Abstract
To evaluate the effect of the 2018 ASCO/CAP guideline in the identification of HER2-positive breast carcinomas (BC) in reflex in situ hybridization (ISH) test. A total of 592 primary invasive BC cases from before and after the publication of the updated ASCO/CAP guideline were evaluated for HER2 amplification by silver ISH according to the 2013 and 2018 guidelines. Cases were mostly (95%) HER2 equivocal by immunohistochemistry (IHC), not centrally reviewed. Other reasons for referring cases were IHC confirmation, IHC discordancy (either between needle-core-biopsy (NCB) and surgical excision specimen (SES) or between different laboratories) and IHC result unexpected for histopathologic features. Cases evaluated with the 2013 guideline (1st cohort) were 14.6% HER2-positive, decreasing significantly after the reclassification with the 2018 guideline due to the exclusion of group 2 cases without HER2 protein overexpression. Cases studied after the implementation of the 2018 guideline (2nd cohort) were 8.7% HER2-positive, a frequency that was not significantly different from the reclassification of the 1st cohort with the 2018 guideline. All cases referred for IHC confirmation had the expected ISH result. Cases with IHC discordancy between NCB and SES were ISH concordant. Only one out of 14 cases with an IHC score 3+ and classified as histological grade 1 or with a Ki67 below 10% was classified as ISH HER2-positive. The 2018 ASCO/CAP guideline resulted in a decrease of HER2-positive cases in reflex ISH test, selecting less patients for anti-HER2-targeted therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the most frequently diagnosed cancer in women worldwide and the leading cause of cancer death in most countries [1]. According to current guidelines, human epidermal growth factor receptor 2 (HER2) quantification must be routinely performed, along with estrogen and progesterone receptor, in all patients with invasive BC, recurrences, and metastases [2, 3].
The overexpression and/or amplification of HER2 in invasive BC has been associated with aggressive clinical behavior but with a high probability of response to HER2-targeted therapy [4,5,6]. Many clinical trials have demonstrated that HER2-targeted therapy given during and/or after chemotherapy results in a significant improvement in disease-free and overall survival only in patients with BCs showing HER2 amplification or overexpression [6,7,8,9]. Consequently, the correct identification of HER2-positive BC selects patients expected to benefit from targeted therapy.
In most laboratories, HER2 evaluation begins with the analysis of protein expression by immunohistochemistry (IHC) resulting in the following scenarios: negative (score 0 or 1+), equivocal (score 2+), positive (score 3+), and indeterminate. If the IHC result is equivocal or indeterminate, reflex testing should be performed with in situ hybridization (ISH) assays for the assessment of HER2 amplification [2].
Recently, the ASCO/CAP (American Society of Clinical Oncology/College of American Pathologists) has updated the HER2 guideline, clarifying the definition of equivocal cases by IHC as well as introducing the criteria for less frequent results (non-classical) in the ISH quantification (for details see below). Currently, a HER2 score 2+ is defined as the presence of weak to moderate complete membrane staining, in at least 10% of tumor cells. Moreover, according to the new guideline, the possibility of a discordancy between IHC and ISH can only be considered in classical groups (1 and 5, amplified and non-amplified, respectively) [2]. The remaining non-classical groups (2 to 4) can express any IHC score (from 0 to 3+). For these latter groups, it is required, henceforth, concomitant IHC to reach a final integrated interpretation. Consequently, it means that the ISH result is no longer an absolute truth, unless it is a classical result. Finally, the equivocal nomenclature by ISH was substituted by the non-classical group 4 [2].
The aim of the present study is to evaluate the effect of the 2018 ASCO/CAP guideline in the identification of HER2-positive BC by the ISH technique.
Materials and methods
Case selection
The cases included formalin-fixed, paraffin-embedded needle core biopsies (NCB) and surgical excision specimens (SES) referred to Ipatimup Diagnostics (national reference center for HER2 ISH) from 27 institutions (from 1 to 108 cases each) for evaluation of HER2 gene amplification with bright-field ISH. HER2 test by IHC was performed in the sending institution, without information regarding pre-analytical conditions. The first cohort comprised 380 primary invasive BC cases studied 1 year prior to the introduction of the 2018 ASCO/CAP HER2 guideline (from June 2017 to May 2018). The second cohort included 212 primary invasive BC cases since the publication of the 2018 ASCO/CAP HER2 guideline (June 2018) until November 2018. This study has been performed in accordance with the national regulative law for the handling of biological specimens from tumor banks, being the samples exclusively available for research purposes in retrospective studies, as well as under the international Helsinki declaration. Ethical approval and informed consent were not required for this study.
Silver in situ hybridization
SISH was performed on 3-μm-thick sections in one block of each case with dual-hapten, dual-color ISH. The dual-probe assay (INFORM HER2 Dual ISH DNA Probe Cocktail Assay; Ventana Medical Systems, Inc., Tucson, AZ, USA), which is Food and Drug Administration-approved, contains an HER2 locus-specific probe (black signal) and a control probe specific for the centromere of chromosome 17 (centromere enumeration probe-CEP17, red signal), which allows detection of HER2 gene amplification by light microscopy. The entire procedure was carried out on an automated staining system (Ventana BenchMark XT Staining System; Ventana Medical Systems, Inc., Tucson, AZ, USA) according to the manufacturer’s instructions. Appropriated positive and negative controls were used in every set of slides.
SISH interpretation
The samples were classified by a pathologist (AP) according to the 2013 and 2018 ASCO/CAP guidelines. Corresponding hematoxylin and eosin (HE) staining was used for the identification of the invasive component of the tumor, and only cells with a minimum of one copy of HER2 and CEP17 each were scored. The number of HER2 signals was estimated in clusters, except for doublets, which counted as a single signal. The evaluation of the samples included scoring of at least 20 nuclei, in two different areas, recording the numbers of HER2 and CEP17 signals. The 2013 ASCO/CAP guideline establishes the result of HER2 gene amplification as: positive when the HER2/CEP17 ratio is ≥ 2.0 or < 2.0 and the average HER2 copy number is ≥ 6.0 signals per cell; equivocal when the HER2/CEP17 ratio is < 2.0 and the average HER2 copy number is ≥ 4.0 and < 6.0 signals per cell; and negative when the HER2/CEP17 ratio is < 2.0 and the average HER2 copy number is < 4.0 signals per cell. The 2018 ASCO/CAP guideline defines HER2 gene amplification as positive (classical group 1) when the HER2/CEP17 ratio is ≥ 2.0 and the average HER2 copy number is ≥ 4.0 signals per cell, and negative (classical group 5) when the HER2/CEP17 ratio is < 2.0 and the average HER2 copy number is < 4.0 signals per cell. Moreover, group 2 is defined as HER2/CEP17 ratio ≥ 2.0 and average HER2 copy number < 4.0 signals per cell; group 3 as HER2/CEP17 ratio < 2.0 and average HER2 copy number ≥ 6.0 signals per cell; and group 4 as HER2/CEP17 ratio < 2.0 and average HER2 copy number ≥ 4.0 and < 6.0 signals per cell. The final classification in groups 2 to 4 (non-classical) depends on the result of IHC analysis and is considered positive if a score 3+ in these groups or a score 2+ in group 3, and negative if otherwise. HER2 genomic heterogeneity (HER2-GH) is defined as tumors with a discrete population of tumor cells with different HER2 gene status. The proportion of amplified areas was quantified by measuring the number of fields (power field of 200×) with HER2 gene amplification divided by the number of fields of invasive carcinoma. A case is considered positive if HER2 gene amplification represents at least 10% of the total tumor cell population.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 24.0 for Windows. The Pearson’s Chi-squared (χ2) test (or the Fisher’s exact test, if appropriate) and McNemar test were used for comparison of qualitative variables, and the t test was used for comparison of quantitative variables. The level of significance was set at P < 0.05.
Results
The first cohort included 311 NCB (81.8%) and 69 SES (18.2%), diagnosed in 376 women (98.9%) and 4 men (1.1%). The age of the patients ranged from 24 to 93 years old, with a median age at diagnosis of 61 years old. The second cohort comprised 178 NCB (84.0%) and 34 SES (16.0%), diagnosed in 208 women (98.1%) and 4 men (1.9%). The age of the patients ranged from 24 to 94 years old, with a median age at diagnosis of 61 years old. The distributions by procedure, gender, age, HER2/CEP17 ratio, average HER2, and CEP17 copy number per cell were not statistically different between the two cohorts (Table S1 and Fig. S1).
In the first cohort, the IHC analysis from the referred cases included 3 cases scored 0 and 1+ (0.8%), 356 cases scored 2+ (93.7%), 16 cases scored 3+ (4.2%), and 5 indeterminate cases (1.3%) (Table S2). According to the 2013 ASCO/CAP guideline, there were 310 HER2-negative cases (85.4%), 53 HER2-positive cases (14.6%), and no equivocal results in the SISH test (Table 1). HER2-GH was detected in 0.6% of the cases (2/363), the proportion of HER2-amplified cells varied from 20 to 30% of the total tumor cell population represented in the sample.
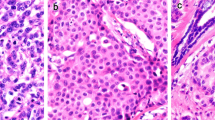
Regarding the 19 cases scored 0, 1+, or 3+ in the first cohort (cases usually not elected for ISH analysis), the reasons for ISH testing were as follows: 6 cases for IHC confirmation (1 case scored 0 and 5 cases scored 3+), 9 NCB cases scored 3+ classified as histological grade 1 (7 cases) or with Ki67 below 10% (2 cases), 3 cases with IHC discordancy between NCB and SES and, lastly, one case scored 0 and 3+ by two different laboratories. All cases sent for IHC confirmation had a concordant result with SISH. In contrast, only one out of the 9 NCB cases scored 3+ and classified as histological grade 1 or with Ki67 below 10% had an HER2 gene amplification. This case had a mucinous pattern and an HER2/CEP17 ratio of 2.96 and an average HER2 copy number of 3.93 (Fig. 1). Concerning the 3 discordant cases (Table 2), cases 1 and 2 were scored 3+ in the NCB but with no protein overexpression in the SES. SISH testing was performed in the NCB and SES of case 1 and in the NCB of case 2, being negative for HER2 amplification in all samples. Case 3 had a score 2+ in the NCB, a score 1+ in the SES and HER2 amplification by SISH in both samples. Finally, the case with IHC discordancy between laboratories presented a HER2/CEP17 ratio of 2.2 and an average HER2 copy number of 2.7, being classified as HER2-positive according to the 2013 ASCO/CAP guideline.
In the second cohort, the IHC analysis from the referred cases comprised 4 cases scored 0 and 1+ (1.9%), 203 cases scored 2+ (95.7%), and 5 cases scored 3+ (2.4%) (Table S2). According to the 2018 ASCO/CAP guideline, there was 190 HER2-negative cases (91.3%) and 18 HER2-positive cases (8.7%) (Table 1), without cases with HER2-GH. The HER2-negative cases included 9 group 2 cases (4.3%) without HER2 protein overexpression (score 2+) (Tables 3 and S3). Moreover, there were no group 3 or group 4 cases.
Regarding the 9 cases scored 0, 1+, or 3+ in the second cohort, the reasons for ISH testing were as follows: 4 cases for IHC confirmation (3 cases scored 0 and 1 case scored 1+) and 5 NCB cases scored 3+ classified as histological grade 1. All the 9 cases were negative for HER2 amplification (group 5) (Table S3).
Comparing the cohorts, we observed a significant decrease of HER2-positive cases from 14.6% to 8.7% between the classification of the first cohort (2013 ASCO/CAP guideline) and the second cohort (2018 ASCO/CAP guideline) (p = 0.038), unlike the difference between the classification of both cohorts according to the 2018 ASCO/CAP guideline (p = 0.274) (Table 1). In the reclassification of the first cohort using the 2018 ASCO/CAP guideline, we observed 321 HER2-negative cases (88.4%) and 42 HER2-positive cases (11.6%), which represented a 20.8% decrease in HER2-positive cases (p = 0.001), corresponding to the exclusion of 11 cases from group 2 without HER2 protein overexpression (Tables 1 and S3). The two group 2 cases with HER2 protein overexpression corresponded to one case sent for IHC confirmation and another case classified as histological grade 1 (for details, see above). All negative cases according to the 2013 guideline remained negative with the 2018 guideline, and there were no cases in groups 3 and 4 (Tables 2 and 3).
Considering both cohorts, there were 21 indeterminate results by SISH (3.6%), not being statistically different between NCB and SES (3.7% (18/489) and 2.9% (3/103), respectively; Fisher’s exact test p = 1.000). The main reason for these results was the lack of well-defined signals (18/21–85.7%), with the formation of a black precipitation in the remainders.
Discussion
In the present study, we aimed to compare the effect of the 2018 ASCO/CAP guideline in the identification of HER2-positive BC by SISH. We found that the updated guideline significantly decreases the number of HER2-positive BC cases due to the exclusion of group 2 cases without HER2 protein overexpression. Group 2 represents BC cases with deletion of the reference probe (CEP17) rather than HER2 amplification, giving rise to an artificially high HER2/CEP17 ratio. The reclassification of the cases from the first cohort with the 2018 ASCO/CAP guideline was able to predict that decrease, as it does not differ significantly from the classification of the second cohort. The difference between both cohorts when classified with the same ASCO/CAP guideline is the application of IHC criteria, which we demonstrate as not playing a major role in the global effect of the new guideline. In our series, the IHC performed externally was not centrally reviewed, which represents a limitation of this work. As such, the results represent the impact of the updated guideline in reflex ISH test when applied to equivocal results by IHC performed in external laboratories. In the setting of ISH test as the first approach in HER2 evaluation, it is expected that the non-classical groups represent a higher proportion of cases, given the usual correlation with negative results by IHC [10, 11]. Since equivocal cases by IHC represent 10 to 20% of BC cases, the overall decrease of HER2 positivity rate to be expected from the updated guideline will be less pronounced [2].
Since the application of the 2013 ASCO/CAP guideline for HER2 test in BC, most studies document an increase in both HER2-positive and HER2-equivocal cases by fluorescence ISH (FISH), the latter group representing 2 to 14% of the cases [12,13,14,15,16,17,18,19]. In 2016, our group also confirmed, using SISH for the first time, that the above mentioned ASCO/CAP guideline resulted in a significant increase of HER2-positive cases [20]. Very recently, a studied from an Irish group found a decrease in HER2-positive cases from the updated guideline in reflex ISH test, also due to the exclusion of group 2 cases (about 7%) as is documented in our series [21]. An Asian group has shown that the 2018 ASCO/CAP guideline significantly increases the HER2-negative cases by FISH at the expense of reclassification of equivocal cases (nearly 15% of the cases) into HER2-negative cases (group 4). Additionally, the same authors also reported an absence of group 2 cases [22]. The explanation for the difference of proportion of groups 2 and 4 in our results and theirs may be related to the size of the series, pre-analytical conditions, or different ISH platforms. As we speculated before, differences between bright-field ISH and FISH, due to the presence of autofluorescence, could result in an increase of both HER2 and CEP17 signals in the latter technique, with consequent higher HER2 copy numbers with the same HER2/CEP17 ratio, and therefore increased number of group 4 and less group 2 cases [20, 23, 24]. Although the majority of the published literature finds concordance rates between SISH and FISH greater than 90%, the recent creation of groups 1 to 5 according to HER2/CEP17 ratio and average HER2 copy number per cell may change this concordance rate [25,26,27,28,29,30,31,32,33,34].
The application of different groups in ISH analysis is one of the most relevant changes from the current guideline. The likelihood of a BC case being HER2 overexpressed increases substantially with average HER2 copy number higher than 6.0, representing cases with significantly higher risk of death from BC compared to both HER2 copy number lower than 4.0 and between 4.0 and 6.0, particularly in the first 5 years after the diagnosis [10, 11, 35]. In contrast, group 2 cases are associated with low HER2 protein expression and without survival benefit from trastuzumab therapy (at variance with group 1 cases), supporting the classification of these cases as HER2-negative [11]. Furthermore, groups 4 is also associated with low HER2 protein expression, not showing significant survival differences between group 5, suggesting that group 4 is also best classified as HER2-negative [11, 36]. Remarkably, when comparing group 4 cases with low-amplified group 1 cases (HER2/CEP17 ratio higher than 2.0 and average HER2 copy number between 4.0 and 6.0), it has been demonstrated that the latter group also correlates with the absence of HER2 protein overexpression and present similar BC-specific survivorship [11, 35, 37]. Taken together, this means that group 5, 4, and low-amplified group 1 have similar survival, although the data lacks clarification regarding response to targeted therapy in latter group.
Bright-field ISH allows a better link between tissue morphology and HER2 gene status in comparison with FISH and, in the present work, we report HER2-GH in less than 1% of the cases [38]. HER2-GH has been shown to be a rare event, including in carcinoma in situ, which has been also confirmed by our group in the past [20, 39,40,41,42]. The 2018 ASCO/CAP guideline maintained the previous definition of HER2-GH from the 2013 guideline, without mentioning to the potential adaptation following the creation of different ISH groups. As such, we assume that HER2-GH is the presence of different ISH groups in the same tumor, and that IHC should also be taken into consideration in this analysis.
In our work, about 95% of the cases received for ISH analysis, in both cohorts, were equivocal or indeterminate by IHC. Regarding the remainder cases, all cases referred for IHC confirmation had an expected ISH result. On the other hand, in both cohorts, only one out of 14 cases score 3+ and classified as histological grade 1 or Ki67 below 10% was HER2-positive by ISH. HER2 gene amplification has been shown to be associated with high histological grade, being rare in grade 1 cases [39, 43, 44]. We note that this case was a NCB and that histological grading may have been underestimated, as it happens in 25% of the cases when compared with the SES [45, 46]. The abovementioned data supports the HER2 guideline recommendation of ISH analysis whenever the histopathologic features disagree with HER2 protein overexpression [2].
In our series, the IHC discordancy between NCB and SES was also a rare reason to perform reflex ISH; nevertheless, there was no ISH discordancy among these cases, suggesting a probable interference of unknown pre-analytical conditions in IHC test. In the first cohort, case 3 had a HER2/CEP17 ratio higher than 2.0 in both NCB and SES, but an average HER2 copy number below 4.0 and higher than 4.0 in NCB and SES, respectively. Both samples were classified as HER2-positive according to the 2013 guideline, which would be different if applying the 2018 guideline (HER2-negative group 2 and HER2-positive group 1 in NCB and SES, respectively). The 2018 guideline acknowledges cases with quantification near decision thresholds admitting a high likelihood of different result by chance when repeating the ISH test, a situation that could potentially be problematic [2]. In fact, cases with quantifications near the decision thresholds represent most of the discordant cases by ISH [41, 47]. In the past, we have demonstrated that counting additional invasive cells can solve most of these difficult cases, reducing the variability of the quantification and increasing the accuracy and robustness of the ISH result [41]. Counting 20 additional invasive cells is the procedure recommended by the current guideline to clarify the abovementioned situations [2]. As we suggested before, even when performed by an experienced observer, the recommendation of counting at least 60 invasive cells in these situations seems to be more appropriate, as suggested by the UK guideline [41, 48].
Lastly, we report a case with total IHC discordancy between different laboratories (positive versus negative). This case from the first cohort was classified according to the 2013 guideline as HER2-positive with an HER2/CEP17 ratio higher than 2.0 and an average HER2 copy number below 4.0. The 2018 guideline would consider this case as group 2 and the IHC analysis would define the result. As such, both laboratories would reclaim their result as the correct one. Although this is a rare situation (only 1 out of nearly 600 cases in the present work), the solution is not provided by the current guideline. As this case was reported just before the introduction of the 2018 guideline, we were asked to review it a few weeks later. We ended up performing an additional IHC analysis and reported a score 2+ (without overexpression), switching it into a HER2-negative case.
Indeterminate cases by SISH were observed in rare situations (fewer than 5% of the cases). Surprisingly, we did not find a higher proportion of these cases in SES in which pre-analytical conditions are thought to be more often compromised. In our practice, every indeterminate case is tested at least twice with different equipment protocols; however, it remains difficult to adapt protocols when pre-analytical information is not provided.
The use of alternative control probes (other than CEP17) has been a matter of debate in the last few years. Apparently, the use of these probes can convert a large proportion of cases from group 4 (previously equivocal) to low-amplified group 1 (from 30 to more than 90% of the cases) and even from group 5 (classical HER2-negative) to group 2 (from about 10 to almost 50% of the cases) mainly due to the frequent heterozygous deletions of the alternative control probes, particularly in group 4 cases [36, 49, 50]. These upgraded HER2-positive cases are associated with low HER2 protein expression and have similar prognosis compared with group 5, therefore being best regarded as HER2-negative cases [36]. Although much has been published regarding group 4 cases, little has been explored concerning group 3 cases, that, by definition, must all be chromosome 17 polysomic (average CEP17 copy number higher than 3.0). Importantly, it has been reported that this rare group might represent both HER2-positive and HER2-negative cases, the latter ones significantly correlating with low HER2 protein expression, providing evidence for the use of IHC to establish HER2 status in group 3 [10, 11].
Lastly, HER2 somatic mutations, mostly in the tyrosine kinase domain, have been identified in BC (2 to 3%) representing an alternative activation of HER2 signaling, particularly in lobular carcinoma [51]. Moreover, this mechanism has been further evaluated in clinical trials for HER2-targeted therapy with small tyrosine kinase inhibitors [52]. These cases are neither HER2 protein overexpressed nor HER2 gene amplified, making IHC and ISH tests useless in their identification. It has been shown that gene sequencing using samples from the primary tumor, metastases, or circulating tumor DNA is suitable for such detection [52].
In conclusion, we report that the updated 2018 HER2 guideline results in a decrease of HER2-positive BC cases using the SISH technique, mostly due to the exclusion of group 2 cases without HER2 overexpression. Therefore, the 2018 ASCO/CAP guideline selects less patients for anti-HER2 targeted-therapy, hopefully selecting the ones who will benefit the most.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M (2018) Human epidermal growth factor receptor 2 testing in breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med 142(11):1364–1382
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134(7):e48–e72
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182
Press MF, Bernstein L, Thomas PA, Meisner LF, Zhou JY, Ma Y, Hung G, Robinson RA, Harris C, El-Naggar A, Slamon DJ, Phillips RN, Ross JS, Wolman SR, Flom KJ (1997) HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol 15(8):2894–2904
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792
Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ (1999) Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17(9):2639–2648
Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20(3):719–726
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353(16):1659–1672
Press MF, Villalobos I, Santiago A, Guzman R, Cervantes M, Gasparyan A, Campeau A, Ma Y, Tsao-Wei DD, Groshen S (2016) Assessing the new American Society of Clinical Oncology/College of American Pathologists Guidelines for HER2 testing by fluorescence in situ hybridization: experience of an academic consultation practice. Arch Pathol Lab Med 140:1250–1258
Press MF, Sauter G, Buyse M, Fourmanoir H, Quinaux E, Tsao-Wei DD, Eiermann W, Robert N, Pienkowski T, Crown J, Martin M, Valero V, Mackey JR, Bee V, Ma Y, Villalobos I, Campeau A, Mirlacher M, Lindsay MA, Slamon DJ (2016) HER2 gene amplification testing by fluorescent in situ hybridization (FISH): comparison of the ASCO-College of American pathologists guidelines with FISH scores used for enrollment in breast Cancer international research group clinical trials. J Clin Oncol 34(29):3518–3528
Long TH, Lawce H, Durum C, Moore SR, Olson SB, Gatter K, Troxell ML (2015) The new equivocal: changes to HER2 FISH results when applying the 2013 ASCO/CAP guidelines. Am J Clin Pathol 144(2):253–262
Muller KE, Marotti JD, Memoli VA, Wells WA, Tafe LJ (2015) Impact of the 2013 ASCO/CAP HER2 guideline updates at an Academic Medical Center that performs primary HER2 FISH testing: increase in equivocal results and utility of reflex immunohistochemistry. Am J Clin Pathol 144(2):247–252
Varga Z, Noske A (2015) Impact of modified 2013 ASCO/CAP guidelines on HER2 testing in breast Cancer. One year experience. PLoS One 10(10):e0140652
Bethune GC, Veldhuijzen van Zanten D, MacIntosh RF, Rayson D, Younis T, Thompson K, Barnes PJ (2015) Impact of the 2013 American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 (HER2) testing of invasive breast carcinoma: a focus on tumours assessed as 'equivocal' for HER2 gene amplification by fluorescence in-situ hybridization. Histopathology 67(6):880–887
Lim TH, Lim AS, Thike AA, Tien SL, Tan PH (2016) Implications of the updated 2013 American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations on human epidermal growth factor receptor 2 gene testing using immunohistochemistry and fluorescence in situ hybridization for breast Cancer. Arch Pathol Lab Med 140(2):140–147
Stoss OC, Scheel A, Nagelmeier I, Schildhaus HU, Henkel T, Viale G, Jasani B, Untch M, Ruschoff J (2015) Impact of updated HER2 testing guidelines in breast cancer--re-evaluation of HERA trial fluorescence in situ hybridization data. Mod Pathol 28(12):1528–1534
Hanna WM, Slodkowska E, Lu FI, Nafisi H, Nofech-Mozes S (2017) Comparative analysis of human epidermal growth factor receptor 2 testing in breast Cancer according to 2007 and 2013 American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations. J Clin Oncol 35(26):3039–3045
Shah MV, Wiktor AE, Meyer RG, Tenner KS, Ballman KV, Green SJ, Sukov WR, Ketterling RP, Perez EA, Jenkins RB (2016) Change in pattern of HER2 fluorescent in situ hybridization (FISH) results in breast cancers submitted for FISH testing: experience of a reference laboratory using US Food and Drug Administration criteria and American Society of Clinical Oncology and College of American Pathologists Guidelines. J Clin Oncol 34(29):3502–3510
Polonia A, Leitao D, Schmitt F (2016) Application of the 2013 ASCO/CAP guideline and the SISH technique for HER2 testing of breast cancer selects more patients for anti-HER2 treatment. Virchows Arch 468(4):417–423
Murray C, D'Arcy C, Gullo G, Flanagan L, Quinn CM, Quinn CM (2018) Human epidermal growth factor receptor 2 testing by fluorescent in situ hybridization: positive or negative? ASCO/College of American Pathologists Guidelines 2007, 2013, and 2018. J Clin Oncol 36(35):3522–3523
Xu B, Shen J, Guo W, Zhao W, Zhuang Y, Wang L (2019) Impact of the 2018 ASCO/CAP HER2 guidelines update for HER2 testing by FISH in breast cancer. Pathol Res Pract 215(2):251–255
Wilkens L, Gerr H, Gadzicki D, Kreipe H, Schlegelberger B (2005) Standardised fluorescence in situ hybridisation in cytological and histological specimens. Virchows Arch 447(3):586–592
Starczynski J, Atkey N, Connelly Y, O'Grady T, Campbell FM, di Palma S, Wencyk P, Jasani B, Gandy M, Bartlett JM (2012) HER2 gene amplification in breast cancer: a rogues’ gallery of challenging diagnostic cases: UKNEQAS interpretation guidelines and research recommendations. Am J Clin Pathol 137(4):595–605
Dietel M, Ellis IO, Hofler H, Kreipe H, Moch H, Dankof A, Kolble K, Kristiansen G (2007) Comparison of automated silver enhanced in situ hybridisation (SISH) and fluorescence ISH (FISH) for the validation of HER2 gene status in breast carcinoma according to the guidelines of the American Society of Clinical Oncology and the College of American Pathologists. Virchows Arch 451(1):19–25
Shousha S, Peston D, Amo-Takyi B, Morgan M, Jasani B (2009) Evaluation of automated silver-enhanced in situ hybridization (SISH) for detection of HER2 gene amplification in breast carcinoma excision and core biopsy specimens. Histopathology 54(2):248–253
Bartlett JM, Campbell FM, Ibrahim M, Wencyk P, Ellis I, Kay E, Connolly Y, O'Grady A, Di Palma S, Starczynski J, Morgan JM, Jasani B, Miller K (2009) Chromogenic in situ hybridization: a multicenter study comparing silver in situ hybridization with FISH. Am J Clin Pathol 132(4):514–520
Papouchado BG, Myles J, Lloyd RV, Stoler M, Oliveira AM, Downs-Kelly E, Morey A, Bilous M, Nagle R, Prescott N, Wang L, Dragovich L, McElhinny A, Garcia CF, Ranger-Moore J, Free H, Powell W, Loftus M, Pettay J, Gaire F, Roberts C, Dietel M, Roche P, Grogan T, Tubbs R (2010) Silver in situ hybridization (SISH) for determination of HER2 gene status in breast carcinoma: comparison with FISH and assessment of interobserver reproducibility. Am J Surg Pathol 34(6):767–776
Koh YW, Lee HJ, Lee JW, Kang J, Gong G (2011) Dual-color silver-enhanced in situ hybridization for assessing HER2 gene amplification in breast cancer. Mod Pathol 24(6):794–800
Lee Y, Ryu Y, Jeong H, Chang H, Kim Y, Kim A (2012) Effectiveness of silver-enhanced in situ hybridization for evaluating HER2 gene status in invasive breast carcinoma: a comparative study. Arch Med Res 43(2):139–144
Park K, Han S, Kim JY, Kim HJ, Kwon JE, Gwak G (2012) Silver-enhanced in situ hybridization as an alternative to fluorescence in situ hybridization for assaying HER2 amplification in clinical breast Cancer. J Breast Cancer 14(4):276–282
Jacquemier J, Spyratos F, Esterni B, Mozziconacci MJ, Antoine M, Arnould L, Lizard S, Bertheau P, Lehmann-Che J, Fournier CB, Krieger S, Bibeau F, Lamy PJ, Chenard MP, Legrain M, Guinebretiere JM, Loussouarn D, Macgrogan G, Hostein I, Mathieu MC, Lacroix L, Valent A, Robin YM, Revillion F, Triki ML, Seaume A, Salomon AV, de Cremoux P, Portefaix G, Xerri L, Vacher S, Bieche I, Penault-Llorca F (2013) SISH/CISH or qPCR as alternative techniques to FISH for determination of HER2 amplification status on breast tumors core needle biopsies: a multicenter experience based on 840 cases. BMC Cancer 13:351
Lim SJ, Cantillep A, Carpenter PM (2013) Validation and workflow optimization of human epidermal growth factor receptor 2 testing using INFORM HER2 dual-color in situ hybridization. Hum Pathol 44(11):2590–2596
Unal B, Karaveli FS, Pestereli HE, Erdogan G (2013) Determination of HER2 gene amplification in breast cancer using dual-color silver enhanced in situ hybridization (dc- SISH) and comparison with fluorescence ISH (FISH). Asian Pac J Cancer Prev 14(10):6131–6134
Biserni GB, Engstrom MJ, Bofin AM (2016) HER2 gene copy number and breast cancer-specific survival. Histopathology 69(5):871–879
Press MF, Seoane JA, Curtis C, Quinaux E, Guzman R, Sauter G, Eiermann W, Mackey JR, Robert N, Pienkowski T, Crown J, Martin M, Valero V, Bee V, Ma Y, Villalobos I, Slamon DJ (2018) Assessment of ERBB2/HER2 status in HER2-equivocal breast cancers by FISH and 2013/2014 ASCO-CAP guidelines. JAMA Oncol 5(3):366–375
Ballard M, Jalikis F, Krings G, Schmidt RA, Chen YY, Rendi MH, Dintzis SM, Jensen KC, West RB, Sibley RK, Troxell ML, Allison KH (2017) 'Non-classical' HER2 FISH results in breast cancer: a multi-institutional study. Mod Pathol 30(2):227–235
Nitta H, Hauss-Wegrzyniak B, Lehrkamp M, Murillo AE, Gaire F, Farrell M, Walk E, Penault-Llorca F, Kurosumi M, Dietel M, Wang L, Loftus M, Pettay J, Tubbs RR, Grogan TM (2008) Development of automated brightfield double in situ hybridization (BDISH) application for HER2 gene and chromosome 17 centromere (CEN 17) for breast carcinomas and an assay performance comparison to manual dual color HER2 fluorescence in situ hybridization (FISH). Diagn Pathol 3:41
Hanna W, Nofech-Mozes S, Kahn HJ (2007) Intratumoral heterogeneity of HER2/neu in breast cancer--a rare event. Breast J 13(2):122–129
Andersson J, Linderholm B, Bergh J, Elmberger G (2004) HER-2/neu (c-erbB-2) evaluation in primary breast carcinoma by fluorescent in situ hybridization and immunohistochemistry with special focus on intratumor heterogeneity and comparison of invasive and in situ components. Appl Immunohistochem Mol Morphol 12(1):14–20
Polonia A, Eloy C, Pinto J, Braga AC, Oliveira G, Schmitt F (2017) Counting invasive breast cancer cells in the HER2 silver in-situ hybridization test: how many cells are enough? Histopathology 71(2):247–257
Polonia A, Oliveira G, Schmitt F (2017) Characterization of HER2 gene amplification heterogeneity in invasive and in situ breast cancer using bright-field in situ hybridization. Virchows Arch 471(5):589–598
Martins D, Sousa B, Lopes N, Gomes M, Veronese L, Albergaria A, Paredes J, Schmitt F (2011) Molecular phenotypes of matched in situ and invasive components of breast carcinomas. Hum Pathol 42(10):1438–1446
Tamimi RM, Baer HJ, Marotti J, Galan M, Galaburda L, Fu Y, Deitz AC, Connolly JL, Schnitt SJ, Colditz GA, Collins LC (2008) Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res 10(4):R67
Rakha EA, Reis-Filho JS, Baehner F, Dabbs DJ, Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani SR, Palacios J, Richardson AL, Schnitt SJ, Schmitt FC, Tan PH, Tse GM, Badve S (2010) Ellis IO breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res 12(4):207
Rakha EA, Ellis IO (2007) An overview of assessment of prognostic and predictive factors in breast cancer needle core biopsy specimens. J Clin Pathol 60(12):1300–1306
Dowsett M, Hanna WM, Kockx M, Penault-Llorca F, Ruschoff J, Gutjahr T, Habben K, van de Vijver MJ (2007) Standardization of HER2 testing: results of an international proficiency-testing ring study. Mod Pathol 20(5):584–591
Rakha EA, Pinder SE, Bartlett JM, Ibrahim M, Starczynski J, Carder PJ, Provenzano E, Hanby A, Hales S, Lee AH, Ellis IO (2015) Updated UK recommendations for HER2 assessment in breast cancer. J Clin Pathol 68(2):93–99
Tse CH, Hwang HC, Goldstein LC, Kandalaft PL, Wiley JC, Kussick SJ, Gown AM (2011) Determining true HER2 gene status in breast cancers with polysomy by using alternative chromosome 17 reference genes: implications for anti-HER2 targeted therapy. J Clin Oncol 29(31):4168–4174
Donaldson AR, Shetty S, Wang Z, Rivera CL, Portier BP, Budd GT, Downs-Kelly E, Lanigan CP, Calhoun BC (2017) Impact of an alternative chromosome 17 probe and the 2013 American Society of Clinical Oncology and College of American Pathologists guidelines on fluorescence in situ hybridization for the determination of HER2 gene amplification in breast cancer. Cancer 123(12):2230–2239
Cancer Genome Atlas N (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418):61–70
Ma CX, Bose R, Gao F, Freedman RA, Telli ML, Kimmick G, Winer E, Naughton M, Goetz MP, Russell C, Tripathy D, Cobleigh M, Forero A, Pluard TJ, Anders C, Niravath PA, Thomas S, Anderson J, Bumb C, Banks KC, Lanman RB, Bryce R, Lalani AS, Pfeifer J, Hayes DF, Pegram M, Blackwell K, Bedard PL, Al-Kateb H, Ellis MJC (2017) Neratinib efficacy and circulating tumor DNA detection of HER2 mutations in HER2 nonamplified metastatic breast Cancer. Clin Cancer Res 23(19):5687–5695
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MC, AC, CE, and AP: designed the research study; MC and AC: retrieved and analyzed the data and wrote the manuscript. AC: performed the laboratory work. CE, AP: conducted HER2 SISH interpretation, analyzed the data, wrote and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
This study has been performed in accordance with the national regulative law for the handling of biological specimens from tumor banks, being the samples exclusively available for research purposes in retrospective studies, as well as under the international Helsinki declaration. Ethical approval and informed consent were not required for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ethical Responsibilities of Authors Section
Virchows Archiv conforms to the ICMJE recommendation for qualification of authorship. The ICMJE recommends that authorship be based on the following 4 criteria:
• Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
• Drafting the work or revising it critically for important intellectual content; AND
• Final approval of the version to be published; AND
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
All individuals listed as co-authors of the manuscript must qualify for every one of the four criteria listed above. Should an individual’s contributions to the manuscript meet three of the criteria or fewer, then they should not be listed as a co-author on the manuscript; instead, their contributions should be acknowledged in the Acknowledgements section of the manuscript.
Electronic supplementary material
ESM 1
(PDF 357 kb)
Rights and permissions
About this article
Cite this article
Curado, M., Caramelo, A.S., Eloy, C. et al. What to expect from the 2018 ASCO/CAP HER2 guideline in the reflex in situ hybridization test of immunohistochemically equivocal 2+ cases?. Virchows Arch 475, 303–311 (2019). https://doi.org/10.1007/s00428-019-02567-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-019-02567-z