Abstract
In this study, we investigated the functional mechanisms of microRNA-193-3p (miR-193-3p) in human gastric cancer. Quantitative RT-PCR (qRT-PCR) was used to assess whether miR-193-3p was aberrantly expressed in gastric cancer cells and clinical samples from gastric cancer patients. Gastric cancer cell line AGS and MKN-45 cells were stably transduced with lentivirus to downregulate endogenous miR-193-3p. The modulation of miR-193-3p downregulation on gastric cancer proliferation, migration, chemo-drug responses, and tumor explant were assessed by MTT, wound-healing, 5-FU chemoresistance and in vivo tumorigenicity assays, respectively. Downstream target of miR-193-3p, phosphatase and tensin homolog (PTEN) in gastric cancer, was assessed by dual-luciferase reporter assay, qRT-PCR, and western blot. PTEN was knocked down by siRNA in AGS and MKN-45 cells to assess its direct impact on miR-193-3p modulation in gastric cancer. MiR-193-3p was aberrantly upregulated in both gastric cell lines and human gastric tumors. In AGS and MKN-45 cells, miR-193-3p downregulation reduced cancer proliferation, migration and 5-FU chemoresistance in vitro, and tumorigenicity in vivo. PTEN was confirmed to be targeted by miR-193-3p in gastric cancer. PTEN inhibition in AGS and MKN-45 cells directly reversed the anti-tumor modulations of miR-193-3p downregulation on gastric cancer proliferation, migration, and 5-FU chemoresistance. We presented clear evidence showing miR-193-3p played critical role in regulating human gastric cancer through direct targeting on PTEN gene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is one of the most aggressive and malignant cancers in the world [1, 2]. In the USA alone, the yearly estimated gastric cancer new cases was around 25,000, and the yearly estimated gastric cancer-related deaths were around 11,000 [2]. The incident rates of gastric cancer are much higher in Asian countries, including China [3]. In Chinese patients, gastric cancer is often diagnosed at advanced stages with evident tumor metastasis [4, 5]. In those patients, the clinical prognosis was very poor with estimated 5-year survival rates to be less than 10 % [4, 5]. Therefore, it is a great challenge to identify novel molecular targets to provide gastric cancer patients with efficient early diagnostic methods and effective therapeutic plans.
MicroRNAs (miRNAs) are short-length (18∼22 nt) noncoding RNAs that bind the complementary sites in the 3′-untranslated region (3′-UTR) of target genes to post-transcriptionally induce gene suppression or protein degradation [6]. In gastric cancer, miRNAs have been shown to be efficient biomarker for cancer prediction, as well as important modulators to regulate cancer proliferation, migration, and metastasis [7]. For instance, a combination of five serum miRNAs, miR-1, miR-20a, miR-27a, miR-34, and miR-423-5p, were shown to be a signature non-invasive diagnostic method for gastric cancer [8]. Also, miR-21 was shown to be an oncogenic initiator for gastric cancer development by promoting cancer proliferation and migration [9]. Within the group of miRNAs to be closely associated with gastric cancer, mature human microRNA 193-3p (miR-193-3p) was demonstrated to be upregulated in gastric cancer cell lines [10]. Yet, the molecular mechanisms of miR-193-3p in gastric cancer have never been characterized.
Gene of phosphatase and tensin homolog (PTEN) is located on human chromosome 10q23.3, and acts as a critical tumor suppressive gene in various cancer types [11]. In gastric cancer, PTEN is generally downregulated in tumors [12, 13], but the definite role of PTEN as tumor suppressor in gastric cancer has yet to be established [14]. Interestingly, in several studies involving the epigenetic regulation of miRNAs, PTEN was suggested to be highly associated with miRNA-mediated gastric cancer development and metastasis [13, 15, 16]. However, it is unknown whether PTEN may directly affect miRNAs regulation on gastric cancer development and metastasis.
In the current study, we firstly examined the expression profile of miR-193-3p in gastric cancer cell lines and clinical samples. We hypothesized that downregulation of miR-193-3p had anti-tumor effects on gastric cancer proliferation, migration, and chemoresistance. We also predicted PTEN to be the downstream target of miR-193-3p in gastric cancer. PTEN gene was then downregulated to investigate its direct impact on miR-193-3p regulation in gastric cancer.
Materials and methods
Ethic statement
In our study, all experimental protocols were carried out in accordance to the principles of the Declaration of Helsinki, as well as approved by the Human Research Board and Ethics Committee at the Yongchuan Hospital, Chongqing Medical University in Chongqing, China. Signed consent forms were obtained from all patients before the onset of the study.
Gastric cancer cell lines and clinical samples
Five gastric cancer cell lines, AGS, NIC-N87, SNU-1. SNU-5, SNU-16 were purchased from the American Type Culture Collection (ATCC) (Shanghai, China). The other four gastric cell lines, MKN-45, SGC-7901, BGC-823, and MKN-28, and a normal gastric epithelial cell line GES-1, were purchased from the cell bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). All cell lines were maintained in DMEM medium (Invitrogen, USA), supplemented with 10 % fetal bovine serum (FBS, Invitrogen, USA) in a tissue incubator of 5 % CO2 at 37 °C.
Paired gastric tumor tissues and adjacent non-tumor tissues were surgically extracted from 14 patients in the Department of Gastrointestinal surgery at Yongchuan Hospital. All tissues were derived from patients without adjuvant treatments to avoid treatment-induced gene change. The adjacent non-tumor tissues were extracted at least 3 cm away from the obvious edge of tumor tissues. Upon extraction, all clinical samples were immediately snap-frozen in liquid nitrogen and kept at −80 °C until further RNA extraction.
RNA isolation and quantitative RT-PCR
Total RNA from gastric cancer cell lines and clinical samples was isolated with a RNeasy mini kit (Invitrogen, USA) according to the manufacturer’s instruction. After verifying the purity of RNA, cDNA was reversely transcribed trough a TaqMan Reverse Transcription Kit (Applied Biosystem, USA) according to the manufacturer’s instruction. For quantitative RT-PCR (qRT-PCR), the TaqMan miRNA Assay (Applied Biosystem, USA) was used to determine the gene expression level of human miR-193-3p. U6 transcript was used and loading template. To determine the mRNA expression level of PTEN, a SYBR Green Real-Time PCR Master Mix Kit (Applied Biosystems, USA) was applied with GAPDH as loading template. All qRT-PCR reactions were carried out on a BIO-RAD CFX96™ system (Bio-Rad, USA).
Lentiviral downregulation of miR-193-3p
Lentiviruses expressing miR-193-3p inhibitor (miR-193-3p-In), or negative control miRNA inhibitor (miR-NC) were purchased from SunBio (SunBio Medical Biotechnology, Shanghai, China). To transduce gastric cancer cell lines AGS and MKN-45 cells, 100 pmol lentiviruses, along with 8 μg/ml polybrene, were added into culture medium overnight a multiplicity of infection (MOI) of 10∼15. On the second day, fresh medium was replenished. Cell cultures were maintained for another 5 to 7 days to stabilize the lentivirus transduction. After that, the transduction efficiency was verified by qRT-PCR.
Cell proliferation assay
After lentiviral transduction was stabilized, AGS and MKN-45 cells were plated in 96-well plates (5000 cells/well). Every 24 h between day 1 and day 5 in culture, 200 μL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/mL) was added into each experimental well for 4 h. After the supernatant was aspirated, 200 μL dimethyl sulfoxide (DMSO, Sigma Aldrich, USA) was added in each well to dissolve formazan crystals. A MTT assay (Invitrogen, USA) was then performed according to the manufacturer’s instruction. Gastric cancer proliferation was measured as the optical density (OD) at a wavelength of 570 nm.
Wound-healing migration assay
AGS and MKN-45 cells were plated in six-well plates (25,000 cells/well). A sterile Eppendorf pipette tip was used to draw across the centerline of cell monolayer to create a wound area. After removing debris, the cultures were replenished with fresh medium and maintained for 24 h. Cell migration into the wound area was examined under phase contrast objectives (×10) on a CK2 inverted microscope (DM16000, Leica, Germany).
Chemoresistance assay
AGS and MKN-45 cells were plated in 96-well plates, and treated with 5-FU with gradient concentrations at 0, 5, 10, 20, 50, and 100 μg/mL for 24 h. A MTT assay was performed, and relative viability was assessed against cells treated with 0 μg/mL 5-FU under control conditions.
Tumorigenicity assay
After lentiviral transduction was stabilized, one million AGS cells were subcutaneously injected into the left flanks of 2-month-old athymic mice. The in vivo tumorigenicity assay was carried out for 5 weeks. Every week, the tumor volumes (mm3) were measured by the equation, length × width2/2. At the end of tumorigenicity assay, mice were sacrificed and the paraffin-embedded sections of tumors were prepared, then subject to immunohistochemistry of Ki-67 (Sigma Aldrich, USA).
Dual-luciferase reporter assay
We used the psiCHECK2 luciferase plasmid (Promega, USA) and inserted the sequence of wild-type (WT) PTEN 3′-UTR between XhoI and NotI restriction enzyme sites. The constructed luciferase plasmid was called luc-PTEN-WT, as it included the original hsa-miR-193-3p binding site. In addition, we used QuickChange Multi Site-Directed Mutagenesis kit (Stratagene, USA) to mutate the hsa-miR-193-3p binding site on PTEN 3′-UTR. Then, the mutated (MT) 3′-UTR sequence was used to create a luciferase plasmid, luc-PTEN-MT without complementary miR-193-3p binding sequence. In a dual-luciferase reporter assay, HEK293T cells were co-transfected with 25 ng/mL either luc-PTEN-WT or luc-PTEN-MT, and 50 nM either miR-193-3p mimics (RiboBio, Guangzhou, China) or non-specific miRNA mimics (miR-NC, RiboBio, Guangzhou, China) for 48 h. The relative luciferase activity was examined through a FL500 microplate fluorescence reader (Bio-Tek, USA) according to the manufacturer’s instructions.
Western blot assay
The protein expression level of PTEN was detected by western blot in AGS and MKN-45 cells. Briefly, the cultured cells were quickly washed with ice-cold PBS and collected into 1.5 mL conical tube to be treated with a lysis buffer (Promega, USA). The proteins (40 μg/sample) were separated in 10 % SDS-PAGE gels and transferred to polyvinylidene fluoride membranes (ThermoFisher, USA). The membranes were blocked with 5 % non-fat dry milk in Tris buffered saline (TBS, Invitrogen, USA) for 2 h at room temperature, then incubated with primary antibody against PTEN (Santa Cruz, USA) in TBS for 24 h at 4 °C. Another antibody against β-actin was used as blot control (Santa Cruz, USA). On the second day, membranes were then incubated with the horseradish-peroxidase conjugated secondary antibodies, for 2 h at room temperature. The blots were visualized using enhanced chemi-luminescence film kit (Amersham, USA) according to the manufacturer’s instructions.
PTEN downregulation assay
A PTEN-targeting siRNA (siRNA/PTEN), and a control scrambled siRNA (siRNA/C) were bought from RiboBio (RiboBio, Guangzhhou, China). To downregulate PTEN gene, AGS and MKN-45 cells were transfected with 100 nM siRNA/PTEN or siRNA/C for 48 h. The efficiency of PTEN knockdown was examined by qRT-PCR.
Statistical analysis
In our study, all experiments were biologically repeated for at least four or five times. The averaged data were presented as mean ± SEM. Statistic comparisons between two data samples was carried out using student’s t test. In addition, statistical comparisons between two groups of data with multiple samples were carried out using one-way ANOVA with a post hoc test. Differences were considered statistically significant if P < 0.05.
Results
miR-193-3p is aberrantly upregulated in gastric cancer
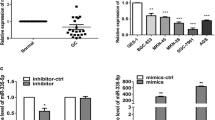
In a recent microarray study, miR-193-3p was reported to be highly expressed in gastric cancer cell lines [10]. In our study, we used a much more accurate molecular method, qRT-PCR to investigate whether miR-193-3p was aberrantly expressed in gastric cancer. We firstly examined the expression of miR-193-3p in nine gastric cancer cell lines. The result of qRT-PCR showed that miR-193-3p was significantly upregulated in all gastric cancer cell lines, including AGS, MKN-45, SGC-7901, BGC-823, NCI-N87, MKN-28, SNU-1, SNU-5, and SNU-16 cells, as compared to a normal gastric epithelial cell line, GES-1 (Fig. 1a, *P < 0.05). We also compared gene expression of miR-193-3p in paired samples, between gastric tumors (T) and their adjacent non-tumor (ANT) gastric tissues in 14 individual patients with gastric cancer. It showed that, in samples from each examined patient, miR-193-3p was always highly expressed in gastric tumors than in normal gastric tissues (Fig. 1b, *P < 0.05).
MiR-193-3p is upregulated in gastric cancer cell lines and clinical tumor samples. a QRT-PCR was carried out to compare miR-193-3p expression levels between 9 gastric cancer cell lines, AGS, MKN-45, SGC-7901, BGC-823, NCI-N87, MKN-28, SNU-1, SNU-5 and SNU-16 cells, and a normal gastric epithelial cell line, GES-1 (*P < 0.05, vs. GES-1) b In 14 patients with gastric cancer, miR-193-3p expression was compared between paired gastric tumor (T) and adjacent non-tumor (ANT) gastric samples (*P < 0.05)
Downregulation of miR-193-3p inhibited gastric cancer cell proliferation and migration
The molecular mechanisms of miR-193-3p have never been characterized in gastric cancer. In our work, we stably transduced two gastric cancer cell lines, AGS and MKN-45 cells with lentivirus carrying miR-193-3p inhibitor (miR-193-3p-In), to specifically downregulate the endogenous expression of miR-193-3p in those cells. In a controlled experiment, AGS and MKN-45 cells were transduced with another lentivirus carrying a negative control miRNA inhibitor (miR-NC). After transduction was stabilized, assessment by qRT-PCR showed that in both AGS and MKN-45 cells, miR-193-3p expression was knocked down by at least 50 % with miR-193-3p-In transduction than with miR-NC transduction (Fig. 2a, *P < 0.05).
Downregulation of miR-193-3p inhibits gastric cancer proliferation and migration. a Two gastric cancer cell lines, AGS and MKN-45 cells were transduced with a lentivirus expressing miR-193-3p inhibitor (miR-193-3p-In) or a lentivirus expressing negative control miRNA inhibitor (miR-NC). After lentiviral transduction was stabilized, qRT-PCR was carried out to examine the efficiency of miR-193-3p knockdown (*P < 0.05). b Lentiviral-infected AGS and MKN-45 cells were examined by a 5-day MTT assay to assess proliferation rates between miR-193-3p downregulated gastric cancer cells (infected with miR-193-3p-In) and the control gastric cancer cells (infected with miR-NC) (**P < 0.05, one-way ANOVA). c Lentiviral-infected AGS and MKN-45 cells were also examined by a 24-h wound-healing assay to assess migration capability. Phase contrast images were shown for the miR-193-3p downregulated cells (infected with miR-193-3p-In) or the control cells (infected with miR-NC) at 0 and 24 h after initial wound. The dotted lines represent the wound edges
Cancer proliferation rates in lentiviral-transduced AGS and MKN-45 cells were examined by a 5-day MTT assay. The results showed that, in miR-193-3p downregulated AGS and MKN-45 cells, cancer proliferation rate was significantly reduced as compared to control cells (Fig. 2b, **P < 0.05, one-way ANOVA). In addition, cancer migrating capability in lentiviral-transduced AGS and MKN-45 cells was also examined, through a wound-healing assay. The result showed that, 24 h after the initial wound, migration into the previously wounded area was significantly inhibited by miR-193-3p downregulation (Fig. 2c).
Therefore, our data clearly demonstrated that downregulation of miR-193-3p had anti-tumor effect on gastric cancer proliferation and migration.
Downregulation of miR-193-3p reduced gastric cancer cell chemoresistance and in vivo tumorigenicity
We investigated the effect of miR-193-3p downregulation on gastric cancer chemoresistance. After lentiviral transduction was stabilized, AGS and MKN-45 cells were treated with series concentrations of 5-FU (0, 5, 10, 20, 50, and 100 μg/mL) for 24 h. The relative viability was compared, using a MTT assay, between cells with miR-193-3p downregulation and control cells. It demonstrated that, miR-193-3p downregulation dramatically decreased gastric cancer cells’ 5-FU chemoresistance in both cell lines (Fig. 3a, **P < 0.05, one-way ANOVA).
Downregulation of miR-193-3p decreased gastric cancer 5-FU resistance and in vivo tumor growth. a Lentiviral-infected AGS and MKN-45 cells were treated with 0, 5, 10, 20, 50, or 100 μg/mL 5-FU for 24 h. Chemoresistance was assessed by a MTT assay. Relative viability was compared against the cells transduced with miR-NC and treated with 0 μg/mL 5-FU (**P < 0.05, one-way ANOVA). b Lentiviral-infected AGS cells (1 × 106/injection) were subcutaneously inoculated into the left flank of 2-month-old female athymic mice. In vivo tumor volumes (mm3) were assessed by the equation of length × width2/2 on a weekly basis for 5 weeks (**P < 0.05, one-way ANOVA). c At the end of tumorigenicity assay, AGS tumors were retrieved, processed with paraffin-embedded sectioning, and Ki-67 immunohistochemistry
We also examined the effect of miR-193-3p downregulation on gastric cancer in vivo tumorigenicity. MiR-193-3p-In or miR-NC transduced AGS cells (1 × 106 cells/injection) were subcutaneously injected into the left flanks of 2-month-old athymic mice. The in vivo growths of gastric tumors were compared on a weekly basis for 5 weeks, by measuring the length and width of the tumors and calculating the tumor volume (mm3) with the equation of length × width2/2. It showed that miR-193-3p downregulation dramatically reduced gastric cancer in vivo tumorigenicity (Fig. 3b, **P < 0.05, one-way ANOVA). This observation was further confirmed by immunohistochemistry of Ki-67 on tumor sections at the end of tumorigenicity assay, showing that in vivo gastric cancer proliferation, indicated by Ki-67 positive immunostaining, was significantly reduced in tumors with miR-193-3p downregulation (Fig. 3c).
PTEN is the downstream target of miR-193-3p in gastric cancer cells
The downstream target of miR-193-3p in regulating gastric cancer was further examined. We cross-examined several miRNA target databases, such as TargetScanHuman (www.targetscan.org), microRNA.org (www.microrna.org), or miRDB (www.mirdb.org), and discovered that PTEN, an active cancer regulator, was a possible target (Fig. 4a).
PTEN is the downstream target of miR-193-3p in gastric cancer. a The putative binding of human miR-193-3p on human PTEN gene 3′-UTR was shown (red). A mutated 3′-UTR sequence of PTEN gene was also demonstrated to nullify the binding of miR-193-3p (gray). b HEK293T cells were co-transfected with luciferase plasmids with wild-type PTEN 3′UTR (luc-PTEN-WT) or with mutant PTEN 3′UTR (luc-PTEN-WT), and miR-193-3p-mimics or non-specific miRNA mimics (miR-NC) for 48 h. The relative luciferase activities were measured by a dual-luciferase assay (*P < 0.05, ∆ P > 0.05). c In AGS and MKN-45 cells, PTEN gene expression levels were compared between the cells transduced with miR-NC lentivirus and the cells transduced with miR-193-3p-In lentivirus (*P < 0.05). d In AGS and MKN-45 cells, western blot was used to compare PTEN protein expression levels between the cells transduced with miR-NC lentivirus and the cells transduced with miR-193-3p-In lentivirus
We cloned the wild type (WT) 3′-UTR of PTEN gene, which includes the putative miR-193-3p binding site, into a psiCHECK2 luciferase plasmid (luc-PTEN-WT). The mutated (MT) 3′-UTR of PTEN gene, with modified 3′-UTR sequence on miR-193-3p binding site, was also inserted into the luciferase cassette (luc-PTEN-MT). These two luciferase plasmids were co-transfected with either miR-193-3p mimics or a negative control miRNA mimics (miR-NC) in the culture of HEK293T cells for 48 h, followed by a dual-luciferase reporter assay to measure the relative luciferase activities. The result showed that, in HEK293T cells transfected with luc-PTEN-WT, there was a significant difference in relative luciferase activity between miR-NC and miR-193-3p-mimics co-transfection (Fig. 4b, *P < 0.05). However, in HEK293T cells transfected with luc-PTEN-MT, there was no difference in relative luciferase activity between miR-NC and miR-193-3p-mimics co-transfection (Fig. 4b, * ∆ < 0.05). Thus, the result of dual-luciferase activity assay confirmed that PTEN gene was the downstream target of miR-193-3p.
The regulatory effect of miR-193-3p on PTEN expression profile in gastric cancers was examined by qRT-PCR and western blot. The result of qRT-PCR showed, in both AGS and MKN-45 cells, PTEN expression was considerably upregulated by miR-193-3p downregulation (Fig. 4c, *P < 0.05). The result of western blot showed, PTEN protein expression was also upregulated by miR-193-3p downregulation (Fig. 4d). These data suggest that PTEN was actively involved in the modulation of miR-193-3p in gastric cancer.
Downregulation of PTEN reversed the anti-tumor effect of miR-193-3p downregulation in gastric cancer
The direct impact of PTEN on miR-193-3p modulated cancer proliferation, migration, and chemoresistance was examined by siRNA-induced PTEN downregulation in gastric cancer cells. We transfected AGS and MKN-45 cells with either a PTEN-targeting siRNA (siRNA/PTEN), or a control scrambled siRNA (siRNA/C). Forty-eight hours after transfection, qRT-PCR confirmed that PTEN expression was significantly downregulated by siRNA/PTEN in both cell lines (Fig. 5a, *P < 0.05). Western blot also demonstrated that PTEN protein was knocked down by siRNA/PTEN (Fig. 5b).
Downregulation of PTEN restored cancer proliferation, migration, and chemoresistance in gastric cancer after miR-193-3p downregulation. a In miR-193-3p downregulated AGS and MKN-45 cells, a PTEN-targeting siRNA (siRNA/PTEN), or a control scrambled siRNA (siRNA/C) was transfected into cells for 48 h. Transfection efficiency was examined by qRT-PCR (*P < 0.05). b In addition, western blot was conducted to verify the transfection efficiency on knocking down PTEN protein. c In AGS and MKN-45 cells transduced with miR-193-3p-In and then siRNAs, a 5-day MTT assay was carried out to compare the proliferation rates between the cells transfected with siRNA/PTEN and the cells transfected siRNA/C (**P < 0.05, one-way ANOVA). d In AGS and MKN-45 cells transduced with miR-193-3p-In and then siRNAs, a wound-healing assay was carried out to compare the migrating capabilities between cells transfected with siRNA/PTEN and the cells transfected siRNA/C (**P < 0.05, one-way ANOVA). e In AGS and MKN-45 cells transduced with miR-193-3p-In and then siRNAs, 5-FU chemoresistance was compared between cells transfected with siRNA/PTEN and the cells transfected siRNA/C
In AGS and MKN-45 cells with stable miR-193-3p downregulation (transduced with miR-193-3p-In), we transfected those cells with siRNAs and re-evaluated cancer proliferation, migration, and chemoresistance. Firstly, the result of 5-day MTT assay showed that, in miR-193-3p downregulated AGS and MKN-45 cells, PTEN downregulation markedly restored cell proliferation rates (Fig. 5c, **P < 0.05, one-way ANOVA). Secondly, the result of wound-healing assay demonstrated that migration capability was also significantly restored by PTEN downregulation in AGS and MKN-45 cells with miR-193-3p downregulation (Fig. 5d). Thirdly, the result of 5-FU chemoresistance assay showed that PTEN downregulation also considerably increased drug resistance in AGS and MKN-45 cells with miR-193-3p downregulation (Fig. 5e, **P < 0.05, one-way ANOVA). Therefore, those functional experiments further demonstrated that PTEN gene was the direct downstream target of miR-193-3p regulation on gastric cancer proliferation, migration, and chemoresistance.
Discussion
MiRNAs have been demonstrated to be important epigenetic regulators in human gastric cancer [7]. The total number of gastric cancer-related miRNAs, by the definition that they are either aberrantly expressed in gastric tumors, or functionally regulating gastric cancer development, would well exceed 300 [7]. Thus, it would certainly require much longer time and much devoted effort to fully unravel the complex molecular pathways of miRNA regulation in gastric cancer. In a previous microarray study surveying seven gastric cancer cell lines, miR-193-3p was shown to be aberrantly upregulated in gastric cancer cells than in normal gastric epithelial cells [10]. In the current study, we firstly demonstrated, using a much more accurate quantitative method of qRT-PCR, that miR-193-3p was upregulated in nine gastric cancer cells as compared to normal gastric epithelial cell line GES-1, as well as upregulated in gastric tumors as compared to paired adjacent gastric tissues in 14 patients with gastric cancer. Thus, our data provided definitive evidence showing that miR-193-3p is predominantly upregulated or over-expressed in both gastric cancer cell lines and human gastric tumors.
Though it was shown in a previous study that miR-193-3p was upregulated in gastric cancer cells [10], the exact mechanism of miR-193-3p in gastric cancer has never been elucidated until this study. Using the method of lentiviral transduction, we demonstrated that lentiviral-induced miR-193-3p downregulation significantly reduced cancer cell proliferation, migration, 5-FU chemoresistance, and in vivo tumorigenicity. Thus, our data suggest that miR-193-3p was mainly acting as an oncogenic effector in gastric cancer. Interestingly, in human non-small cell lung cancer (NSCLC), miR-193-3p was shown to be downregulated in cancer cells, and miR-193-3p was acting as a tumor suppressor by inhibiting NSCLC proliferation and migration [17]. Therefore, it seems like miR-193-3p may associate with complex or even distinctly different signaling pathways in different cancer types to act as either oncogene or tumor suppressive gene.
Also in the current study, we further identified PTEN was the direct target of miR-193-3p in gastric cancer. We demonstrated in dual-luciferase assay that miR-193-3p bound the complementary binding site on 3′-UTR of human PTEN gene. We also showed that, PTEN expression was inversely regulated by miR-193-3p downregulation in gastric cancer. Most importantly, through the functional experiments of siRNA-mediated PTEN downregulation, we demonstrated that PTEN directly regulate the tumor suppressive effect of miR-193-3p on gastric cancer proliferation, migration, and chemoresistance. The association of PTEN and miRNA regulation in gastric cancer had been suggested in previous studies [13, 15, 16]. However, though PTEN was shown to be regulated by miR-21, miR-221/222, and miR-19a/b, none of the studies showed direct evidence of PTEN affecting the tumor regulatory effects by miR-21, miR-221/222, or miR-19a/b [13, 15, 16]. Thus, the results of our study not only reinforce the notion that PTEN is a tumor suppressor in gastric cancer, but also present novel finding that PTEN is directly involved in epigenetic regulation of miRNA in gastric cancer.
Overall, we revealed novel mechanistic functions of miR-193-3p in regulating human gastric cancer both in vitro and in vivo. In addition, we showed PTEN was directly and inversely correlated with miR-193-3p regulation in gastric cancer. These data might help in identifying new therapeutic targets for the treatment of patients with gastric cancer.
References
Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–13.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29.
Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20(4):633–49.
Yang L, Parkin DM, Ferlay J, Li L, Chen Y. Estimates of cancer incidence in China for 2000 and projections for 2005. Cancer Epidemiol Biomarkers Prev. 2005;14(1):243–50.
Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12(1):17–20.
Carrington JC, Ambros V. Role of microRNAs in plant and animal development. Science. 2003;301(5631):336–8.
Shrestha S, Hsu SD, Huang WY, Huang HY, Chen W, Weng SL, et al. A systematic review of microRNA expression profiling studies in human gastric cancer. Cancer Med. 2014;3(4):878–88.
Liu R, Zhang C, Hu Z, Li G, Wang C, Yang C, et al. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur J Cancer. 2011;47(5):784–91.
Zhang Z, Li Z, Gao C, Chen P, Chen J, Liu W, et al. Lu H: miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab Invest. 2008;88(12):1358–66.
Li H, Xie S, Liu M, Chen Z, Liu X, Wang L, et al. The clinical significance of downregulation of mir-124-3p, mir-146a-5p, mir-155-5p and mir-335-5p in gastric cancer tumorigenesis. Int J Oncol. 2014;45(1):197–208.
Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22(14):2954–63.
Fei G, Ebert MP, Mawrin C, Leodolter A, Schmidt N, Dietzmann K, et al. Reduced PTEN expression in gastric cancer and in the gastric mucosa of gastric cancer relatives. Eur J Gastroenterol Hepatol. 2002;14(3):297–303.
Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY. Yan M: microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol Rep. 2012;27(4):1019–26.
Sato K, Tamura G, Tsuchiya T, Endoh Y, Sakata K, Motoyama T, et al. Analysis of genetic and epigenetic alterations of the PTEN gene in gastric cancer. Virchows Arch. 2002;440(2):160–5.
Chun-Zhi Z, Lei H, An-Ling Z, Yan-Chao F, Xiao Y, Guang-Xiu W, et al. MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell proliferation and radioresistance by targeting PTEN. BMC Cancer. 2010;10:367.
Wang F, Li T, Zhang B, Li H, Wu Q, Yang L, et al. MicroRNA-19a/b regulates multidrug resistance in human gastric cancer cells by targeting PTEN. Biochem Biophys Res Commun. 2013;434(3):688–94.
Yu T, Li J, Yan M, Liu L, Lin H, Zhao F, et al. MicroRNA-193a-3p and -5p suppress the metastasis of human non-small-cell lung cancer by downregulating the ERBB4/PIK3R3/mTOR/S6K2 signaling pathway. Oncogene. 2015;34(4):413–23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
None.
Rights and permissions
About this article
Cite this article
Jian, B., Li, Z., Xiao, D. et al. Downregulation of microRNA-193-3p inhibits tumor proliferation migration and chemoresistance in human gastric cancer by regulating PTEN gene. Tumor Biol. 37, 8941–8949 (2016). https://doi.org/10.1007/s13277-015-4727-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4727-x