Abstract
We aimed to document the pathological characteristics of breast cancer (BC) cases with different scores of HER2 by immunohistochemistry (IHC), as well as to establish a relationship between HER2 expression and HER2 amplification by in situ hybridization (ISH). A cohort of 258 primary BC cases was evaluated for HER2 gene amplification with bright-field ISH. All HER2-negative and HER2-positive cases by IHC were concordant with the ISH classification. BC cases with score of 0 had lower average of HER2 copy number compared to cases with score of 1 + . HER2-equivocal cases by IHC had intermediate pathological characteristics between HER2-negative and HER2-positive cases. About 12% of HER2-equivocal cases were classified as ISH-positive. HER2-equivocal cases with HER2 gene amplification had proliferation index, HER2/CEP17 ratio, and average of HER2 copy number between HER2-equivocal cases without HER2 gene amplification and HER2-positive cases by IHC. Additionally, HER2-equivocal cases with HER2 amplification had score of 2 + in at least 50% of the total tumor area, with a proportion of ISH-positive cases increasing with the amount of score of 2 + present in the tumor. The quantification of score of 2 + in the tumor predicted the ISH classification with an AUC of 0.902. A logistic regression model using the same HER2 quantification and the nuclear score was able to increase the abovementioned prediction to an AUC of 0.929. As such, we were able to link HER2 quantification by IHC and morphological analysis with HER2 amplification by ISH.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Human epidermal growth factor receptor 2 (HER2) status, along with estrogen receptor (ER) and progesterone receptor (PgR), must be routinely determined in all patients with invasive breast cancer (BC) to predict response to target therapy [1, 2]. The evaluation of HER2 by immunohistochemistry (IHC) gives rise to four categories: negative (score of 0), negative (score of 1 +), equivocal (score of 2 +), and positive (score of 3 +). After the performance of reflex in situ hybridization (ISH) test in equivocal cases, the positive cases by IHC (score of 3 +) and the equivocal cases with HER2 amplification by ISH (score of 2 + with HER2 amplification) are grouped together clinically into a general category of HER2-positive BC [1]. It has been shown that HER2-targeted therapy improves progression-free survival and overall survival only in patients with HER2-positive BC, which represents about 15% of all BC cases [3,4,5,6,7,8,9].
The practical clinical dichotomization of HER2 classification has the purpose to identify patients who will likely benefit from HER2 target therapy. However, it has been shown that HER2-negative, HER2-equivocal, and HER2-positive BC cases by IHC represent a spectrum of HER2 expression with different clinical and pathological characteristics [10, 11].
Recently, HER2-negative cases with score of 1 + and equivocal cases without HER2 amplification have been proposed to group together into a new category of HER2-low BC [12, 13]. This classification originates from the demonstration of response to target therapy in HER2-low BC using antibody–drug conjugates (ADCs) [14, 15].
In this study, we aim to document the pathological characteristics of the different HER2 categories by IHC in a cohort of BC cases. Additionally, we also establish a relationship between the quantification of HER2 expression by IHC with the quantification of HER2 gene amplification by ISH.
Materials and methods
Case selection
A cohort of primary BC cases was retrieved from the archives of Ipatimup Diagnostics from January 2014 to December 2020. From a total of 554 cases, 138 BC cases (24.9%) had equivocal HER2 result by IHC (score of 2 +) with available ISH result. Additionally, BC cases were consecutively collected from January 2014 until 40 cases were reached for each of the remaining IHC categories (score of 0, 1 + , and 3 + , with a total of 120 cases). The cases included formalin-fixed paraffin-embedded needle core biopsies (NCB) and surgical excision specimens (SES) and all had evaluation of HER2 amplification with bright-field ISH.
Immunohistochemistry
HER2 IHC was performed in 3-μm-thick sections in one representative block of each case with rabbit monoclonal primary antibody (PATHWAY anti-HER2/neu (4B5); Ventana Medical Systems, Inc., Tucson, AZ, USA) and Optiview DAB IHC Detection Kit (Ventana Medical Systems, Inc., Tucson, AZ, USA). The entire procedure was carried out on an automated staining system (Ventana BenchMark XT Staining System; Ventana Medical Systems, Inc., Tucson, AZ, USA) according to the manufacturer’s instructions. Appropriate positive and negative controls were used in every set of slides.
Bright-field in situ hybridization
ISH was performed on 4-μm-thick sections in the representative block used for IHC of each case with dual-hapten, dual-color ISH. The INFORM HER2 Dual ISH DNA Probe Cocktail Assay (Ventana Medical Systems, Inc., Tucson, Arizona) was used in equivocal cases (102) from January 2014 to April 2019 and the VENTANA HER2 Dual ISH DNA Probe Cocktail Assay (Ventana Medical Systems, Inc., Tucson, AZ, USA) was used in equivocal cases (36) from May 2019 to December 2020, as well as in all the HER2-negative (score of 0 and 1 +) and HER2-positive cases (score of 3 +). Both assays are Food and Drug Administration-approved, containing a HER2 locus-specific probe (black signal) and a control probe specific for the centromere of chromosome 17 (centromere enumeration probe-CEP17, red signal) that allows detection of HER2 amplification by light microscopy. The entire procedure was carried out on an automated staining system (Ventana BenchMark XT Staining System; Ventana Medical Systems, Inc., Tucson, AZ, USA) according to the manufacturer’s instructions. Appropriate positive and negative controls were used in every set of slides. Optimal staining consists of an absence of non-specific background staining, distinct nuclear morphology, and clear and specific signals within the nucleus.
IHC and ISH interpretation
The samples were evaluated by a pathologist (AP) according to the 2018 ASCO/CAP guideline for HER2 in BC [1]. Additionally, in HER2-equivocal cases, the proportion of score of 2 + was quantified by counting the number of fields (power field of 200 ×) with score of 2 + divided by the number of fields of invasive carcinoma. Corresponding hematoxylin and eosin staining was used for the identification of the invasive component of the tumor, and the IHC slide was used to guide the ISH evaluation in the area with strongest intensity. Only cells with a minimum of one copy of HER2 and CEP17 each were scored. The number of HER2 signals was estimated in clusters, except for doublets, which counted as a single signal. The evaluation of the samples included scoring of at least 20 nuclei in two different areas, with an additional 20 cells if HER2/CEP17 ratio falls between 1.8 and 2.2.
The 2018 BC guideline defines HER2 gene amplification as positive (classical group 1) when the HER2/CEP17 ratio is ≥ 2.0 and the average HER2 copy number is ≥ 4.0 signals per cell, and negative (classical group 5) when the HER2/CEP17 ratio is < 2.0 and the average HER2 copy number is < 4.0 signals per cell. Moreover, group 2 is defined as HER2/CEP17 ratio ≥ 2.0 and average HER2 copy number < 4.0 signals per cell; group 3 as HER2/CEP17 ratio < 2.0 and average HER2 copy number ≥ 6.0 signals per cell; and group 4 as HER2/CEP17 ratio < 2.0 and average HER2 copy number ≥ 4.0 and < 6.0 signals per cell. The final classification in groups 2 to 4 (non-classical groups) depends on the result of IHC analysis and is considered positive if a score 3 + in these groups or a score 2 + in group 3, and negative if otherwise [1].
HER2 genetic heterogeneity (HER2-GH) was documented, defined in the 2018 ASCO/CAP guideline as a discrete aggregated population of tumor cells with HER2 amplification. A case is considered positive if HER2 gene amplification represents at least 10% of the total tumor cell population [1].
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 27.0 for Windows. The Pearson’s chi-square (χ2) test (or the Fisher’s exact test, if appropriate) was used for comparison of qualitative variables, and the Mann–Whitney U-test (MWUT) was used for comparison of quantitative variables. The level of significance was set at p < 0.05.
A logistic regression model was created to predict the classification of the ISH test in cases with equivocal result (score of 2 +). The variation explained by the model was measured by the Nagelkerke R2 and the goodness of fit by the Hosmer–Lemeshow test. The model was evaluated using sensitivity, specificity, negative predictive value, positive predictive value, and area under the receiver operating characteristic (ROC) curve (AUC).
Results
The cohort included 213 needle core biopsies and 45 surgical specimens, diagnosed in 254 women and 4 men. The age of the patients ranged from 33 to 95 years old, with a median age at diagnosis of 58.5 years old. Invasive carcinoma of no special type (NST) was the most frequent histologic type (89.9%), whereas lobular carcinoma (6.6%) and micropapillary carcinoma (1.5%) were the most frequent special types. Most tumors were classified as grade 2 (55.8%), followed by grade 3 (30.2%) and grade 1 (14%). Vascular invasion was only observed in a minority of cases (5%). Estrogen receptor (ER) was positive in 232 cases (89.9%) and progesterone receptor (PR) in 191 cases (74%). Cohort characteristics are summarized in Table 1.
All HER2-negative cases by IHC (score of 0 and 1 +) were classified as group 5 by ISH (Table 2). In HER2-equivocal cases by IHC (score of 2 +), 17 cases (12.3%) were classified as group 1, 2 cases (1.45%) as group 2, 2 cases (1.45%) as group 4, and 117 cases (84.8%) as group 5 by ISH (Tables 2 and S1). Additionally, of the 17 cases classified as group 1, the average of HER2 copy number varied between 4.18 and 8.23, with 8 cases averaging between 4.0 and 5.0 (47.1%).
All, but one, HER2-positive cases by IHC (score of 3 +) were classified as group 1 by ISH (Table 2). The exception was one case classified as group 4 by ISH with HER2/CEP17 ratio of 1.91 and average of HER2 copy number of 5.02, which was also the lowest HER2 copy number observed (non-outlier) in cases with score of 3 + (Table S1). HER2-GH was only observed in 3 HER2-equivocal cases (2.2%), with amplification ranging from 10 to 90% of the total tumor cell population represented in the sample (Table S2).
In HER2-negative cases by IHC, score of 0 and 1 + cases had similar pathological characteristics, with the only significant difference being a lower average of HER2 copy number (median of 1.64 and 1.88, respectively; p < 0.001) in cases with score of 0 (Table 3).
Comparing to HER2-negative cases (score of 0 and 1 +), HER2-equivocal cases had higher median age at diagnosis (53 and 65, respectively; p = 0.001), higher histologic grade (13.8% grade 3 and 30.4% grade 3, respectively; p = 0.017), higher nuclear score (26.3% score 3 and 55.8% score 3, respectively; p < 0.001), higher mitotic score (2.5% score 3 and 9.4% score 3, respectively; p = 0.002), and higher Ki67 quantification (median of 30% and 40%, respectively; p = 0.006), as well as higher average of HER2 copy number (median of 1.77 and 2.08, respectively; p < 0.001) and higher HER2/CEP17 ratio (median of 1.01 and 1.18, respectively; p < 0.001) (Table S3).
In HER2-equivocal cases, we observed that 86 cases (62.3%) had score of 2 + in less than 50% of the total tumor area and that all ISH-positive cases had score of 2 + in at least 50% of the total tumor area (17/52; 32.7%). In HER2-equivocal cases without HER2 amplification by ISH, we did not observe any difference between cases with score of 2 + in less than 50% of the tumor and cases with score of 2 + in at least 50% of the tumor. However, comparing to cases with score of 1 + , cases with score of 2 + in less than 50% of the tumor had higher median age at diagnosis (54.5 and 65, respectively; p = 0.014), higher nuclear score (30% score 3 and 54.7% score 3, respectively; p = 0.010), and higher HER2/CEP17 ratio (median of 1.06 and 1.13, respectively; p = 0.025) (Table 3). Additionally, we observed that the proportion of ISH-positive cases increased with the amount of score of 2 + present in the tumor, rising from 17.7% in cases with score of 2 + in 50% of the tumor to 50% in cases with score of 2 + in at least 90% of the tumor (Fig. 1A and Table S4). Noteworthily, the quantification of score of 2 + in the tumor could predict the classification of ISH test with an AUC of 0.902 (0.849–0.956 95% CI) (Fig. 1B). Establishing a cut-off of at least 50% of score of 2 + in the tumor, we observed a sensitivity of 100%, specificity of 71.1%, a negative predictive value of 100%, and a positive predictive value of 32.7% (Fig. 2).
A Relationship between the quantification of score of 2 + by IHC in the total tumor area and the proportion of ISH-positive cases; B ROC curve using the amount of score of 2 + by IHC to predict the classification of the ISH test; C ROC curve of a logistic regression model to predict the classification of the ISH test using the amount of score of 2 + by IHC and the nuclear score
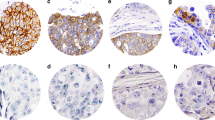
A–C HE, IHC, and ISH, respectively, from a case with score of 2 + in 30% of the tumor without HER2 amplification; D–F HE, IHC, and ISH, respectively, from a case with score of 2 + in 70% of the tumor without HER2 amplification; G–I HE, IHC, and ISH, respectively, from a case with score of 2 + in 60% of the tumor with HER2 amplification
Subsequently, we designed a logistic regression model to predict the classification of ISH test using the quantification of score of 2 + in the tumor and the nuclear score, which was the only pathological characteristic significantly different between ISH positive and ISH negative in cases with score of 2 + in at least 50% of the tumor (88.2% score 3 and 42.9% score 3, respectively; p = 0.002), excluding the obvious HER2/CEP17 ratio and the average of HER2 copy number (Table 3). The interaction of the quantification of score of 2 + in the tumor with the nuclear score in the regression analysis was not statistically significant (p = 0.220) and hence was not included in the final model (Table 4). The presence of high nuclear score (score of 3) increased 22.87 times the probability of being ISH positive, whereas the increase of 10% in the proportion of score of 2 + in the tumor increased 2.39 times the probability of being ISH positive. The output of the model could predict the ISH classification with an AUC of 0.929 (0.865–0.992 95% CI) (Fig. 1C). Establishing a cut-off of 0.10, we observed a sensitivity of 94.1%, specificity of 82.6%, a negative predictive value of 99.0%, and a positive predictive value of 43.2%. The model was able to select 101 cases (73.2%) with low probability of being ISH positive with only 1 false-negative result (Table S5). The model showed that if a case has a nuclear score less than 3, the proportion of score of 2 + in the tumor must be higher than 80% so that the probability of being ISH positive is higher than the above defined cut-off.
Comparing to HER2-equivocal cases, HER2-positive (score of 3 +) cases had lower median age at diagnosis (65 and 53, respectively; p < 0.001), higher histologic grade (30.4% grade 3 and 62.5% grade 3, respectively; p = 0.001), higher tubular score (73.2% score 3 and 92.5% score 3, respectively; p = 0.031), higher mitotic score (9.4% score 3 and 15% score 3, respectively; p = 0.030), higher Ki67 quantification (median of 40% and 70%, respectively; p < 0.001), lower ER positivity (91.3% and 77.5%, respectively; p = 0.025), and lower PR positivity (75.4% and 50%, respectively; p = 0.002), as well as higher average of HER2 copy number (median of 2.08 and 9.57, respectively; p < 0.001) and higher HER2/CEP17 ratio (median of 1.18 and 5.82, respectively; p < 0.001) (Table S3). However, comparing to HER2-equivocal/ISH-positive cases, HER2-positive (score of 3 +) cases had only higher Ki67 quantification (median of 50% and 70%, respectively; p = 0.011), as well as higher average of HER2 copy number (median of 5.02 and 9.57, respectively; p < 0.001) and higher HER2/CEP17 ratio (median of 3.08 and 5.82, respectively; p < 0.001) (Table 3).
Discussion
HER2 assessment usually begins with the evaluation of protein expression by IHC, with equivocal results requiring ISH reflex test, for the quantification of HER2 gene amplification [1]. In this study, all HER2-negative cases by IHC (score of 0 and 1 +) were classified as group 5 by ISH, meaning that all have average of HER2 copy number lower than 4.0. However, although almost all HER2-positive cases by IHC (score of 3 +) were classified as group 1 by ISH, all cases had average of HER2 copy number higher than 5.0. Previously, it has been shown that HER2 overexpression in BC is strongly associated with an average of HER2 copy number higher than 6.0 [16, 17]. Our results also document a clear difference of average of HER2 copy number between negative and positive cases by IHC.
BC cases with score of 0 and 1 + had similar pathological characteristics, except for a lower average of HER2 copy number in cases with score of 0. To our knowledge, this feature has never been reported, and the loss of the HER2 gene in a proportion of tumor cells could explain the absence of HER2 expression in these cases. Moreover, the expression of HER2 with score of 1 + appears to not be enough to trigger aggressive pathological features, such as higher histologic grade or proliferation index, suggesting that these cases might not be HER2-addicted. Additionally, it has been shown in in vitro modeling that increasing expression of HER2 in cell lines is associated with an increasing delivery of HER2-targeted doxorubicin to the nucleus, with a threshold effect seen at about 200,000 HER2 receptors/cell [18]. BC cases with more than this amount of HER2 receptors per cell usually express scores of HER2 by IHC of at least 2 + [19]. As such, it is unlikely that BC cases with HER2 scores of just 1 + will benefit from antibody–drug conjugates (ADCs).
The current guidelines expect that the non-classical ISH groups should comprise up to 5% of the reflex tests, a proportion observed in this study and previously confirmed by our group [1, 20, 21]. Additionally, HER2-GH was documented in about 2% of the cases and, in this work, only observed in HER2-equivocal cases, similar to what has been reported [20,21,22,23]. Of note, the cohort used in this study has an obvious bias given the selection of an equivalent number of cases with scores of 0, 1 + , and 3 + , which does not represent real clinical practice.
Interestingly, 4 out of 5 non-classical ISH groups had HER2 expression with score of 2 + . All these cases had either a HER2/CEP17 ratio near the threshold of 2.0 or an average of HER2 copy number near the threshold of 4.0. However, the case with HER2 expression with score of 3 + had an average of HER2 copy number higher than 5.0. Recently, we showed that the average margin of error of HER2/CEP17 ratio and of HER2 copy number is not below 0.20, unless more than 100 invasive cells are evaluated [21]. Given that in this work no case had such conditions, the margin of error implies that our non-classical cases with score of 2 + had quantifications crossing the decision thresholds and could have been classified as ISH group 5, as well as the case with score of 3 + that could have been classified as ISH group 1. As a rule of thumb, cases with HER2/CEP17 ratio between 1.8 and 2.2, even with 100 invasive cells evaluated, are very likely to have a result with margins of error crossing the decision thresholds. Hopefully, image analysis tools will be able to evaluate thousands of cells and reduce the margins of error to insignificant values.
In HER2-equivocal cases by IHC, about 12% were classified by ISH positive, which is in line with current literature [20, 21, 24, 25]. HER2-equivocal cases had higher histologic grade and higher proliferation index compared to HER2-negative cases. Similarly, HER2-positive cases by IHC had higher histologic grade and proliferation index compared to HER2-equivocal cases. Remarkably, HER2-equivocal cases with HER2 gene amplification have proliferation index, HER2/CEP17 ratio, and average of HER2 copy number between HER2-equivocal cases without HER2 gene amplification and HER2-positive cases by IHC. Moreover, about 50% of these cases had an average of HER2 copy number between 4.0 and 5.0, which can be seen as low-amplification status as well as low expression of HER2 (score of 2 +). This intermediate profile supports the notion that these cases can be regarded as true HER2-low, which has been demonstrated by several studies as cases with lower response to targeted therapy [11, 26,27,28,29].
Curiously, HER2-equivocal cases with score of 2 + in less than 50% of the tumor had similar characteristics compared to HER2-equivocal cases with score of 2 + in more than 50% of the tumor and without HER2 amplification, although it is still unclear the different response rate to ADCs of these cases. Given the semi-quantitative nature of IHC, the current HER2 assay by IHC may not be the optimal test to precisely measure the amount of HER2 required to obtain an acceptable clinical response to ADCs, probably requiring more accurate quantitative methods for that purpose.
Finally, in this study, we observed that all HER2-equivocal cases with HER2 amplification had score of 2 + in at least 50% of the total tumor area and that the proportion of ISH-positive cases increased with the amount of score of 2 + present in the tumor. In fact, the quantification of score of 2 + in the tumor could predict the classification of the ISH test with a high AUC (above 0.9) and a cut-off of at least 50% of score of 2 + in the tumor would achieve a sensitivity of 100% (as well as a negative predictive value of 100%), implying that cases below the cut-off could be excluded from reflex ISH analysis without any loss of identification of HER2-positive cases. Given that in our study these cases represented about 60% of all HER2-equivocal cases, this exclusion could result in a very significant saving for health services. Afterward, we constructed a logistic regression model to increase the prediction of the classification of the ISH test using the same feature and the nuclear score. The model could predict the ISH classification with a slightly higher AUC than the quantification of score of 2 + in the tumor alone, selecting about 70% of all HER2-equivocal cases as having low probability of being ISH positive. In 101 selected cases, only one case had HER2 amplification, achieving a negative predictive value of 99.0%. The use of these features to predict the result of the ISH test in HER2-equivocal cases is dependent, among other factors, on IHC variability, and interpretation of both IHC and nuclear grade. Although image analysis systems could assist in the evaluation of both IHC and nuclear grade, they do not interfere in the pre-analytical and analytical phases of ancillary tests, which remains a pillar of laboratory results. Concluding, we show the pathological characteristics of the spectrum of HER2 expression in BC, linking IHC quantification and morphological analysis to predict the result of HER2 amplification by ISH.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch Pathol Lab Med 142(11):1364–1382
Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR, Chavez-MacGregor M, Perlmutter J, Perou CM, Regan MM, Rimm DL, Symmans WF, Torlakovic EE, Varella L, Viale G, Weisberg TF, McShane LM, Wolff AC (2020) Estrogen and progesterone receptor testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists guideline update. Arch Pathol Lab Med 144(5):545–563
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182
Press MF, Pike MC, Chazin VR, Hung G, Udove JA, Markowicz M, Danyluk J, Godolphin W, Sliwkowski M, Akita R et al (1993) Her-2/neu expression in node-negative breast cancer: direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease. Cancer Res 53(20):4960–4970
Press MF, Bernstein L, Thomas PA, Meisner LF, Zhou JY, Ma Y, Hung G, Robinson RA, Harris C, El-Naggar A, Slamon DJ, Phillips RN, Ross JS, Wolman SR, Flom KJ (1997) HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol 15(8):2894–2904
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792
Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ (1999) Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17(9):2639–2648
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353(16):1659–1672
Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20(3):719–726
Tsai YF, Tseng LM, Lien PJ, Hsu CY, Lin YS, King KL, Wang YL, Chao TC, Liu CY, Chiu JH, Yang MH (2019) HER2 immunohistochemical scores provide prognostic information for patients with HER2-type invasive breast cancer. Histopathology 74(4):578–586
Zhao J, Krishnamurti U, Zhang C, Meisel J, Wei Z, Suo A, Aneja R, Li Z, Li X (2020) HER2 immunohistochemistry staining positivity is strongly predictive of tumor response to neoadjuvant chemotherapy in HER2 positive breast cancer. Pathol Res Pract 216(11):153155
Marchio C, Annaratone L, Marques A, Casorzo L, Berrino E, Sapino A (2021) Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2-low carcinomas and beyond. Semin Cancer Biol 72:123–135
Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, Marra A, Viale G, Trapani D, Cardoso F, Penault-Llorca F, Viale G, Andre F, Curigliano G (2020) HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol 38(17):1951–1962
Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V, Macpherson IR, Boni V, Rolfo C, de Vries EGE, Rottey S, Geenen J, Eskens F, Gil-Martin M, Mommers EC, Koper NP, Aftimos P (2019) Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol 20(8):1124–1135
Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, Moreno-Aspitia A, Doi T, Sagara Y, Redfern C, Krop IE, Lee C, Fujisaki Y, Sugihara M, Zhang L, Shahidi J, Takahashi S (2020) Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-Low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol 38(17):1887–1896
Press MF, Villalobos I, Santiago A, Guzman R, Cervantes M, Gasparyan A, Campeau A, Ma Y, Tsao-Wei DD, Groshen S (2016) Assessing the new American Society of Clinical Oncology/College of American Pathologists guidelines for HER2 testing by fluorescence in situ hybridization: experience of an academic consultation practice. Arch Pathol Lab Med 140(11):1250–1258
Press MF, Sauter G, Buyse M, Fourmanoir H, Quinaux E, Tsao-Wei DD, Eiermann W, Robert N, Pienkowski T, Crown J, Martin M, Valero V, Mackey JR, Bee V, Ma Y, Villalobos I, Campeau A, Mirlacher M, Lindsay MA, Slamon DJ (2016) HER2 gene amplification testing by fluorescent in situ hybridization (FISH): comparison of the ASCO-College of American Pathologists guidelines with FISH scores used for enrollment in Breast Cancer International Research Group clinical trials. J Clin Oncol 34(29):3518–3528
Hendriks BS, Klinz SG, Reynolds JG, Espelin CW, Gaddy DF, Wickham TJ (2013) Impact of tumor HER2/ERBB2 expression level on HER2-targeted liposomal doxorubicin-mediated drug delivery: multiple low-affinity interactions lead to a threshold effect. Mol Cancer Ther 12(9):1816–1828
Onsum MD, Geretti E, Paragas V, Kudla AJ, Moulis SP, Luus L, Wickham TJ, McDonagh CF, MacBeath G, Hendriks BS (2013) Single-cell quantitative HER2 measurement identifies heterogeneity and distinct subgroups within traditionally defined HER2-positive patients. Am J Pathol 183(5):1446–1460
Curado M, Caramelo AS, Eloy C, Polonia A (2019) What to expect from the 2018 ASCO/CAP HER2 guideline in the reflex in situ hybridization test of immunohistochemically equivocal 2+ cases? Virchows Arch 475(3):303–311
Polonia A, Caramelo A (2021) HER2 in situ hybridization test in breast cancer: quantifying margins of error and genetic heterogeneity. Mod Pathol 34(8):1478–1486
Polonia A, Leitao D, Schmitt F (2016) Application of the 2013 ASCO/CAP guideline and the SISH technique for HER2 testing of breast cancer selects more patients for anti-HER2 treatment. Virchows Arch 468(4):417–423
Polonia A, Oliveira G, Schmitt F (2017) Characterization of HER2 gene amplification heterogeneity in invasive and in situ breast cancer using bright-field in situ hybridization. Virchows Arch 471(5):589–598
Murray C, D’Arcy C, Gullo G, Flanagan L, Quinn CM, Quinn CM (2018) Human epidermal growth factor receptor 2 testing by fluorescent in situ hybridization: positive or negative? ASCO/College of American Pathologists Guidelines 2007, 2013, and 2018. J Clin Oncol 36(35):3522–3523
Xu B, Shen J, Guo W, Zhao W, Zhuang Y, Wang L (2019) Impact of the 2018 ASCO/CAP HER2 guidelines update for HER2 testing by FISH in breast cancer. Pathol Res Pract 215(2):251–255
Krystel-Whittemore M, Xu J, Brogi E, Ventura K, Patil S, Ross DS, Dang C, Robson M, Norton L, Morrow M, Wen HY (2019) Pathologic complete response rate according to HER2 detection methods in HER2-positive breast cancer treated with neoadjuvant systemic therapy. Breast Cancer Res Treat 177(1):61–66
Wu Z, Xu S, Zhou L, Yin W, Lin Y, Du Y, Wang Y, Jiang Y, Yin K, Zhang J, Lu J (2018) Clinical significance of quantitative HER2 gene amplification as related to its predictive value in breast cancer patients in neoadjuvant setting. Onco Targets Ther 11:801–808
Hurvitz SA, Caswell-Jin JL, McNamara KL, Zoeller JJ, Bean GR, Dichmann R, Perez A, Patel R, Zehngebot L, Allen H, Bosserman L, DiCarlo B, Kennedy A, Giuliano A, Calfa C, Molthrop D, Mani A, Chen HW, Dering J, Adams B, Kotler E, Press MF, Brugge JS, Curtis C, Slamon DJ (2020) Pathologic and molecular responses to neoadjuvant trastuzumab and/or lapatinib from a phase II randomized trial in HER2-positive breast cancer (TRIO-US B07). Nat Commun 11(1):5824
Katayama A, Miligy IM, Shiino S, Toss MS, Eldib K, Kurozumi S, Quinn CM, Badr N, Murray C, Provenzano E, Callagy G, Martyn C, Millican-Slater R, Purdie C, Purnell D, Pinder SE, Oyama T, Shaaban AM, Ellis I, Lee AHS, Rakha EA (2021) Predictors of pathological complete response to neoadjuvant treatment and changes to post-neoadjuvant HER2 status in HER2-positive invasive breast cancer. Mod Pathol 34(7):1271–1281
Author information
Authors and Affiliations
Contributions
AP and AC: designed the research study. AC and CC: responsible for the execution of the IHC and ISH technique. AP: retrieved and analyzed the data, wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Polónia, A., Canelas, C. & Caramelo, A. The spectrum of HER2 expression in breast cancer: linking immunohistochemistry quantification with in situ hybridization assay. Virchows Arch 480, 1171–1179 (2022). https://doi.org/10.1007/s00428-022-03290-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-022-03290-y