in the laboratory of our hospital between January 2012 and June 2013 were included.
GFR measurement and laboratory methods
GFR determination was measured either by renal inulin or plasmatic iohexol clearance depending on the indication and clinical condition of the patients. Plasmatic iohexol clearance, which does not require urinary samples, was preferred in children younger than 6 years of age or patients with urological problems. By contrast, inulin clearance was chosen when a history of iodine allergy, extracellular overload, or severe level of CKD was present. However, as most studies indicate close agreement between GFR measured by inulin clearance and clearance of iohexol, measured as standard renal clearance or plasma disappearance [23], we consider the two methods as equivalent ones. A standard technique of renal clearance of inulin (polyfructosan, Inutest®, Fresenius Kabi, Graz, Austria) was used as previously described [9, 11]. The measurement of plasma and urine polyfructosan concentrations were performed using an enzymatic method which demonstrated very good specificity and reproducibility (within-run precision <1 % and between-run precision <3.5 %) [11]. Iohexol clearance was performed according to a standard technique of single-bolus injection method. Briefly, an IV injection of about 6 mL/1.73 m2 (with a maximum dose of 6 mL) of iohexol (Omnipaque, 300 mg/mL) was administered, and blood samples were drawn from the contralateral arm after 120, 180, and 240 min. The GFR was calculated from the slope of plasma concentrations using a one-compartment model corrected using the Bröchner-Mortensen formula [3]. Plasma iohexol concentration was determined using HPLC according to the method published by Cavalier et al. [5] which showed high analytical performances. External quality control was provided by Equalis (Uppsala, Sweden) every 3 months. The results were expressed per 1.73 m2 body surface area (Dubois formula: BSA = height0.725 × weight0.425 × 0.007184) [10].
All Pcr determinations were obtained with an enzymatic method (Multigent Creatinine Enzymatic kit purchased from Abbott Diagnostics) on the Architect cSystemS. The creatinine calibrators (ClinChem Cal) are traceable to the National Institute of Standards and Technology, Creatinine Standard Reference Materials 914 (verified with NIST SRM 967). Indicated quality control data showed intra- and interassay precision (CV) of 0.6 and 2.6 %, respectively at a creatinine concentration of 78 and 58 μmol/L, respectively.
Statistical analysis
The agreement between mGFR and eGFR values was evaluated by estimation of the mean bias (mean eGFR − mGFR), the agreement limits, and the 10 % (P10) and 30 % (P30) accuracies as recommended by Earley et al. [12] and according to the Clinical Practice Guidelines of the Kidney Disease Outcomes Quality Initiative (KDOQI) [18]. A random intercept model was used to estimate the mean bias and the standard deviation (SD) of the bias. This allowed for repeated measurements in the same patients and estimates of intra-patient and inter-patient variances. The concordance correlation coefficient (CCC) was used to test agreement [8, 15]. The ability of the formulas to predict a mGFR < 60, <75 and <90 mL/min/1.73 m2 was assessed using area under the ROC curves (AUC).
To compare the mean biases, the CCCs, the P10, and the P30, respectively, a paired t test, the bootstrap 95 % confidence intervals of the differences between CCCs, and a McNemar’s test were used.
Based on the paper of Hoste et al. [14], a performance score for the eGFR formulas was calculated based on the following rules:
-
(i)
absolute bias <5: score +5; between 5 and 10: score +3; between 10 and 15: score +1,
-
(ii)
P30 > 80: score +5; between 70 and 80: score +3; between 60 and 70: score +1
-
(iii)
P10 > 40: score +5; between 30 and 40: score +3; between 20 and 30: score +1.
All the analyses were performed using R for Windows, version 3.0.2. A value of p < 0.05 was considered for statistical significance.
Results
Determination of median Pcr value in Lyon (Q-Lyon) according to age class and sex in the clinical laboratory data set population
Mean ± SD, median, and percentile 2.5 and 97.5 % of Pcr were determined for each sex and for all 1 year interval age classes (Table 2). Comparison of the median values for each age and sex classes between the Belgian results of Pcr [21, 22] and those of our laboratory by a linear regression slope showed a slight but significantly higher median result of Pcr in the Belgian population (slope 1.06, p < 0.0001). There is a 6 % systematic deviation between median Pcr values (Q values) between Lyon and Belgium with lower estimates for Lyon.
Performance of the GFR estimating equations in the population of children with a mGFR
The performance of the height-independent equations (Pottel–Lyon and Pottel–Belgium) and of the height-dependent equations (Schwartz 2009, Schwartz–Lyon, and Pottel–L–Belgium) and formulas has been evaluated in a population of 359 children (438 measurements) referred to our department for GFR determination. Characteristics of the children were indicated in Table 3. Schwartz–Lyon and Pottel–Lyon showed the best performance in the whole population and in patients with a GFR <90 mL/min/1.73 m2 giving significantly higher P30 and CCC and lower bias in comparison with other equations (Table 4). In patients with a GFR ≥ 90 mL/min/1.73 m2, the performance of the five predicting GFR equations is similar. To note, hyperfiltration (GFR ≥135 mL/min/1.73 m2) [4] that was observed in 39 patients (42 measurements, mean GFR = 153 mL/min/1.73 m2) decreased performance (bias, P10 and P30) of all studied equations (data not shown). A performance score for the five different eGFR formulas in the whole population and in each subgroup (defined by a GFR < 90 or ≥90 mL/min/1.73 m2 was calculated (Table 4). In the overall cohort, as well in the two mGFR subgroups, the Schwartz–Lyon and the Pottel–Lyon get the highest score.
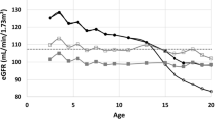
As shown by the areas under the ROC curves of GFR estimating equations (Table 5), all the studied equations have similar performance in identifying patients with renal dysfunction (mGFR < 60 or <75 mL/min/1.73 m2) in the present study. However, when mGFR < 90 mL/min per 1.73 m2, the AUC of Schwartz 2009 and Schwartz–Lyon formulas were significantly higher than those of the others formulas. Bland and Altman graphs show bias changes according to mGFR: the overestimation of mGFR increased when mGFR decreased with all equations (Fig. 1).
Bland and Altman plots showing, for each formula, the estimated GFR minus the measured GFR bias in function of the measured GFR as gold standard in the whole population of children with a mGFR determination. The solid line represents the mean ratio. The broken lines represent the 95 % limits of agreement (i.e., ±1.96 × the standard deviation)
Finally, the performance of eGFR equations was evaluated according to weight and size subgroups (Table 6). All the equations have better performance in the normal group of weight or size. However, Pottel–Lyon and Schwartz–Lyon have a better score in all the subgroups, followed by the Pottel–L–Belgium equation. The performance of Schwartz 2009 and Pottel–Belgium are rather similar.
Determination of eGFR in the testing data set of children
All the first Pcr measurements of 13,117 children (53.3 % of males) aged 8.7 ± 5.2 years (1–18) were included. Mean Pcr was 55 ± 28 μmol/L. Among them, 328 (2.5 %) and 949 (7.2 %) children have a Pcr value less the 2.5th and above the 97.5th percentile for age and sex, respectively. P97.5 corresponds to a mean eGFR of 76.4 ± 3.4 mL/min/1.73 m2 (70–83) with Pottel–Lyon and a Pcr/Q of 1.41 ± 0.06 (1.3–1.5). Three thousand one hundred and four (23.7 %) and 908 (6.9 %) of children have a Pottel–Lyon estimated eGFR <90 and <75 mL/min/1.73 m2, respectively.
Mean ± SD eGFR estimated with Pottel–Lyon was significantly lower than those estimated with Pottel–Belgium (106.3 ± 28.6 and 115.1 ± 30.9 mL/min/1.73 m2, respectively, p < 0.0001). The difference between the two estimating formulae was −8.4 ± 3.8 % and do not change with the Pcr/Q ratio. The use of Q-Belgium to estimate eGFR leads to a change of classification between decreased (eGFR < 90) to normal eGFR (eGFR ≥ 90) in 1315 children. However, mean Pottel–Lyon estimated eGFR of these 1315 patients was 86.3 ± 2.7 mL/min/1.73 m2 (78.6–89.9) that is close to the limit of 90.
Discussion
Pcr measurement is one of the most frequent biochemical parameters measured in patients even in children. However, eGFR is rarely determined by the laboratory due to the lack of height information, a necessary parameter in the Schwartz 2009 formula. To help clinicians, reference intervals of Pcr according to sex and age are provided such as those provided by Pottel et al. or Ceriotti et al. [6, 22]. However, identifying a mild or moderate CKD is challenging, and the use of a height-independent formula might be useful.
According to international recommendations, Pcr measurement in children should be performed by an enzymatic method with calibration traceable to IDMS reference methodology [17] in order that results could be compared in all laboratories of most countries. Therefore, provided that the studied population is similar to the reference group, the use of GFR estimating equations with identical coefficients could be used with a relative confidence. In the present study, the predicting GFR equations (i.e., Schwartz 2009 and Pottel–Belgium or Pottel–L–Belgium) established in other countries (USA and Belgium, respectively) with comparable Pcr method of measurements and in comparable populations (Western countries children), give similar performance results. The good performance of Schwartz 2009, Pottel–Belgium, and Pottel–L–Belgium in our study is in accordance with previous reports [1, 11, 14, 24, 26].
However, a 6 % systematic deviation between median Pcr values (Q values) between Lyon and Belgium was observed with a mean difference between Q values for Lyon and Belgium of −2.7 ± 1.5 μmol/L (−6 to 0), p < 0.0001, leading to a small but significant difference in the mean eGFR estimation in the testing population with either the Pottel–Lyon or the Pottel–Belgium. Despite standardization of Pcr measurement method, this systematic deviation might be partly explained by the use of various manufacturer techniques of Pcr measurement (Roche enzymatic assay in Belgium, Abbott enzymatic assay in Lyon). In addition, differences (i.e., median size and height) between children from different European countries cannot be ruled out as suggested by Bonthuis et al. who reported a difference in mean height between Belgium and France [2]. These small but significant differences might explain the better performance of Pottel–Lyon which uses the locally adapted coefficient for our own children population. However, these differences are not significant on a clinical point of view. The same points (i.e., difference of Pcr measurement method and/or anthropometric differences between children population) could explain the small but significant better performance of the locally adapted Schwartz–Lyon formula compared to Schwartz 2009. However, the performance of the adapted Pottel–Lyon and Schwartz–Lyon equations is similar.
The height-independent Pottel equation is built on the concept of a population-normalized Pcr and median Pcr (Q value) for age and sex can reliably be obtained from large hospital laboratory databases, as the majority of patients in these databases have normal kidney function [20]. In addition, with the systematic exclusion of patients with serial measurements and the use of specific statistical methods (a nonparametric method, the Bhattacharya procedure, and a nonlinear fit of the cumulative Gaussian distribution) which allowed to distinguish the normal from the abnormal data of Pcr, we can establish reliable and robust data of normal Pcr according to sex and age in a healthy population of children. However, in the testing data set as 7.2 % of children have a Pcr value above the 97.5 percentile for age and sex. This result could be explained by the fact that all first Pcr measurements of the pediatric hospital database were included, that is an increased proportion of children with an elevated Pcr compared to a normal healthy population.
In our population, 2155 (16.4 %) of children have a mild CKD according to the Pottel–Lyon equation (stage 2 of KDIGO classification) with a Pcr below the 97.5th percentile. In another way, 23.7 % of children of our testing set population have a Pottel–Lyon eGFR < 90 mL/min/1.73 m2 and 6.9 % an eGFR < 75 mL/min/1.73 m2. These results are in accordance with those of Fadrowski et al. [13] who found that 8.9 % of adolescents from the National Health and Nutrition Examination Survey (NHANES), in the USA, had an eGFR < 75 mL/min/1.73 m2 (the reporting cutoff used by pediatric eGFR calculators on the National Kidney Disease Education Program (NKDEP) web site) when the bedside Schwartz 2009 formulae is used. Chavers et al. found comparable results with the Chronic Kidney Disease in Children formula, which includes Pcr, cystatin C, and blood urea nitrogen concentration [7].
Finally, we can demonstrate that all the studied equations have a better performance in children with a normal weight and/or size for age and that the performance of most of the equations decreases in children with extreme size and/or BMI.
Therefore, the height-independent equation, with or without an adaptation to the local laboratory, could be used as screening tool in a general population and as a warning sign for a possible underlying kidney problem. Pediatric considerations of KDIGO guidelines [19] recommend to report an individual child’s eGFR value of less than 60 mL/min/1.73 m2 as “decreased.” However, according to KDIGO [16], pediatric practice would suggest a higher level of kidney function for referral than that for adults, although there is no specific value in children. As suggested in a recent paper [19], abnormal GFR in children and adolescents starts below 75 mL/min/1.73 m2; we can suggest that all children with an eGFR < 75 mL/min per 1.73 m2 could be indicated to the practitioner as potentially decreased GFR. This may trigger the attention of the general practitioner and may be the reason for sending this particular child to the pediatric nephrologist as for adult patients with the MDRD or CKD-EPI.
Several strengths of the present study can be pointed out: 1) the use of a reference standard method for GFR measurement (i.e., inulin or iohexol clearance) to evaluate performance of the various GFR estimating equations; 2) the use of an IDMS-standardized Pcr to validate the Pottel–Belgium, the Pottel–L–Belgium, and Schwartz 2009 equations; and 3) the demonstration of the practicability of determining an adapted height-independent equation to the local method of determination of Pcr and to the anthropometric characteristics of the French children population and its performance. However, the study presents also some limitations: 1) the lower age limit of 2.7 years of the children with a measured GFR limiting the validity of our results for children aged between 1 and 3 years; 2) the effect of ethnicity could not be tested because this information was not regularly available for all patients.
Conclusion
The performance of the height-independent equation is similar to those of the Schwartz 2009 or Pottel–L–Belgium equation in a Western country population of children when IDMS-standardized Pcr determination is used. Therefore, this equation could be easily used by every laboratory to give an eGFR in children and provide a screening tool to detect CKD in a general population of children.
This study demonstrates that the Q coefficient can be locally determined by large laboratories leading to a better performance of the locally adapted Pottel equation. A locally adapted equation might be very useful when enzymatic Pcr determination is not possible and/or in other population preventing the inappropriate use of formula established in Western countries patients.
Abbreviations
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- BSA:
-
Body surface area
- CCC:
-
Concordance correlation coefficient
- CKD:
-
Chronic kidney disease
- CKD-EPI:
-
Chronic kidney disease epidemiology collaboration equation
- eGFR:
-
Estimation of glomerular filtration rate
- GFR:
-
Glomerular filtration rate
- IDMS-traceable Pcr determination:
-
Traceable to an isotope dilution mass spectrometry reference measurement procedure
- KDIGO:
-
Kidney disease improving global outcomes
- LOA:
-
Limit of agreement
- MDRD:
-
Modification of diet in renal disease equation
- mGFR:
-
Measured GFR
- Pcr:
-
Plasma creatinine
- P10:
-
10 % accuracy
- P30:
-
30 % accuracy
- ROC:
-
Receiver-operator characteristic
- SDS:
-
Standard deviation score
- WHO:
-
World Health Organization
References
Blufpand HN, Westland R, van Wijk JA, Roelandse-Koop EA, Kaspers GJ, Bokenkamp A (2013) Height-independent estimation of glomerular filtration rate in children: an alternative to the Schwartz equation. J Pediatr 163:1722–1727
Bonthuis M, van Stralen KJ, Verrina E, Edefonti A, Molchanova EA, Hokken-Koelega AC, Schaefer F, Jager KJ (2012) Use of national and international growth charts for studying height in European children: development of up-to-date European height-for-age charts. PLoS One 7:e42506
Brochner-Mortensen J (1972) A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest 30:271–274
Cachat F, Combescure C, Cauderay M, Girardin E, Chehade H (2015) A systematic review of glomerular hyperfiltration assessment and definition in the medical literature. Clin J Am Soc Nephrol 10:382–389
Cavalier E, Rozet E, Dubois N, Charlier C, Hubert P, Chapelle JP, Krzesinski JM, Delanaye P (2008) Performance of iohexol determination in serum and urine by HPLC: validation, risk and uncertainty assessment. Clin Chim Acta Int J Clin Chem 396:80–85
Ceriotti F, Boyd JC, Klein G, Henny J, Queralto J, Kairisto V, Panteghini M, Intervals ICoR, Decision L (2008) Reference intervals for serum creatinine concentrations: assessment of available data for global application. Clin Chem 54:559–566
Chavers BM, Rheault MN, Foley RN (2011) Kidney function reference values in US adolescents: national health and nutrition examination survey 1999–2008. Clin J Am Soc Nephrol 6:1956–1962
Crawford SB, Kosinski AS, Lin HM, Williamson JM, Barnhart HX (2007) Computer programs for the concordance correlation coefficient. Comput Methods Prog Biomed 88:62–74
De Souza VC, Rabilloud M, Cochat P, Selistre L, Hadj-Aissa A, Kassai B, Ranchin B, Berg U, Herthelius M, Dubourg L (2012) Schwartz formula: is one k-coefficient adequate for all children? PLoS One 7:e53439
Dubois D, Dubois EF (1916) A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17:863–871
Dubourg L, Hadj-Aissa A, Ferrier B (2010) Adaptation of an enzymatic polyfructosan assay to clinical practice. Anal Biochem 405:266–268
Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K (2012) Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med 156:785–795
Fadrowski JJ, Neu AM, Schwartz GJ, Furth SL (2011) Pediatric GFR estimating equations applied to adolescents in the general population. Clin J Am Soc Nephrol 6:1427–1435
Hoste L, Dubourg L, Selistre L, De Souza VC, Ranchin B, Hadj-Aissa A, Cochat P, Martens F, Pottel H (2014) A new equation to estimate the glomerular filtration rate in children, adolescents and young adults. Nephrol Dial Transplant Off Publ Eur Dial Transplant Assoc Eur Renal Assoc 29:1082–1091
King TS, Chinchilli VM, Carrasco JL (2007) A repeated measures concordance correlation coefficient. Stat Med 26:3095–3113
Members-KB (2013) Chapter 5: referral to specialists and models of care. Kidney Int Suppl 3:112–119
Members KB (2013) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3:19–62
National Kidney F (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39:S1–S266
Pottel H, Hoste L, Delanaye P (2014) Abnormal glomerular filtration rate in children, adolescents and young adults starts below 75 mL/min/1.73 m. Pediatr Nephrol 30:821–828
Pottel H, Hoste L, Martens F (2012) A simple height-independent equation for estimating glomerular filtration rate in children. Pediatr Nephrol 27:973–979
Pottel H, Mottaghy FM, Zaman Z, Martens F (2010) On the relationship between glomerular filtration rate and serum creatinine in children. Pediatr Nephrol 25:927–934
Pottel H, Vrydags N, Mahieu B, Vandewynckele E, Croes K, Martens F (2008) Establishing age/sex related serum creatinine reference intervals from hospital laboratory data based on different statistical methods. Clin Chim Acta Int J Clin Chem 396:49–55
Schwartz GJ, Furth SL (2007) Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol 22:1839–1848
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Sempé M, Pédron G, Roy-Pernot M-P (1979) Auxologie: méthode et séquences. Théraplix, Paris
Staples A, LeBlond R, Watkins S, Wong C, Brandt J (2010) Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr Nephrol 25:2321–2326
Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M (2013) Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158:825–830
Conflict of interest
The authors declare that they have no conflict of interest.
Authors Contribution
All authors have contributed in various degrees to the collection of data, to the research design, to the analysis performance and to the manuscript writing: V. De Souza and L. Dubourg designed the study, collected the data, performed the analysis and wrote the manuscript; H. Pottel and L. Hoste developed the methodology and wrote the manuscript; O. Dolomanova, R. Cartier, L. Selistre, B. Ranchin, A. Hadj-Aïssa, S. Lemoine and P. Cochat designed the study, collected the data and discussed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jaan Toelen
Revisions received: 06 January 2015 / 27 February 2015
Rights and permissions
About this article
Cite this article
De Souza, V., Pottel, H., Hoste, L. et al. Can the height-independent Pottel eGFR equation be used as a screening tool for chronic kidney disease in children?. Eur J Pediatr 174, 1225–1235 (2015). https://doi.org/10.1007/s00431-015-2523-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-015-2523-7