Abstract
The aims of this study were to evaluate and compare the HER2 gene amplification status in invasive and adjacent in situ breast carcinoma, using bright-field in situ hybridization, and to document the possible presence of HER2 genetic heterogeneity (HER2-GH) in both components. A cohort of 100 primary invasive carcinomas (IC) associated with carcinoma in situ (CIS) were evaluated for HER2 gene amplification by SISH according to the 2013 ASCO/CAP HER2 guideline. A second cohort of all the cases with HER2-GH since the introduction of the updated ASCO/CAP HER2 guideline was also characterized, and an evaluation of the HER2 gene amplification in the CIS component, if present, was also done. In the first cohort, the HER2 amplification in the IC was negative in 87% of the cases and positive in 13% of the cases, without the presence of HER2-GH. All the cases had an associated CIS with the same HER2 status as IC, with four cases of CIS presenting HER2-GH. In the CIS, we observed a significant relationship of HER2 gene amplification with high nuclear grade. In the four cases with HER2-GH in CIS, two cases presented HER2 gene amplification in the IC. The second cohort included 12 cases with HER2-GH in a total of 1243 IC cases (0.97%). Additionally, we identified two cases associated with non-amplified CIS. HER2-GH is a rare event in IC and can already be present in CIS, not being an important step in the acquisition of invasive features.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human epidermal growth factor receptor 2 (HER2) is amplified and/or overexpressed in about 15 to 20% of invasive breast cancer (BC), being associated with worse clinical outcome and predictive of benefit from HER2-targeted therapy [1,2,3]. The incidence rate of carcinoma in situ (CIS) of the breast, the immediate precursor of invasive carcinoma (IC), has stabilized since the beginning of the millennium in women older than 50 years, but continues to increase about 2% every year in younger women [4]. Several studies have shown that HER2 amplification can already be present in CIS and that frequently HER2 status is concordant with the invasive component [5, 6].
Heterogeneity has been noticed in almost all types of cancer, including BC, being related to several aspects of disease progression and clinical outcome [7]. The first recommendation regarding HER2 genetic heterogeneity (HER2-GH) was published in 2009 as an extension of the 2007 American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) HER2 guidelines after the acknowledgment that some tumors displayed intratumoral heterogeneity and such cases could originate discrepant results between immunohistochemistry (IHC) and in situ hybridization (ISH) analysis [8]. At that time, HER2-GH was defined as HER2 gene amplification in 5 to 50% of invasive cancer cells. Importantly, the definition was based on studies that did not include clinical outcome, being the first step to investigate the clinical significance of HER2-GH and the possible role of target therapy in this setting [9, 10]. Thereafter, numerous studies have shown that HER2-GH could be present in BC from 5 to 40% of the cases [11, 12]. Additionally, it was shown that HER2-GH was more frequent in cases near the threshold of positivity and that heterogeneity measured in individual cells is not informative of clonal heterogeneity within a tumor population [13].
Currently, the definition of HER2-GH has changed from individual cells to discrete population of tumor cells with HER2 gene amplification. According to the 2013 ASCO/CAP HER2 guideline, a tumor is considered HER2 positive if HER2 gene amplification is present in at least 10% of the total tumor cell population [14].
The aims of the present study are to compare the HER2 gene amplification status in invasive and adjacent in situ BC, using bright-field ISH, and to document the possible presence of heterogeneity in both components.
Materials and methods
Case selection
The cases included formalin-fixed, paraffin-embedded needle core biopsies (NCB) and surgical excision specimens (SES) referred to Ipatimup Diagnostics with an equivocal HER2 result (score of 2+) in IC by IHC for performance of an evaluation of HER2 amplification with bright-field ISH. There was no information regarding patient treatment.
The first cohort included 100 primary invasive BC cases associated with CIS retrieved from the archives from November 2015 to July 2016 to determine the concordance of HER2 gene amplification in both components. During this period, 347 cases with an equivocal HER2 result by IHC were evaluated for HER2 gene amplification. The cohort comprised 66 NCB and 34 SES, all diagnosed in women. The age of the patients ranged from 31 to 83 years old, with a median age at diagnosis of 54 years. The majority of the histological types were invasive carcinomas of no special type (NST), with 14% of the cases being classified as grade 1, 66% as grade 2, and 20% as grade 3 (Table 1).
The second cohort included all cases with HER2-GH (primary invasive or metastatic BC) since the introduction of the 2013 ASCO/CAP HER2 guideline (November 2013) to October 2016. An evaluation of HER2 gene amplification in the CIS component, if present, was also done. The cohort comprised 10 NCB and 2 SES with HER2-GH in a total of 1243 cases (0.97%), 11 of which were primary invasive BC and one lymph node metastasis. The age of the patients ranged from 42 to 74 years old, with a median age at diagnosis of 58 years, and two cases were diagnosed in men. All histological types but one were IC, NST, with eight cases being classified as grade 2 and four cases as grade 3 (Table 2).
All cases were reviewed for histological type and grade (Nottingham Histologic Score) in the IC. The characterization of the CIS included nuclear grade, the presence of necrosis, and microcalcifications.
This study has been performed in accordance with the national regulative law for the handling of biological specimens from tumor banks, being the samples exclusively available for research purposes in retrospective studies, as well as under the international Helsinki declaration.
Silver in situ hybridization
SISH technique was performed on 3-μm-thick sections in one block of each case with dual-hapten, dual-color ISH. The dual-probe assay (INFORM HER2 Dual ISH DNA Probe Cocktail Assay; Ventana Medical Systems, Inc., Tucson, AZ, USA), which is Food and Drug Administration-approved, contains an HER2 locus-specific probe (black signal) and a control probe specific for the centromere of chromosome 17 (centromere enumeration probe-CEP17, red signal), which allows detection of HER2 gene amplification by light microscopy. The entire procedure was carried out on an automated staining system (Ventana BenchMark XT Staining System; Ventana Medical Systems, Inc., Tucson, AZ, USA) according to the manufacturer’s instructions. Appropriated positive and negative controls were used in every set of slides.
SISH interpretation
The evaluation of the samples included scoring of at least 20 nuclei, in two different areas, recording the number of HER2 and CEP17 signals. Corresponding hematoxylin and eosin (H&E) staining was used for the identification of the invasive and in situ components of the tumor, and only cells with a minimum of one copy of HER2 and CEP17 each were scored. The number of HER2 signals was estimated in clusters, except for doublets which counted as a single signal. The samples were classified by a pathologist (AP) according to the 2013 ASCO/CAP ISH criteria for HER2 gene amplification: positive when the HER2/CEP17 ratio is ≥2.0 or <2.0 and the average HER2 copy number is ≥6.0 signals per cell; equivocal when the HER2/CEP17 ratio is <2.0 and the average HER2 copy number is ≥4.0 and <6.0 signals per cell; and negative when the HER2/CEP17 ratio is <2.0 and the average HER2 copy number is <4.0 signals per cell [14].
HER2-GH is defined as tumors with discrete population of tumor cells with different HER2 gene status [14]. The proportion of amplified areas was quantified by measuring the number of fields (power field of 200×) with HER2 gene amplification divided by the number of fields of invasive or in situ carcinoma.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 24.0 for Windows. The Pearson’s Chi-squared (χ2) test (or the Fisher’s exact test, if appropriate) was used for comparison of qualitative variables and the Mann-Whitney U (MWU) test, the t test, and Pearson’s correlation coefficient (PCC) were used for comparison of quantitative variables. The level of significance was set at p < 0.05.
Results
In the first cohort, the HER2 amplification in the IC was negative in 87% of the cases and positive in 13% of the cases, without the presence of HER2-GH. All the cases had an associated CIS with the same HER2 status than IC, with four cases of CIS presenting HER2-GH. Because one case presented both lobular and ductal CIS, we characterized 101 types of CIS, where 92.1% were ductal CIS (DCIS) and 2.9% of the cases were classified as low grade, 41.6% as intermediate grade, and 55.5% as high grade. We also observed necrosis in 42.6% and microcalcifications in 27.7% (Table 1).
HER2 amplification in the IC was not related with the procedure, the age of the patients or the histological grade. However, in the CIS, we observed a significant relationship of HER2 amplification with high nuclear grade (18.9 vs 4.4%; p = 0.030), without an association with the remaining characteristics (Table 3).
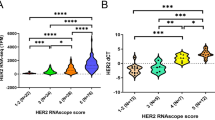
The distribution of HER2/CEP17 ratio and average of HER2 and CEP17 copy number per cell were not statistically different between the IC and CIS (Table S1). Additionally, we observed a high correlation of HER2/CEP17 ratio and average HER2 copy number per cell between IC and CIS (PCC = 0.981; p < 0.001 and PCC = 0.929; p < 0.001, respectively) (Fig. 1 and S1).
In the four cases with HER2-GH in CIS, only one case was identified in NCB and two cases presented HER2 gene amplification in the IC (Figs. 2 and 3). The proportion of cells with HER2 gene amplification in the CIS varied between 30 and 60% of the total CIS represented in the sample, being all high-grade DCIS (for details, see Table 4 and S2).
Carcinoma in situ with HER2 genetic heterogeneity associated with HER2-negative invasive carcinoma. Case 4 (1st cohort): a1 LCIS, H&E 200×; a2 non-amplified LCIS, SISH 400×; b1 invasive lobular carcinoma, H&E 200×; b2 non-amplified invasive lobular carcinoma, SISH 400×; c1 DCIS, H&E 200×; c2 amplified DCIS, SISH, 400×
In the second cohort, the proportion of cells with HER2 amplification varied between 1 and 50% of the total tumor cell population represented in the sample. In the negative component, HER2/CEP17 ratio varied between 1.00 and 1.52, and in the positive component between 2.06 and 9.17 (Table S3).
The primary IC cases had similar morphological features in the amplified and non-amplified components. The lymph node metastasis case represented a primary lobular carcinoma of the breast with an equivocal HER2 result (score of 2+) by IHC and no HER2 gene amplification by SISH (case 2). The metastasis showed two types of neoplastic cells, one similar with the primary lesion and another one with more pleomorphic nuclei. After additional IHC in the metastasis, it was found that the latter areas showed strong and complete membranous staining in 40% of the cells (score of 3+) that were confirmed by SISH as HER2 amplified (Fig. 4).
HER2-negative invasive carcinoma associated with HER2 genetic heterogeneity in the lymph node metastasis. Case 2 (2nd cohort): a1 invasive lobular carcinoma, H&E 200×; a2 non-amplified invasive lobular carcinoma, SISH, 400×; b1, c1 same lymph node metastasis, H&E 200×; b2 non-amplified area in lymph node metastasis; c2 amplified area in lymph node metastasis, SISH 400×
Additionally, in the primary IC cases, we identified two cases associated with non-amplified DCIS (cases 11 and 12—Table 5 and S4). Case 12 was classified as equivocal by IHC (score of 2+) because it showed strong and complete membranous staining in small groups of tumor cells, along with scattered single cells, representing less than 10% of the total tumor cell population in the sample. After SISH analysis, the same component presented HER2 gene amplification, consequently being classified as HER2 ISH negative (Fig. 5).
Invasive carcinoma with HER2 genetic heterogeneity associated with HER2-negative carcinoma in situ. Case 12 (2nd cohort): a1 DCIS, H&E 200×; a2 non-amplified DCIS, SISH 400×; b1 invasive carcinoma, NST, H&E 200×; b2 HER2 genetic heterogeneity in invasive carcinoma (HER2 gene amplification in small groups of tumor cells (circle areas) along with scattered single cells (arrows))
Discussion
The aim of the present study was to compare the amplification status of HER2 gene between IC and CIS and search for HER2-GH in both components. Our results show that in all the cases, we observed the same HER2 status in both IC and adjacent CIS, according to previous studies [15, 16]. Although, in this work, we only consider cases with an equivocal HER2 result by IHC that can bias the results, these are the cases that require reflex testing making the issue of HER2-GH in ISH evaluation more important in this setting.
Bright-field ISH allows to better correlate tissue morphology and HER2 gene status, clearly identifying HER2-GH in IC and CIS [17]. In CIS, HER2-GH was recognized more frequently in SES, in which more tissue is available for evaluation. Interestingly, in these cases, only half of IC presented HER2 gene amplification, confirming that this amplification is not relevant for the transition from CIS to IC. In the literature, it has been documented that in HER2-positive cases, a significant increase occurs in HER2 copy number between primary BC and metastatic lesions [18]. The high correlation of HER2/CEP17 ratio and HER2 copy number between IC and CIS suggests that the described genetic instability of HER2 gene amplification is only present in metastatic stages.
In our study, HER2 gene amplification in IC was not associated with histological grade, contrary to what has been published [6, 19]. Current evidence shows that histological grading of NCB can only be concordant with SES in about 75% of the cases [20, 21]. Most of the discordant cases are upgraded in the SES, generally due to an underscored of the mitotic frequency on NCB [21, 22]. Although our first cohort included a slight increase of grade 2 tumors compared with expected values in the literature, it might be the result of the large number of NCB, which can underestimate the histological grade and compromise the statistical relationship with HER2 gene amplification [20]. Nevertheless, HER2 gene amplification in CIS was significantly associated with high nuclear grade, as previously documented [6, 19].
Before the introduction of the first definition of HER2-GH, intratumoral heterogeneity was applied to discrete population of cells and reported as a rare event [23, 24]. Recently, we also showed the presence of HER2-GH in IC, according to the updated ASCO/CAP HER2 guideline, to be extremely infrequent [25]. In the present work, we identified it in about 1% of the cases, including cases of male patients.
HER2-GH in the IC was observed more often in NCB, because most evaluations are performed by this procedure, which represent the first biological material on which the hormone receptors (HR) and HER2 markers should be first determined [14, 26]. The predominant histologic type was invasive carcinomas, NST, with no cases classified as grade 1, as previously noticed [23]. Although we had no information regarding previous treatment, the fact that most IC cases with HER2-GH were found in NCB shows us that this rare event can occur in patients without previous treatment.
All primary invasive BC cases presented similar histological characteristics in the amplified and non-amplified areas, with HER2 gene amplification in a minor component, supporting the idea that most cases develop in a single tumor that acquired HER2 gene amplification during tumor progression. Even though molecular analysis has shown that cases with HER2-GH in the IC can be the result of two distinct tumors (also known as collision tumors), most cases appear to be clonally related resulting from clonal divergence from a single tumor, as previously shown [27, 28]. However, it remains to be shown if the same process can occur in CIS.
In case 2 (second cohort), the HER2-GH was found in the lymph node metastasis rather than the primary tumor. This can be the result of tumor evolution in the lymph node metastasis or, eventually, the representation of an independent tumor that was not identified in the SES. Nevertheless, this case illustrates the importance of pathologists in selecting tumor areas with less differentiation and higher nuclear pleomorphism, either in the primary IC or in the lymph node metastases, which most likely are going to be HER2 amplified. Additionally, according to the 2013 ASCO/CAP guideline, the HER2 test should also be repeated if results are discordant with histopathologic findings [14].
Although it has been previously reported that HRs and HER2 conversion by IHC can occur between primary and metastatic lesions, the former is much more frequent (preferentially from HR-positivity in the primary tumors to HR-negativity in the metastasis) [18, 29]. The conversion phenomenon could be explained by HER2-GH in the primary tumor, which can be more frequently found if more than one block is tested [30, 31]. In this study, we were restricted to the analysis of only one block, which can underestimate the prevalence of HER2-GH. Additionally, we also not considered cases with HER2-GH by IHC (score 3+ in >10 and <100%). Interestingly, it has also been shown that patients with HER2-GH have worse outcome compared to patients with homogeneous amplified or non-amplified HER2 gene, suggesting that mixed tumors behave more aggressively [32, 33].
Furthermore, it has been described that the majority of ICs with HER2-GH have non-amplified DCIS, consistent with our study, again supporting the idea that IC originates from CIS and that HER2 gene amplification can also be acquired in later stages [23]. Cases with HER2-GH in the IC associated with amplified DCIS probably represent distinct tumors, given the fact that the loss of the HER2 gene amplification is an unlikely event.
Finally, regarding case 12 (second cohort), which presented HER2 gene amplification in less than 10% of the total tumor cell population, a comment was made in the report recommending repetition of HER2 test by IHC in the SES to find and accurately quantify the HER2 positive component. Moreover, focally amplified small populations can be overlooked and IHC should be used to guide ISH analysis, searching for areas of potential amplification [14]. All cases exhibiting HER2-GH on NCB by ISH should have HER2 test repeated on the SES, according to the updated United Kingdom guidelines [34]. Additionally, clinical trials have not so far been based on the new 10% cut-off for ISH as provided by the 2013 ASCO/CAP guideline [35,36,37,38,39]. In fact, the presence of HER2 gene amplification was enough for inclusion of patients to HER2-targeted therapy, irrespective of the proportion of amplified cells. Fortunately, the rare presence of HER2-GH probably did not influence the clinical results given that any random group of cells evaluated will represent, most of the times, the whole tumor [40]. However, it remains to be demonstrated what is the minimal proportion of amplified tumor cell population that achieves clinical response to HER2-targeted therapy.
In conclusion, we show that HER2-GH is a rare event in IC and can already be present in CIS, not being an important step in the acquisition of invasive features. As far as we know, this is the first time that HER2-GH, according to the updated ASCO/CAP HER2 guideline, has been evaluated in both IC and adjacent CIS using bright-field ISH. Although intratumoral heterogeneity of HER2 gene amplification can have clinical significance, not only affecting the selection of patients but also explaining some of the variability of the response to targeted therapy, this is a rare event in breast cancer cases.
Authors’s contributions
AP: designed the research study, conducted HER2 SISH interpretation, retrieved and analyzed the data, and wrote the manuscript. GO: performed the laboratory work. FS: designed the research study and critically revised the manuscript. All authors read and approved the final manuscript.
References
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182
Press MF, Bernstein L, Thomas PA, Meisner LF, Zhou JY, Ma Y, Hung G, Robinson RA, Harris C, El-Naggar A, Slamon DJ, Phillips RN, Ross JS, Wolman SR, Flom KJ (1997) HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol 15(8):2894–2904
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792
DeSantis C, Ma J, Bryan L, Jemal A (2013) Breast cancer statistics, 2013. CA Cancer J Clin 64(1):52–62
Steinman S, Wang J, Bourne P, Yang Q, Tang P (2007) Expression of cytokeratin markers, ER-alpha, PR, HER-2/neu, and EGFR in pure ductal carcinoma in situ (DCIS) and DCIS with co-existing invasive ductal carcinoma (IDC) of the breast. Ann Clin Lab Sci 37(2):127–134
Martins D, Sousa B, Lopes N, Gomes M, Veronese L, Albergaria A, Paredes J, Schmitt F Molecular phenotypes of matched in situ and invasive components of breast carcinomas. Hum Pathol 42(10):1438–1446
Polyak K Heterogeneity in breast cancer. J Clin Invest 121(10):3786–3788
Vance GH, Barry TS, Bloom KJ, Fitzgibbons PL, Hicks DG, Jenkins RB, Persons DL, Tubbs RR, Hammond ME (2009) Genetic heterogeneity in HER2 testing in breast cancer: panel summary and guidelines. Arch Pathol Lab Med 133(4):611–612
Tubbs RR, Hicks DG, Cook J, Downs-Kelly E, Pettay J, Hartke MB, Hood L, Neelon R, Myles J, Budd GT, Moore HC, Andresen S, Crowe JP (2007) Fluorescence in situ hybridization (FISH) as primary methodology for the assessment of HER2 status in adenocarcinoma of the breast: a single institution experience. Diagn Mol Pathol 16(4):207–210
Miller DVJR, Lingle WL, Davidson NE, Kaufman PA, Martino S, Dakhil SR, Perez EA (2004) Focal HER2/neu amplified clones partially account for discordance between immunohistochemistry and fluorescence in-situ hybridization results: data from NCCTG N9831 intergroup adjuvant trial. J Clin Oncol 22(14S)
Allison KH, Dintzis SM, Schmidt RA (2011) Frequency of HER2 heterogeneity by fluorescence in situ hybridization according to CAP expert panel recommendations: time for a new look at how to report heterogeneity. Am J Clin Pathol 136(6):864–871
Hanna WM, Ruschoff J, Bilous M, Coudry RA, Dowsett M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G (2013) HER2 in situ hybridization in breast cancer: clinical implications of polysomy 17 and genetic heterogeneity. Mod Pathol 27(1):4–18
Chang MC, Malowany JI, Mazurkiewicz J, Wood M (2012) 'Genetic heterogeneity' in HER2/neu testing by fluorescence in situ hybridization: a study of 2,522 cases. Mod Pathol 25(5):683–688
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013
Kobayashi M, Ooi A, Oda Y, Nakanishi I (2002) Protein overexpression and gene amplification of c-erbB-2 in breast carcinomas: a comparative study of immunohistochemistry and fluorescence in situ hybridization of formalin-fixed, paraffin-embedded tissues. Hum Pathol 33(1):21–28
Latta EK, Tjan S, Parkes RK, O'Malley FP (2002) The role of HER2/neu overexpression/amplification in the progression of ductal carcinoma in situ to invasive carcinoma of the breast. Mod Pathol 15(12):1318–1325
Nitta H, Hauss-Wegrzyniak B, Lehrkamp M, Murillo AE, Gaire F, Farrell M, Walk E, Penault-Llorca F, Kurosumi M, Dietel M, Wang L, Loftus M, Pettay J, Tubbs RR, Grogan TM (2008) Development of automated brightfield double in situ hybridization (BDISH) application for HER2 gene and chromosome 17 centromere (CEN 17) for breast carcinomas and an assay performance comparison to manual dual color HER2 fluorescence in situ hybridization (FISH). Diagn Pathol 3:41
Fabi A, Di Benedetto A, Metro G, Perracchio L, Nistico C, Di Filippo F, Ercolani C, Ferretti G, Melucci E, Buglioni S, Sperduti I, Papaldo P, Cognetti F, Mottolese M HER2 protein and gene variation between primary and metastatic breast cancer: significance and impact on patient care. Clin Cancer Res 17(7):2055–2064
Tamimi RM, Baer HJ, Marotti J, Galan M, Galaburda L, Fu Y, Deitz AC, Connolly JL, Schnitt SJ, Colditz GA, Collins LC (2008) Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res 10(4):R67
Rakha EA, Reis-Filho JS, Baehner F, Dabbs DJ, Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani SR, Palacios J, Richardson AL, Schnitt SJ, Schmitt FC, Tan PH, Tse GM, Badve S, Ellis IO Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res 12(4):207
Rakha EA, Ellis IO (2007) An overview of assessment of prognostic and predictive factors in breast cancer needle core biopsy specimens. J Clin Pathol 60(12):1300–1306
Denley H, Pinder SE, Elston CW, Lee AH, Ellis IO (2001) Preoperative assessment of prognostic factors in breast cancer. J Clin Pathol 54(1):20–24
Hanna W, Nofech-Mozes S, Kahn HJ (2007) Intratumoral heterogeneity of HER2/neu in breast cancer--a rare event. Breast J 13(2):122–129
Andersson J, Linderholm B, Bergh J, Elmberger G (2004) HER-2/neu (c-erbB-2) evaluation in primary breast carcinoma by fluorescent in situ hybridization and immunohistochemistry with special focus on intratumor heterogeneity and comparison of invasive and in situ components. Appl Immunohistochem Mol Morphol 12(1):14–20
Polonia A, Leitao D, Schmitt F (2016) Application of the 2013 ASCO/CAP guideline and the SISH technique for HER2 testing of breast cancer selects more patients for anti-HER2 treatment. Virchows Arch 468(4):417–423
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134(7):e48–e72
Lehmann-Che J, Amira-Bouhidel F, Turpin E, Antoine M, Soliman H, Legres L, Bocquet C, Bernoud R, Flandre E, Varna M, de Roquancourt A, Plassa LF, Giacchetti S, Espie M, de Bazelaire C, Cahen-Doidy L, Bourstyn E, Janin A, de The H, Bertheau P Immunohistochemical and molecular analyses of HER2 status in breast cancers are highly concordant and complementary approaches. Br J Cancer 104(11):1739–1746
Ng CK, Martelotto LG, Gauthier A, Wen HC, Piscuoglio S, Lim RS, Cowell CF, Wilkerson PM, Wai P, Rodrigues DN, Arnould L, Geyer FC, Bromberg SE, Lacroix-Triki M, Penault-Llorca F, Giard S, Sastre-Garau X, Natrajan R, Norton L, Cottu PH, Weigelt B, Vincent-Salomon A, Reis-Filho JS (2015) Intra-tumor genetic heterogeneity and alternative driver genetic alterations in breast cancers with heterogeneous HER2 gene amplification. Genome Biol 16:107
Hoefnagel LD, van de Vijver MJ, van Slooten HJ, Wesseling P, Wesseling J, Westenend PJ, Bart J, Seldenrijk CA, Nagtegaal ID, Oudejans J, van der Valk P, van der Groep P, de Vries EG, van der Wall E, van Diest PJ Receptor conversion in distant breast cancer metastases. Breast Cancer Res 12(5):R75
Lewis JT, Ketterling RP, Halling KC, Reynolds C, Jenkins RB, Visscher DW (2005) Analysis of intratumoral heterogeneity and amplification status in breast carcinomas with equivocal (2+) HER-2 immunostaining. Am J Clin Pathol 124(2):273–281
Perez EA, Press MF, Dueck AC, Jenkins RB, Kim C, Chen B, Villalobos I, Paik S, Buyse M, Wiktor AE, Meyer R, Finnigan M, Zujewski J, Shing M, Stern HM, Lingle WL, Reinholz MM, Slamon DJ Immunohistochemistry and fluorescence in situ hybridization assessment of HER2 in clinical trials of adjuvant therapy for breast cancer (NCCTG N9831, BCIRG 006, and BCIRG 005). Breast Cancer Res Treat 138(1):99–108
Seol H, Lee HJ, Choi Y, Lee HE, Kim YJ, Kim JH, Kang E, Kim SW, Park SY (2012) Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod Pathol 25(7):938–948
Kurozumi S, Padilla M, Kurosumi M, Matsumoto H, Inoue K, Horiguchi J, Takeyoshi I, Oyama T, Ranger-Moore J, Allred DC, Dennis E, Nitta H HER2 intratumoral heterogeneity analyses by concurrent HER2 gene and protein assessment for the prognosis of HER2 negative invasive breast cancer patients. Breast Cancer Res Treat 158(1):99–111
Rakha EA, Pinder SE, Bartlett JM, Ibrahim M, Starczynski J, Carder PJ, Provenzano E, Hanby A, Hales S, Lee AH, Ellis IO, Updated UK Recommendations for HER2 assessment in breast cancer. J Clin Pathol 68(2):93–99
Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Anton A, Lluch A, Kennedy J, O'Byrne K, Conte P, Green M, Ward C, Mayne K, Extra JM (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 23(19):4265–4274
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353(16):1659–1672
Robert N, Leyland-Jones B, Asmar L, Belt R, Ilegbodu D, Loesch D, Raju R, Valentine E, Sayre R, Cobleigh M, Albain K, McCullough C, Fuchs L, Slamon D (2006) Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol 24(18):2786–2792
Gianni L, Eiermann W, Semiglazov V, Lluch A, Tjulandin S, Zambetti M, Moliterni A, Vazquez F, Byakhov MJ, Lichinitser M, Climent MA, Ciruelos E, Ojeda B, Mansutti M, Bozhok A, Magazzu D, Heinzmann D, Steinseifer J, Valagussa P, Baselga J (2014) Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol 15(6):640–647
Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr, Martino S, Rastogi P, Gralow J, Swain SM, Winer EP, Colon-Otero G, Davidson NE, Mamounas E, Zujewski JA, Wolmark N (2014) Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 32(33):3744–3752
Polonia A, Eloy C, Pinto J, Braga AC, Oliveira G, Schmitt F (2017) Counting invasive breast cancer cells in the HER2 silver in-situ hybridization test: how many cells are enough? Histopathology. doi:10.1111/his.13208
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study has been performed in accordance with the national regulative law for the handling of biological specimens from tumor banks, being the samples exclusively available for research purposes in retrospective studies, as well as under the international Helsinki declaration.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Polónia, A., Oliveira, G. & Schmitt, F. Characterization of HER2 gene amplification heterogeneity in invasive and in situ breast cancer using bright-field in situ hybridization. Virchows Arch 471, 589–598 (2017). https://doi.org/10.1007/s00428-017-2189-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-017-2189-9