Abstract
The aim of this study is to assess the impact of changes of the 2013 ASCO/CAP guideline on the results of HER2 testing in breast cancer. A series of 916 primary invasive breast cancer cases, assessed as HER2 2+ by IHC in part using the 2007 and in part the 2013 ASCO/CAP criteria, was evaluated for HER2 amplification status by SISH and classified according to both 2007 and 2013 ASCO/CAP ISH guideline criteria. We observed a significant increase of HER2-positive cases (12.4 to 16.8 %) and a decrease of HER2-equivocal cases (3.6 to 0.7 %). Of the cases studied, 52.1 % fulfilled both criteria of HER2/CEP17 ratio and average HER2 copy number per cell to be classified as HER2-positive. Reclassification of the cases from before the introduction of the new ASCO/CAP guideline with the 2013 ISH criteria resulted in an increase of cases with a HER2-positive status (12.4 to 14.2 %) and in a decrease of HER2-equivocal cases (3.6 to 1.6 %). The 2013 ASCO/CAP guideline selects more patients for anti-HER2 targeted therapy, mostly based on the modifications of criteria to evaluate ISH-HER2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the western world, breast cancer (BC) is the most commonly diagnosed malignancy among women, representing about 30 % of all new cancer cases, and after lung cancer the second leading cause of cancer death [1, 2]. The current cancer care guidelines for BC recommend that estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2) status must be routinely determined in all patients with invasive BC, BC recurrence and BC metastases [3, 4]. These guidelines were published to help improve laboratory performance in the determination of these markers, which provide useful predictive information regarding response to targeted therapy.
HER2, located on the long arm of chromosome 17 (17q12), is amplified and/or overexpressed in about 15 to 20 % of invasive BC. Cases with a HER2-positive status represent a clinically important subset of BC associated with poor outcome but also with a high likelihood of response to HER2-targeted therapy [5–8]. Several studies have shown that anti-HER2 therapy given during and/or after chemotherapy results in a significant improvement in disease-free and overall survival [9–11]. Therefore, HER2 is a helpful marker for therapy decision making in patients with BC and appropriate evaluation of HER2 status ensures that the right patient receives the right treatment [3].
At present, HER2 protein expression is determined in BC samples by immunohistochemistry (IHC) resulting in three possible outcomes: negative (score 0 or 1+), equivocal (score 2+), and positive (score 3+). If the IHC result is equivocal, reflex testing should be performed on the same specimen using an alternative assay, such as in situ hybridization (ISH) [3].
The new 2013 ASCO/CAP (American Society of Clinical Oncology/College of American Pathologists) guideline has updated the definition of HER2-positive status by modifying both IHC and ISH criteria, reducing the thresholds for post-analytical interpretation of positive results in comparison with the previous 2007 ASCO/CAP guideline [3, 12]. In the new guideline, a HER2 score 3+ is defined as the presence of complete and intense membrane staining, in at least 10 % of tumor cells [3]. This represented a return to the IHC criteria originally used in the first-generation clinical trials [13]. A similar approach was used regarding ISH criteria (see below).
In this study, we aim to compare the impact of the change from the 2007 to the 2013 ASCO/CAP guidelines on the result of HER2 amplification test in BC.
Materials and methods
Cases
A series of 916 primary invasive BC cases was retrieved from the archives of Ipatimup Diagnostics, including cases evaluated 1 year before (494 cases from November 2012 to October 2013) and 1 year after (422 cases from December 2013 to November 2014) the publication of the new ASCO/CAP guideline (November 2013). All BC cases (core biopsies and surgical specimens) had been fixed in 10 % formalin, embedded in paraffin, and were referred to our institution (national reference center for HER2 ISH) with an equivocal IHC HER2 score (2+) to perform the HER2 amplification assay with a HER2 Dual ISH DNA Probe with a silver marker (SISH).
Ethics approval and informed consent were not required for this study.
SISH
SISH testing was performed on 3-μm sections of formalin-fixed, paraffin-embedded tissue of all BC cases using dual-hapten, dual-color ISH (DDISH). The dual-probe assay (INFORM HER2 Dual ISH DNA Probe Cocktail Assay; Ventana Medical Systems, Inc., Tucson, Arizona) contains a HER2 locus-specific probe and a control probe specific for the centromere of chromosome 17 (CEP17). The entire procedure was carried out on an automated staining system (Ventana BenchMarkTM XT Staining System) according to the manufacturer’s instructions. Positive and negative controls were used for each staining run.
Evaluation of the results included recording the number of HER2 and CEP17 signals in at least 20 nuclei in two different areas. The samples were classified by pathologists (AP and FS) according to the 2007 and 2013 ISH criteria for HER2 amplification. Corresponding hematoxylin and eosin staining were used for the identification of the invasive component of the tumor.
The 2007 ASCO/CAP guideline defines HER2 amplification as positive at a HER2/CEP17 ratio >2.2, equivocal at a HER2/CEP17 ratio ≤2.2 and ≥1.8, and negative at a HER2/CEP17 ratio <1.8 [12]. The 2013 ASCO/CAP guideline establishes the result of HER2 amplification as positive at a HER2/CEP17 ratio ≥2.0 or a HER2/CEP17 ratio <2.0 and an average HER2 copy number per cell of ≥6.0, equivocal when HER2/CEP17 ratio <2.0 and average of HER2 copy number ≥4.0 and <6.0 signals per cell, and negative when HER2/CEP17 ratio <2.0 and average HER2 copy number of <4.0 signals per cell [3].
Chromosome 17 polysomy was defined as an average of ≥3.0 CEP17 signals per cell [14]. Genomic heterogeneity was also recorded and considered present if a discrete population of tumor cells with HER2 amplification represented at least 10 % of the total tumor cell population [3].
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 21.0 for Windows. The Pearson’s chi-square (χ 2) test and McNemar test were used for comparison of qualitative variables and the t test for quantitative variables. The level of significance was set at p < 0.05.
Results
The 916 BC cases concerned 97.2 % women and 1.2 % men. The age ranged from 24 to 103 years, with a median age at diagnosis of 59 years.
The distribution of gender, age, HER2/CEP17 ratio, and average HER2 copy number per cell were not statistically different between the pre- and post-new guideline cases (Table 1 and Fig. 1). The only parameters that changed significantly with the new guideline were the average CEP17 copy number per cell and the presence of chromosome 17 polysomy (4.1 to 0.9 %; p = 0.003; Table 1).
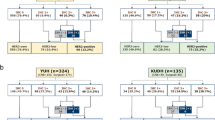
Table 2 and Fig. 2a present the results of HER2 test performed on the pre-new guideline cases (using the ISH criteria from the 2007 ASCO/CAP guideline): 415 cases (84.0 %) HER2-negative, 18 cases (3.6 %) HER2-equivocal, and 61 cases (12.4 %) HER2-positive. Table 2 and Fig. 2b present the results of HER2 test performed on the post-new guideline cases (using the ISH criteria from the 2013 ASCO/CAP guideline): 348 cases (82.5 %) HER2-negative, 3 cases (0.7 %) HER2-equivocal, and 71 cases (16.8 %) HER2-positive. The differences are statistically significant (Table 2—statistical analysis A; p = 0.003). We also observed that 52.1 % of the positive cases (37/71) fulfill both criteria of HER2/CEP17 ratio ≥2.0 and average of HER2 copy number per cell ≥6.0 (Table 3 and Fig. 2b). We furthermore classified the pre- and post-new guideline cases using the 2007 and 2013 ISH criteria and observed a slight but non-significant increase in HER2-positive cases and a similar decrease in HER2-equivocal cases (Table 2—statistical analysis B and C; p = 0.185 and p = 0.261, respectively).
In the reclassification of the two case series using the 2007 and 2013 ISH criteria, we observed an increase in HER2-positive cases (12.4 to 14.2 % and 15.9 to 16.8 %, respectively) and a decrease in HER2-equivocal cases (3.6 to 1.6 % and 2.4 to 0.7 %, respectively). This was statistically significant in the pre-new guideline cases (Table 2—statistical analysis D; p = 0.011) and near significant in post-new guideline cases (Table 2—statistical analysis E; p = 0.071).
In the pre-new guideline cases, the 2013 ISH criteria reclassified 22 (4.5 %) of the cases, 9 as HER2-positive (from HER2-equivocal), 7 as HER2-negative (from HER2-equivocal), and 6 as HER2-equivocal (from HER2-negative). All HER2-positive cases according to the 2007 guideline remained HER2 positive with the 2013 guideline (Table 4).
In the post-new guideline cases, genomic heterogeneity was detected in 0.47 % of the cases (2/422), the proportion of HER2 amplified cells varying between 25 and 40 % of the tumor cell population.
Discussion
Our center (Ipatimup) is one of the reference centers for SISH test of BC in Portugal. In our center, the introduction of the updated ASCO/CAP guideline for HER2 test by SISH resulted in a significant increase of positive cases (12.4 to 16.8 %) and decrease of equivocal cases (3.6 to 0.7 %).
Several studies recently reported an increase of HER2-positive cases evaluated by FISH but also an increase of HER2-equivocal cases with the introduction of 2013 ASCO/CAP guideline [15–19]. However, the study by the group of Garbar et al. had results similar to ours using FISH, with an increase of HER2-positive cases and a slight decrease in HER2-equivocal cases [20]. The explanation for these differences is not clear, but this might be related to the number of cases, pre-analytical conditions, and different ISH platforms. We did not review centrally the IHC performed externally, which might explain the decrease in equivocal cases in comparison with recent literature.
As yet, the published concordance rates between SISH and FISH vary between 92 and 99 %, the majority fulfilling the ASCO/CAP validation requirement of a concordance rate exceeding 95 % (Table 5) [21–30]. However, the requirement in the 2013 ASCO/CAP guideline to determine the average of HER2 copy number (first applied to bright field ISH and now applied to the FISH test) introduces a problem that did not exist previously. Autofluorescence in FISH might result in overestimation of both HER2 and CEP17 signals, resulting in HER2/CEP17 ratios below 2.0 and average of HER2 copy numbers above 4 per cell and an increase of equivocal HER2 results [31, 32]. If an increase of HER2-equivocal cases by FISH and a decrease of HER2-equivocal cases by SISH is confirmed, the concordance rate of these two ISH tests might decrease to under 95 %. This would open up the question which of these techniques provides the most reliable information on HER2 amplification status.
For nearly half of the cases studied (52.1 %), both criteria (HER2/CEP17 ratio and average of HER2 copy number per cell) were fulfilled to allow them to be classified as HER2-positive. This is particularly relevant given the fact that half the cases would be excluded from targeted therapy if HER2 amplification would be evaluated using just the HER2 probe (as some methods do).
Classification of the pre-new guideline and post-new guideline case series with the 2007 and 2013 criteria did not result in significant changes in the HER2 test results. This suggests that modifying the threshold in IHC, from 30 to 10 % of cells with moderate staining, had little effect on the HER2 amplification test results. Lee et al. found that cases with equivocal IHC (score 2+) in 10–30 % of the cells had a probability of being amplified of 5–12 % [33]. It is then not surprising that inclusion of these cases does not significantly change the HER2 amplification test results.
In contrast, classification of pre-new guideline and post-new guideline cases with different ISH criteria (2007 and 2013) resulted in significant changes in HER2 amplification test results. Our findings suggest that the 2013 modified ISH criteria had a stronger impact on the test results than the modified IHC criteria. We found that the 2013 ISH criteria resulted in reclassification of 4.5 % of the cases. Other publications have shown a reclassification rate of up to 15 % of cases [16, 17].
Polysomy of chromosome 17 changed from 4.1 to 0.9 % with the introduction of the 2013 ASCO/CAP guideline, which is probably due to modification of the definition of equivocal IHC HER2 staining (score 2+) rather than a change in the biology of the tumors. Several studies have shown that polysomy of chromosome 17 (measured on the basis of CEP17) varies between 3 and 49 % of the cases, depending on the definitions of polysomy and on the method used [12, 14, 34, 35]. The approach is based on the notion that CEP17 copy number is a surrogate marker for chromosome 17 copy number. However, molecular karyotyping has revealed that an increased CEP17 signal number is usually due to gain of the pericentromeric region rather than to duplication of the entire chromosome [36–41]. CEP17 might therefore not be a good marker for polysomy 17, making true polysomy 17 probably a rare event in BC. Nevertheless, CEP17 amplification can still be the cause of misleading HER2 amplification and false-negative test results, excluding patients from anti-HER2 targeted therapy [34].
Tumors with polysomy 17 are thought to be different from non-HER2 amplified tumors, associated with a more aggressive clinical behavior and not responsive to conventional therapy [14, 42]. However, in BC, the relationship between polysomy of chromosome 17 and the response to anti-HER2 therapy remains to be determined [43–45].
We found the presence of genomic heterogeneity to be rare as observed in just 0.47 % of cases. Several studies have addressed this issue in the past and reported genomic heterogeneity in 5 to 40 % of BC cases [14, 46–49]. Studies on the relationship between genomic heterogeneity and prognosis have shown that tumors with a HER2 amplification in at least 30 % of the cells have a reduced disease-free survival [48, 49]. However, the definition of genomic heterogeneity has also changed from individual cells (between 5 and 50 % of tumor cells with HER2 amplification) to discrete populations of tumor cells (at least 10 % of the total tumor cell population with HER2 amplification) [3, 50]. Additional work is needed to determine the prevalence of genomic heterogeneity with this new definition and the response to anti-HER2 targeted therapy in these patients.
In conclusion, we show that the new HER2 guideline results in an increased number of HER2-positive and a decreased number of HER2-equivocal cases using the SISH technique, primarily because of modifications of ISH rather than of IHC criteria. As a consequence, the 2013 ASCO/CAP guideline selects more patients for anti-HER2 targeted therapy.
References
DeSantis C, Ma J, Bryan L, Jemal A (2013) Breast cancer statistics, 2013. CA Cancer J Clin 64(1):52–62
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134(7):e48–72
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182
Press MF, Pike MC, Chazin VR, Hung G, Udove JA, Markowicz M, Danyluk J, Godolphin W, Sliwkowski M, Akita R et al (1993) Her-2/neu expression in node-negative breast cancer: direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease. Cancer Res 53(20):4960–4970
Press MF, Bernstein L, Thomas PA, Meisner LF, Zhou JY, Ma Y, Hung G, Robinson RA, Harris C, El-Naggar A, Slamon DJ, Phillips RN, Ross JS, Wolman SR, Flom KJ (1997) HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol 15(8):2894–2904
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792
Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ (1999) Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17(9):2639–2648
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353(16):1659–1672
Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20(3):719–726
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145
Shah S, Chen B (2010) Testing for HER2 in breast cancer: a continuing evolution. Patholog Res Int 2011:903202
Hanna WM, Ruschoff J, Bilous M, Coudry RA, Dowsett M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G (2013) HER2 in situ hybridization in breast cancer: clinical implications of polysomy 17 and genetic heterogeneity. Mod Pathol 27(1):4–18
Long TH, Lawce H, Durum C, Moore SR, Olson SB, Gatter K, Troxell ML The New Equivocal: Changes to HER2 FISH Results When Applying the (2013) ASCO/CAP guidelines. Am J Clin Pathol 144(2):253–262
Pu X, Shi J, Li Z, Feng A, Ye Q (2015) Comparison of the 2007 and 2013 ASCO/CAP evaluation systems for HER2 amplification in breast cancer. Pathol Res Pract 211(6):421–425
Bethune GC, Veldhuijzen van Zanten D, MacIntosh RF, Rayson D, Younis T, Thompson K, Barnes PJ (2015) Impact of the 2013 American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 (HER2) testing of invasive breast carcinoma: a focus on tumours assessed as ‘equivocal’ for HER2 gene amplification by fluorescence in-situ hybridization. Histopathology. doi:10.1111/his.12723
Muller KE, Marotti JD, Memoli VA, Wells WA, Tafe LJ Impact of the (2013) ASCO/CAP HER2 guideline updates at an academic medical center that performs primary HER2 FISH testing: increase in equivocal results and utility of reflex immunohistochemistry. Am J Clin Pathol 144(2):247–252
Varga Z, Noske A (2015) Impact of modified 2013 ASCO/CAP guidelines on HER2 testing in breast cancer. One year experience. PLoS One 10(10):e0140652
Garbar C, Savoye AM, Mascaux C, Brabencova E, Cure H (2014) The human epidermal growth factor receptor 2 screening tests for breast cancer suggested by the new updated recommendation of the American Society of Clinical Oncology/College of American Pathologists will involve a rise of the in-situ hybridization tests for the European laboratories of pathology. ISRN Oncol 2014:793695
Dietel M, Ellis IO, Hofler H, Kreipe H, Moch H, Dankof A, Kolble K, Kristiansen G (2007) Comparison of automated silver enhanced in situ hybridisation (SISH) and fluorescence ISH (FISH) for the validation of HER2 gene status in breast carcinoma according to the guidelines of the American Society of Clinical Oncology and the College of American Pathologists. Virchows Arch 451(1):19–25
Shousha S, Peston D, Amo-Takyi B, Morgan M, Jasani B (2009) Evaluation of automated silver-enhanced in situ hybridization (SISH) for detection of HER2 gene amplification in breast carcinoma excision and core biopsy specimens. Histopathology 54(2):248–253
Bartlett JM, Campbell FM, Ibrahim M, Wencyk P, Ellis I, Kay E, Connolly Y, O’Grady A, Di Palma S, Starczynski J, Morgan JM, Jasani B, Miller K (2009) Chromogenic in situ hybridization: a multicenter study comparing silver in situ hybridization with FISH. Am J Clin Pathol 132(4):514–520
Papouchado BG, Myles J, Lloyd RV, Stoler M, Oliveira AM, Downs-Kelly E, Morey A, Bilous M, Nagle R, Prescott N, Wang L, Dragovich L, McElhinny A, Garcia CF, Ranger-Moore J, Free H, Powell W, Loftus M, Pettay J, Gaire F, Roberts C, Dietel M, Roche P, Grogan T, Tubbs R (2010) Silver in situ hybridization (SISH) for determination of HER2 gene status in breast carcinoma: comparison with FISH and assessment of interobserver reproducibility. Am J Surg Pathol 34(6):767–776
Koh YW, Lee HJ, Lee JW, Kang J, Gong G (2011) Dual-color silver-enhanced in situ hybridization for assessing HER2 gene amplification in breast cancer. Mod Pathol 24(6):794–800
Lee Y, Ryu Y, Jeong H, Chang H, Kim Y, Kim A (2012) Effectiveness of silver-enhanced in situ hybridization for evaluating HER2 gene status in invasive breast carcinoma: a comparative study. Arch Med Res 43(2):139–144
Park K, Han S, Kim JY, Kim HJ, Kwon JE, Gwak G (2012) Silver-enhanced in situ hybridization as an alternative to fluorescence in situ hybridization for assaying HER2 amplification in clinical breast cancer. J Breast Cancer 14(4):276–282
Jacquemier J, Spyratos F, Esterni B, Mozziconacci MJ, Antoine M, Arnould L, Lizard S, Bertheau P, Lehmann-Che J, Fournier CB, Krieger S, Bibeau F, Lamy PJ, Chenard MP, Legrain M, Guinebretiere JM, Loussouarn D, Macgrogan G, Hostein I, Mathieu MC, Lacroix L, Valent A, Robin YM, Revillion F, Triki ML, Seaume A, Salomon AV, de Cremoux P, Portefaix G, Xerri L, Vacher S, Bieche I, Penault-Llorca F (2013) SISH/CISH or qPCR as alternative techniques to FISH for determination of HER2 amplification status on breast tumors core needle biopsies: a multicenter experience based on 840 cases. BMC Cancer 13:351
Lim SJ, Cantillep A, Carpenter PM (2013) Validation and workflow optimization of human epidermal growth factor receptor 2 testing using INFORM HER2 dual-color in situ hybridization. Hum Pathol 44(11):2590–2596
Unal B, Karaveli FS, Pestereli HE, Erdogan G (2013) Determination of HER2 gene amplification in breast cancer using dual-color silver enhanced in situ hybridization (dc-SISH) and comparison with fluorescence ISH (FISH). Asian Pac J Cancer Prev 14(10):6131–6134
Wilkens L, Gerr H, Gadzicki D, Kreipe H, Schlegelberger B (2005) Standardised fluorescence in situ hybridisation in cytological and histological specimens. Virchows Arch 447(3):586–592
Starczynski J, Atkey N, Connelly Y, O’Grady T, Campbell FM, di Palma S, Wencyk P, Jasani B, Gandy M (2012) Bartlett JM HER2 gene amplification in breast cancer: a rogues’ gallery of challenging diagnostic cases: UKNEQAS interpretation guidelines and research recommendations. Am J Clin Pathol 137(4):595–605
Lee AH, Key HP, Bell JA, Hodi Z, Ellis IO (2011) Breast carcinomas with borderline (2+) HER2 immunohistochemistry: percentage of cells with complete membrane staining for HER2 and the frequency of HER2 amplification. J Clin Pathol 64(6):490–492
Moelans CB, Reis-Filho JS, van Diest PJ (2011) Implications of rarity of chromosome 17 polysomy in breast cancer. Lancet Oncol 12(12):1087–1089
Jiang H, Bai X, Zhao T, Zhang C, Zhang X (2014) Fluorescence in situ hybridization of chromosome 17 polysomy in breast cancer using thin tissue sections causes the loss of CEP17 and HER2 signals. Oncol Rep 32(5):1889–1896
Yeh IT, Martin MA, Robetorye RS, Bolla AR, McCaskill C, Shah RK, Gorre ME, Mohammed MS, Gunn SR (2009) Clinical validation of an array CGH test for HER2 status in breast cancer reveals that polysomy 17 is a rare event. Mod Pathol 22(9):1169–1175
Marchio C, Lambros MB, Gugliotta P, Di Cantogno LV, Botta C, Pasini B, Tan DS, Mackay A, Fenwick K, Tamber N, Bussolati G, Ashworth A, Reis-Filho JS, Sapino A (2009) Does chromosome 17 centromere copy number predict polysomy in breast cancer? A fluorescence in situ hybridization and microarray-based CGH analysis. J Pathol 219(1):16–24
Gunn S, Yeh IT, Lytvak I, Tirtorahardjo B, Dzidic N, Zadeh S, Kim J, McCaskill C, Lim L, Gorre M, Mohammed M (2010) Clinical array-based karyotyping of breast cancer with equivocal HER2 status resolves gene copy number and reveals chromosome 17 complexity. BMC Cancer 10:396
Staaf J, Jonsson G, Ringner M, Vallon-Christersson J, Grabau D, Arason A, Gunnarsson H, Agnarsson BA, Malmstrom PO, Johannsson OT, Loman N, Barkardottir RB, Borg A (2010) High-resolution genomic and expression analyses of copy number alterations in HER2-amplified breast cancer. Breast Cancer Res 12(3):R25
Varga Z, Tubbs RR, Wang Z, Sun Y, Noske A, Kradolfer D, Bosshard G, Jochum W, Moch H, Ohlschlegel C (2011) Co-amplification of the HER2 gene and chromosome 17 centromere: a potential diagnostic pitfall in HER2 testing in breast cancer. Breast Cancer Res Treat 132(3):925–935
Moelans CB, de Weger RA, van Diest PJ (2009) Absence of chromosome 17 polysomy in breast cancer: analysis by CEP17 chromogenic in situ hybridization and multiplex ligation-dependent probe amplification. Breast Cancer Res Treat 120(1):1–7
Vanden Bempt I, Van Loo P, Drijkoningen M, Neven P, Smeets A, Christiaens MR, Paridaens R, De Wolf-Peeters C (2008) Polysomy 17 in breast cancer: clinicopathologic significance and impact on HER-2 testing. J Clin Oncol 26(30):4869–4874
Hofmann M, Stoss O, Gaiser T, Kneitz H, Heinmoller P, Gutjahr T, Kaufmann M, Henkel T, Ruschoff J (2008) Central HER2 IHC and FISH analysis in a trastuzumab (Herceptin) phase II monotherapy study: assessment of test sensitivity and impact of chromosome 17 polysomy. J Clin Pathol 61(1):89–94
Orlando L, Del Curto B, Gandini S, Ghisini R, Pietri E, Torrisi R, Balduzzi A, Cardillo A, Dellapasqua S, Veronesi P, Viale G, Goldhirsch A, Colleoni M (2008) Topoisomerase IIalpha gene status and prediction of pathological complete remission after anthracycline-based neoadjuvant chemotherapy in endocrine non-responsive Her2/neu-positive breast cancer. Breast 17(5):506–511
Krishnamurti U, Hammers JL, Atem FD, Storto PD, Silverman JF (2009) Poor prognostic significance of unamplified chromosome 17 polysomy in invasive breast carcinoma. Mod Pathol 22(8):1044–1048
Allison KH, Dintzis SM, Schmidt RA (2011) Frequency of HER2 heterogeneity by fluorescence in situ hybridization according to CAP expert panel recommendations: time for a new look at how to report heterogeneity. Am J Clin Pathol 136(6):864–871
Chang MC, Malowany JI, Mazurkiewicz J, Wood M (2012) ‘Genetic heterogeneity’ in HER2/neu testing by fluorescence in situ hybridization: a study of 2,522 cases. Mod Pathol 25(5):683–688
Seol H, Lee HJ, Choi Y, Lee HE, Kim YJ, Kim JH, Kang E, Kim SW, Park SY (2012) Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod Pathol 25(7):938–948
Bartlett AI, Starcyznski J, Robson T, Maclellan A, Campbell FM, van de Velde CJ, Hasenburg A, Markopoulos C, Seynaeve C, Rea D, Bartlett JM (2011) Heterogeneous HER2 gene amplification: impact on patient outcome and a clinically relevant definition. Am J Clin Pathol 136(2):266–274
Vance GH, Barry TS, Bloom KJ, Fitzgibbons PL, Hicks DG, Jenkins RB, Persons DL, Tubbs RR, Hammond ME (2009) Genetic heterogeneity in HER2 testing in breast cancer: panel summary and guidelines. Arch Pathol Lab Med 133(4):611–612
Acknowledgments
We thank Drª Orquídea Ribeiro for assistance in statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing of interests.
Rights and permissions
About this article
Cite this article
Polónia, A., Leitão, D. & Schmitt, F. Application of the 2013 ASCO/CAP guideline and the SISH technique for HER2 testing of breast cancer selects more patients for anti-HER2 treatment. Virchows Arch 468, 417–423 (2016). https://doi.org/10.1007/s00428-016-1903-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-016-1903-3