Abstract
The tripartite motif protein tripartite motif-containing 24 (TRIM24) is involved in human cancer progression. However, the expression pattern and biological roles of TRIM24 in human gastric cancer have not been studied. Here, we report that expression of TRIM24 protein was upregulated in 65 of 133 gastric cancer specimens. TRIM24 upregulation positively correlated with clinical stage, local invasion, and poor patient prognosis. We overexpressed TRIM24 by transfection in SGC-7901 cells and used an siRNA strategy to knock down TRIM24 in MKN-1 cells. MTT and colony formation assays showed that transfection of TRIM24 plasmid accelerated, while its depletion inhibited cell proliferation rate. TRIM24 overxpression also induced chemoresistance to 5-FU in gastric cancer cells. Further analysis showed that TRIM24 overexpression upregulated cyclin D1 and Akt phosphorylation. Akt inhibitor LY294002 reversed the role of TRIM24 on chemoresistance. In conclusion, our study shows that TRIM24 is overexpressed in human gastric cancer and accelerates cell growth as well as induce chemoresistance, possibly through the Akt pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the third most lethal cancer in male and the fifth in female individuals [1]. Despite improved prognosis resulting from earlier diagnosis, radical surgery, and development of adjuvant therapy, the 5-year survival rate still remains below 30 % [2]. Oncogenes and tumor suppressor genes have a vital role in the development and progression of gastric cancer, and genetic heterogeneity has been proven to influence the prognosis markedly. Therefore, it is important to identify novel molecular mediators conferring malignant potential to gastric cancer cells that may be used as tumor markers in predicting risk of gastric cancer progression [3–5].
TRIM24 is also known as transcription intermediary factor 1-alpha (TIF1α) and was identified as a coregulator of retinoid signaling [6–8]. Aberrant expression of TRIM24 might promote tumor development. TRIM24 is a target of chromosomal translocations to form oncogenic fusion proteins in acute promyelocytic leukemia, papillary thyroid carcinoma, and myeloproliferative syndrome [9–11]. TRIM24 was reported to bind chromatin and estrogen receptor to activate estrogen-dependent genes associated with cellular proliferation and tumor development [12, 13]. Elevated expression of TRIM24 promotes progression of prostate cancer and negatively correlates with survival of breast cancer patients [13, 14]. TRIM24 is overexpressed in nonsmall cell lung cancer, and its depletion inhibited cell growth [15]. TRIM24 accelerates malignant progression and serves as a prognostic factor in glioma [16]. These findings suggest that TRIM24 is an importnat oncogene in tumor development.
In the present study, we examined expression pattern of TRIM24 in 133 gastric cancer specimens and analyzed its correlation with clinicopathological factors. We also investigated its effects on proliferation and chemoresistance in gastric cancer cell lines.
Materials and methods
Patients and specimens
The study protocol was approved by the institutional reviewer board of China Medical University. Tumor tissue specimens of 133 cases of primary gastric cancer and 20 adjacent normal gastric tissue samples were obtained from patients diagnosed with gastric cancer who underwent resection in the First Affiliated Hospital of China Medical University between 2006 and 2010. The histological diagnosis was evaluated on sections stained with hematoxylin and eosin according to the World Health Organization (WHO) classification guidelines. Clinical and histopathological data including histopathological diagnosis and tumor grade were extracted from medical records. All cases in this study received radical total or subtotal gastrectomy with D2/D3 lymph node dissection. Pathological examination revealed no tumor involvement of the resection margins in surgical specimens (R0: no residual tumor based on R category in AJCC classification). None of our study patients had received preoperative chemotherapy or radiotherapy. After surgery, patients received four cycles or more of chemotherapy (chemotherapy regimens: FOLFOX4 program).
Cell culture and transfection
MKN-1 and SGC-7901 cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in DMEM (Invitrogen, Carlsbad, CA, USA) containing 10 % fetal calf serum (Invitrogen), 100 IU/ml penicillin (Sigma, St. Louis, MO, USA), and 100 μg/ml streptomycin (Sigma). Cells were grown on sterilized culture dishes and were passaged every 2 days with 0.25 % trypsin (Invitrogen).
The plasmid of pCMV6-TRIM24 was purchased from Origene (Origene, Rockville, USA). Plasmid was transfected into cells using Attractene Transfection (Qiagen, Hilden, Germany). Empty vector was used as a negative control. Cells were harvested 48 h later after transfection.
On-TargetPlus SMARTpool small interfering RNA (siRNA) for TRIM24 (M-005387-03-0005) and ON-TARGETplus Non-targeting siRNA #1 (D-001810-01-20) were purchased from Dharmacon. The cells were transfected with siRNA using the DharmaFECT 1 (0.20 μl/well; ThermoFisher Scientific) according to the manufacturer’s protocol.
Immunohistochemistry
Surgically excised tumor specimens were fixed with 10 % neutral formalin and embedded in paraffin, and 4-μm-thick sections were prepared. Immunostaining was performed using the avidin–biotin–peroxidase complex method (Ultrasensitive™, MaiXin, Fuzhou, China). The sections were deparaffinized in xylene, rehydrated with graded alcohol, and then boiled in 0.01 M citrate buffer (pH 6.0) for 2 min in an autoclave. Hydrogen peroxide (0.3 %) was applied to block endogenous peroxide activity, and the sections were incubated with normal goat serum to reduce nonspecific binding. Tissue sections were incubated with TRIM24 rabbit polyclonal antibody (1:150 dilution; Proteintech) at 4 °C overnight. Rabbit immunoglobulin (at the same concentration as for the antigen-specific antibody) was used as a negative control. Prostate cancer specimens were used as positive control, which were found TRIM24 staining positive in a previous study [15]. Biotinylated goat antirabbit serum IgG was used as a secondary antibody. After washing, the sections were incubated with streptavidin–biotin conjugated with horseradish peroxidase, and the peroxidase reaction was developed with 3, 3′-diaminobenzidine tetrahydrochloride. Counterstaining with hematoxylin was performed, and the sections were dehydrated in ethanol before mounting.
Two independent blinded investigators examined all tumor slides randomly. Five views were examined per slide, and 100 cells were observed per view at ×400 magnification. Immunostaining of TRIM24 was scored on a semiquantitative scale by evaluating the intensity and percentage of tumor cells according to previous studies [15]. Nuclear immunostaining in tumor cells was considered as positive staining. We counted 400 tumor cells and calculated the percentage of positively stained cells. The intensity of TRIM24 staining was scored as 0 (no signal), 1 (moderate), and 2 (strong). Percentage scores were assigned as 1, 1–25; 2, 26–50; 3, 51–75; and 4, 76–100 %. The scores of each tumor sample were multiplied to give a final score of 0–8; tumor samples scored 4–8 were considered as TRIM24 upregulated.
Quantitative real-time PCR (SYBR green method)
Quantitative real-time PCR was performed using SYBR Green PCR master mix (Applied Biosystems) in a total volume of 20 μl on 7900HT Fast Real-Time PCR System (Applied Biosystems) as follows: 95 °C for 30 s, 40 cycles of 95 °C for 5 s, and 60 °C for 30 s. A dissociation step was performed to generate a melting curve to confirm the specificity of the amplification. B-Actin was used as the reference gene. The relative levels of gene expression were represented as ΔCt = Ct gene – Ct reference, and the fold change of gene expression was calculated by the 2-ΔΔCt method. Experiments were repeated in triplicate. The primer sequences are as follows: TRIM24 forward, 5′ CGCCACCCAAGTTGGAGT 3′, TRIM24 reverse, 5′ GCTGGGAACCTCAGTAGTGTCCT 3′; β-actin forward, 5′ ATAGCACAGCCTGGATAGCAACGTAC 3′, β-actin reverse, 5′ CACCTTCTACAATGAGCTGCGTGTG 3′.
Western blot analysis
Total proteins from cells were extracted in lysis buffer (Pierce, Rockford, IL, USA), quantified using the Bradford method, and 50 μg protein samples were separated by SDS-PAGE. Samples were transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA) and incubated overnight at 4 °C with antibody against TRIM24 (1: 500; Proteintech, Chicago, USA), p-Akt, Akt, cyclin D1 (1:1000, Cell Signaling Technology, Boston, USA), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:1000; Santa Cruz, USA). After incubation with peroxidase-coupled antimouse/rabbit IgG (1:1000, Cell Signaling Technology, USA) at 37 °C for 2 h, bound proteins were visualized using ECL (Pierce) and detected using a DNR BioImaging System (DNR, Jerusalem, Israel). Relative protein levels were quantified using GAPDH as loading control.
Colony formation and MTT assays
For colony formation assay, cells were transfected with plasmid for 48 h and plated into three 6-cm cell culture dishes (1000 per dish). Cells were incubated for 12 days in medium containing 10 % FBS. Plates were washed with PBS and stained with Giemsa. The number of colonies with more than 50 cells was counted. The colonies were manually counted using a microscope.
For 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, 24 h after transfection cells were plated in 96-well plates at a concentration of approximately 3000 cells per well and cultured for 5 days. For quantitation of cell viability, 20 μl of 5 mg/ml MTT (thiazolyl blue) solution was added to each well and incubated for 4 h at 37 °C. The medium was removed from each well, and the resulting MTT formazan was solubilized in 150 μl of DMSO. Each solution was measured spectrophotometrically at 490 nm.
Statistical analysis
SPSS version 11.5 for Windows was used for all statistical analyses. A χ 2 test was used to examine possible correlations between TRIM24 expression and clinicopathological factors. The Kaplan–Meier method was used to estimate the probability of patient survival, and differences in the survival of subgroups of patients were compared using Mantel’s log-rank test. Student’s t test was used to compare densitometry data on focus numbers between control and TRIM24-transfected cells. All p values are based on a two-sided statistical analysis, and p < 0.05 was considered to indicate statistical significance.
Results
Expression pattern and clinical significance of TRIM24 in gastric cancer tissue
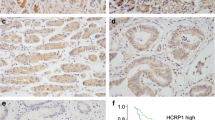
We examined TRIM24 protein expression in 133 gastric cancer specimens (including 110 tubular adenocarcinomas, 5 papillary adenocarcinomas, 12 mucinous adenocarcinomas, and 6 poorly cohesive adenocarcinomas) and 20 samples of normal gastric mucosa (Fig. 1). In normal gastric mucosa, cytoplasm of differentiated surface epithelia and mucosal glands showed weak or negative staining. Sixty-five of 133 primary gastric cancers showed TRIM24 upregulation, with immunoreactivity located in the nuclear compartment of carcinoma cells. Correlations between TRIM24 upregulation and clinicopathological characteristics are shown in Table 1. TRIM24 upregulation in the primary gastric cancer correlated significantly with advanced clinical stage (p = 0.0104). The rate of TRIM24 upregulation in the T3–T4 group was higher than that in the T1–T2 group (p = 0.0092). In addition, significant positive correlation was observed between TRIM24 upregulation and tumor relapse (p = 0.0003). There was no significant correlation between TRIM24 levels with other parameters as listed in Table 1. Kaplan–Meier analysis and the log-rank test were used to calculate the effect of TRIM24 expression on patient survival. The log-rank test showed that overall survival was significantly different between these two groups. Patients with low TRIM24 expression had longer overall survival, whereas those with high TRIM24 expression had shorter survival (Fig. 1g, log-rank, p < 0.0001).
Expression pattern of TRIM24 protein in gastric cancer tissues. a Negative nuclear TRIM24 staining in normal gastric tissue. b Negative nuclear TRIM24 staining in a case of papillary adenocarcinoma. c Strong nuclear TRIM24 expression in a case of tubular adenocarcinoma. d Negative nuclear staining of TRIM24 in a case of tubular adenocarcinoma. e Positive TRIM24 expression in a case of poorly cohesive adenocarcinoma (signet ring cell carcinoma). f Negative TRIM24 expression in a case of mucinous adenocarcinoma (magnification, ×400). g TRIM24 upregulation correlated with poor prognosis of gastric cancer patients
TRIM24 overexpression accelerates cell growth in gastric cancer cell lines
We checked TRIM24 protein and messenger RNA (mRNA) expression in five gastric cancer cell lines (BGC-823, AGS, SGC-7901, MKN-1, and HGC-27) and found that TRIM24 expression was relatively low in SGC-7901 cells and relatively high in MKN-1 cells (Fig. 2a). Plasmid transfection was performed in SGC-7901 cells, and siRNA knockdown was carried out in MKN-1 cells. As shown in Fig. 2b, plasmid transfection significantly upregulated TRIM24 at both protein and mRNA levels, and siRNA treatment downregulated its expression. To investigate the impact of TRIM24 on cell proliferation, MTT and colony formation assays were performed. MTT assay showed that TRIM24 transfection caused significant increase in proliferation rate of SGC-7901 cells, while siRNA depletion inhibited proliferation of MKN-1 cells (Fig. 3a). Consistent with the MTT results, colony formation assay showed that TRIM24 overexpression increased, while its depletion decreased colony formation ability (Fig. 3b).
Expression of TRIM24 in gastric cancer cell lines. a Protein and mRNA expression of TRIM24 in gastric cancer cell lines. MKN-1 cell line has relatively high expression and SGC-7901 cell line has relatively low expression. b Western blot and real-time PCR analysis showed that siRNA treatment markedly decreases TRIM24 levels in MKN-1 cells and plasmid transfection significantly increased its level in SGC-7901 cells
Biological roles of TRIM24 on gastric cancer cell proliferation and chemoresistance. a MTT assay showed that TRIM24 knockdown inhibited proliferation in MKN-1 cell line. TRIM24 overexpression promoted proliferation in SGC-7901 cell line (p < 0.05). b TRIM24 knockdown decreased colony numbers while its overexpression increased colony formation ability (p < 0.05). c MTT assay showed that TRIM24 overexpression enhanced cell viability and its depletion inhibited cell viability in gastric cancer cells treated with 5-Fu (2 and 4 μg/ml, for 48 h). d. TRIM24 knockdown decreased while its overexpression upregulated cyclin D1 and p-Akt expression
TRIM24 increases chemoresistance of gastric cancer cells
To investigate the role of TRIM24 on chemoresistance of gastric cancer cells, we treated TRIM24 overexpressed cells with different concentrations of 5-FU (2 and 4 μg/ml, 48 h). Relative cell viability was determined by MTT assay. Compared with the control group, TRIM24 transfection increased viability of SGC-7901 cells. In contrast, TRIM24 siRNA treatment decreased cell viability (Fig. 3c). These data indicate that TRIM24 enhances resistance of gastric cancer cells against 5-FU.
TRIM24 regulates Akt signaling pathway
To investigate the possible mechanism of TRIM24 on proliferation and chemoresistance, we examined changes of cyclin D1 and Akt phosphorylation in TRIM24-overexpressed and depleted cancer cells. We found that the levels of cyclin D1 and p-Akt were upregulated after TRIM24 transfection and downregulated after siRNA depletion (Fig. 3d). To validate the involvement of Akt activation in TRIM24-mediated cell proliferation and chemoresistance, we used LY294002 (20 μmol/l, 48 h after transfection), an Akt inhibitor, on SGC-7901 cells. Akt inhibitor treatment did not block the effect of TRIM24 on proliferation (Fig. 4b). As shown in Fig. 4c, Akt inhibitor significantly blocked the effect of TRIM24 on chemoresistance. These results indicate that inhibition of Akt counteracts the chemoresistance-enhancing effect of TRIM24.
TRIM24 enhances gastric cancer chemoresistance via Akt. a Akt inhibitor LY294002 (20 μmol/l, 48 h after transfection) blocked Akt phosphorylation in both control and TRIM24 transfected cells. b MTT assay showed that Akt inhibitor treatment did not block the role of TRIM24 on cell proliferation. c Effect of Akt inhibitor on chemoresistance. SGC-7901/Empty vector and SGC-7901/TRIM24 cells were treated with Akt inhibitor. Akt inhibitor treatment counter-acts the upregulatory effect of TRIM24 on chemoresistance
Discussion
Gastric cancer is one of the most common cancers worldwide, and it has been one of the most serious public health problems. Although researchers have found that many aberrantly expressed genes in gastric cancer contribute to the malignant behavior, novel molecular markers that can identify tumor progression and predict the aggressive phenotype at the level of the individual patient are still urgently needed [17, 18]. Upregulation of TRIM24 expression had been implicated in several human cancers. TRIM24 gene upregulation in breast cancer is associated with poor patient prognosis. TRIM24 overexpression was also reported in advanced head and neck squamous cell carcinoma and correlates with adverse overall survival.TRIM24 protein was found to be overexpressed in human lung cancer and correlated with TNM stage, poor differentiation, and Ki67 labeling index. TRIM24 is overexpressed in human malignant glioma and serves as an independent predictive factor for overall and progression-free survival [9–11, 13, 15]. To data, its expression pattern and clinical significance in human gastric cancer remain unexplored. Here, we examined TRIM24 in 133 gastric cancer specimens by immunohistochemistry. We found that TRIM24 expression is upregulated in almost 50 % of the cases and associated with local invasion and clinical stage, suggesting that its expression is correlated with malignant behavior. In addition, we demonstrated that TRIM24 upregulation is correlated with poor overall survival of gastric cancer patients. These data are in agreement with previous reports and suggest that overexpression of TRIM24 protein may be a common feature in gastric cancer and can serve as a prognostic marker to identify patients with poor clinical outcome.
Previous studies also suggested that TRIM24 siRNA knockdown inhibits cell growth and invasion. To further reveal the function of TRIM24 in gastric cancer, plasmid transfection and siRNA knockdown were performed in SGC-7901 and MKN-1 cells. MTT and colony formation assay were employed to assess its role on cancer growth. In agreement with previous studies, TRIM24 transfection promoted gastric cancer cell proliferation, and its depletion inhibited cell growth. In addition, TRIM24 overexpression increased the level of cyclin D1. In addition to its effect on cell proliferation, we found that TRIM24 overexpression modifies cancer cell survival upon 5-FU treatment, suggesting that TRIM24 might be a potential target for gastric cancer chemotherapy.
Chemoresistance of cancer cells involves a host of processes and interaction of multiple genes. Previous studies indicated that TRIM24 regulates Akt signaling in human glioma [16]. In this study, we examined how TRIM24 overexpression and knockdown changes Akt phosphorylation in gastric cancer cells. We found that TRIM24 overexpression increased Ser 473 phosphorylation of Akt, while its knockdown decreased phosphorylation. In addition, an Akt inhibitor partly reversed the enhancing effect of TRIM24 on chemoresistance. Akt is involved in the regulation of chemoresistance to many drugs including 5-FU [19–21]. These data strongly suggest that TRIM24 regulates cancer cell chemoresistance through activation of the Akt pathway.
In summary, we show that TRIM24 expression is upregulated in human gastric cancer in association with local invasion, clinical stage, and poor prognosis. TRIM24 accelerates malignant cell growth and induces chemoresistance, possibly through the Akt pathway. We propose that TRIM24 upregulation might identify patients at high risk and serve as a novel therapeutic target for gastric cancer.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Brenner H, Rothenbacher D, Arndt V (2009) Epidemiology of stomach cancer. Methods Mol Biol 472:467–477
Jia B, Liu H, Kong Q, Li B (2012) RKIP expression associated with gastric cancer cell invasion and metastasis. Tumour Biol 33(4):919–925
Jia Y, Dong B, Tang L, Liu Y, Du H, Yuan P, Wu A, Ji J (2012) Apoptosis index correlates with chemotherapy efficacy and predicts the survival of patients with gastric cancer. Tumour Biol 33(4):1151–1158
Oishi Y, Watanabe Y, Yoshida Y, Sato Y, Hiraishi T, Oikawa R, Maehata T, Suzuki H, Toyota M, Niwa H, Suzuki M, Itoh F (2012) Hypermethylation of Sox17 gene is useful as a molecular diagnostic application in early gastric cancer. Tumour Biol 33(2):383–393
Le Douarin B, Nielsen AL, Garnier JM, Ichinose H, Jeanmougin F, Losson R, Chambon P (1996) A possible involvement of TIF1 alpha and TIF1 beta in the epigenetic control of transcription by nuclear receptors. EMBO J 15(23):6701–6715
Le Douarin B, Nielsen AL, You J, Chambon P, Losson R (1997) TIF1 alpha: a chromatin-specific mediator for the ligand-dependent activation function AF-2 of nuclear receptors? Biochem Soc Trans 25(2):605–612
Le Douarin B, Zechel C, Garnier JM, Lutz Y, Tora L, Pierrat P, Heery D, Gronemeyer H, Chambon P, Losson R (1995) The N-terminal part of TIF1, a putative mediator of the ligand-dependent activation function (AF-2) of nuclear receptors, is fused to B-raf in the oncogenic protein T18. EMBO J 14(9):2020–2033
Klugbauer S, Rabes HM (1999) The transcription coactivator HTIF1 and a related protein are fused to the RET receptor tyrosine kinase in childhood papillary thyroid carcinomas. Oncogene 18(30):4388–4393
Zhong S, Delva L, Rachez C, Cenciarelli C, Gandini D, Zhang H, Kalantry S, Freedman LP, Pandolfi PP (1999) A RA-dependent, tumour-growth suppressive transcription complex is the target of the PML-RARalpha and T18 oncoproteins. Nat Genet 23(3):287–295
Belloni E, Trubia M, Gasparini P, Micucci C, Tapinassi C, Confalonieri S, Nuciforo P, Martino B, Lo-Coco F, Pelicci PG, Di Fiore PP (2005) 8p11 myeloproliferative syndrome with a novel t(7;8) translocation leading to fusion of the FGFR1 and TIF1 genes. Genes Chromosomes Cancer 42(3):320–325
Katzenellenbogen BS (1996) Estrogen receptors: bioactivities and interactions with cell signaling pathways. Biol Reprod 54(2):287–293
Tsai WW, Wang Z, Yiu TT, Akdemir KC, Xia W, Winter S, Tsai CY, Shi X, Schwarzer D, Plunkett W, Aronow B, Gozani O, Fischle W, Hung MC, Patel DJ, Barton MC (2010) TRIM24 links a non-canonical histone signature to breast cancer. Nature 468(7326):927–932
Chambon M, Orsetti B, Berthe ML, Bascoul-Mollevi C, Rodriguez C, Duong V, Gleizes M, Thenot S, Bibeau F, Theillet C, Cavailles V (2011) Prognostic significance of TRIM24/TIF-1alpha gene expression in breast cancer. Am J Pathol 178(4):1461–1469
Li H, Sun L, Tang Z, Fu L, Xu Y, Li Z, Luo W, Qiu X, Wang E (2012) Overexpression of TRIM24 correlates with tumor progression in non-small cell lung cancer. PLoS One 7(5):e37657
Zhang LH, Yin AA, Cheng JX, Huang HY, Li XM, Zhang YQ, Han N, and Zhang X (2015) TRIM24 promotes glioma progression and enhances chemoresistance through activation of the PI3K/Akt signaling pathway. Oncogene 34:600–610
Jeong SH, Ko GH, Cho YH, Lee YJ, Cho BI, Ha WS, Choi SK, Kim JW, Lee CW, Heo YS, Shin SH, Yoo J, Hong SC (2012) Pyrophosphatase overexpression is associated with cell migration, invasion, and poor prognosis in gastric cancer. Tumour Biol 33(6):1889–1898
Yu HF, Zhao G, Ge ZJ, Wang DR, Chen J, Zhang Y, Zha TZ, Zhang K, Zhang M, Tan YF, Zhou SJ, Jiang C (2012) High RIN1 expression is associated with poor prognosis in patients with gastric adenocarcinoma. Tumour Biol 33(5):1557–1563
Xie C, Fu L, Han Y, Li Q, Wang E (2014) Decreased ARID1A expression facilitates cell proliferation and inhibits 5-fluorouracil-induced apoptosis in colorectal carcinoma. Tumour Biol 35:7921–7792
Yothaisong S, Dokduang H, Techasen A, Namwat N, Yongvanit P, Bhudhisawasdi V, Puapairoj A, Riggins GJ, Loilome W (2013) Increased activation of PI3K/AKT signaling pathway is associated with cholangiocarcinoma metastasis and PI3K/mTOR inhibition presents a possible therapeutic strategy. Tumour Biol 34(6):3637–3648
Shin JY, Kim JO, Lee SK, Chae HS, Kang JH (2010) LY294002 may overcome 5-FU resistance via down-regulation of activated p-AKT in Epstein–Barr virus-positive gastric cancer cells. BMC Cancer 10:425
Conflict of interest
No potential conflicts of interest were disclosed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miao, ZF., Wang, ZN., Zhao, TT. et al. TRIM24 is upregulated in human gastric cancer and promotes gastric cancer cell growth and chemoresistance. Virchows Arch 466, 525–532 (2015). https://doi.org/10.1007/s00428-015-1737-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1737-4