Abstract
Rho-associated coiled-coil-containing protein kinase 2 (ROCK2) has been known as an effector for the small GTPase Rho and plays an important role in tumor progression and metastasis. However, the effect of ROCK2 in gastric cancer (GC) has not been identified. This study showed that ROCK2 expression significantly increased in clinical GC tissues compared with adjacent non-cancer tissues. Immunohistochemistrical analysis showed that high expression of ROCK2 was correlated with tumor grade, tumor–node–metastasis stage, infiltration depth, lymph node invasion and Ki-67, and predicted poor prognosis in 135 gastric cancer specimens. In addition, we found that upregulated ROCK2 promoted proliferation, metastasis and invasion of GC cells, while ROCK2 knockdown led to the opposite results in vitro by Cell Counting Kit-8 (CCK-8) assay, colony formation assays, flow cytometric analysis and trans-well assays. Our findings supported that ROCK2 was a significant protein in the progress of GC and would provide a novel promising therapeutic strategy against human GC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the fifth most common cancer and the third malignant tumor leading cause of cancer deaths worldwide, causing an estimated more than 720,000 deaths around the world every year [1, 2]. Diagnosis and treatment methods have improved year by year, and earlier diagnosis and improved treatment have led to prolonged survival [3]. However, the prognosis of patients with non-curable factors such as hepatic, peritoneal or distant lymph node metastases is still poor, and most patients survival does not exceed 12 months [4, 5].Therefore, it is needful to know the molecular mechanisms and recognize novel molecules that take part in the metastatic and invasion regulation of gastric cancer to provide new opportunities for the diagnosis and treatment of gastric cancer.
Rho-associated coiled-coil-forming protein kinase 2 (ROCK2) belongs to a family of serine/threonine kinases that regulate important cellular processes, such as cell morphology, shape, adhesion and migration [6–8]. ROCK2 expression increased in several types of cancers. For example, ROCK2 was associated with growth and invasion of non-small cell lung cancer [9], ROCK2 enhanced cell motility and invasiveness in HCC cells [10], and ROCK2 promoted tumor invasion and metastasis in colorectal cancer [11]. However, the expression of ROCK2 in GC and its specific mechanism of action in the process of GC proliferation, invasion and metastasis remain unclear.
In this study, we elaborated a comprehensive analysis of ROCK2 and its biological characteristics in the metastases of gastric cancer for the first time. First, we investigated the expression of ROCK2 in gastric cancer tissues by Western blot analysis and immunohistochemical assay. Secondly, we examined the correlation between ROCK2 expression and clinicopathological parameters. Thirdly, we analyzed the role of ROCK2 on regulating the ability of proliferation, metastasis and invasion. Fourthly, we detected the ability of ROCK2 in affecting the expression of proliferation, metastasis and invasion-related proteins. These findings were firstly reported in gastric cancer cells and might provide a novel insight into developing experimental therapies in gastric cancer.
Materials and methods
Tissue samples
A total of 135 gastric cancer tissue samples and adjacent non-cancer tissues were selected from patients who underwent surgery between 2007 and 2013 at Department of Pathology, Nantong Tumor Hospital. These tissues were stored at −80 °C after surgical removal rapidly. For histological examination, all GC tissue samples were fixed in 10% buffered formalin and embedded in paraffin for sectioning. Resected specimens were classified according to the Seventh Edition of the TNM Classification for Gastric Cancer [12]. All the clinicopathological information included age, gender, tumor grade, TNM stage, infiltration depth, lymph node invasion and nerve invasion. The approval of removed tissues for research purposes was obtained from the Ethics Committee of Nantong Tumor Hospital, and the signed informed consent was obtained from each patient. The primary clinical characteristics and pathologic variables are summarized in Table 1.
Cell culture and transient transfection
MGC803, BGC823 and MKN45 human gastric cancer cells (the cell library of the Chinese Academy of Sciences) and GES-1 human gastric epithelial cells (Shanghai Bioleaf Biotech Co., Ltd) were maintained in RPMI-1640 containing 10% fetal bovine serum (GIBCO-BRL, Grand Island, NY, USA) supplemented with 2 ml glutamine, at 37 °C and 5% CO2. ROCK2 small-interfering RNA (siRNA) was designed and synthesized by Shanghai Genechem (China). The siRNA targeting ROCK2 sequences was as follows: 5′-GGAACTGCAAGACCAACTT-3′. A non-specific, scrambled siRNA with a sequence of 5′-UUCUCCGAACGUGUCACGU-3′ was used as a negative control. The ROCK2-Flag plasmid was purchased from Shanghai Genechem (China). Cells were stably transfected with siRNA or ROCK2-Flag using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol.
Western blot and antibodies
Western blot assay was used to detect some proteins. Cell or tissue lysates were subjected to SDS-PAGE, and proteins were transferred to polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA). The membranes were blocked in Tris-buffered saline (TBS, pH 7.4) containing 5% nonfat milk and 0.1% Tween-20 for 2 h. After that, the PVDF membranes were incubated with the primary antibody for 4 h, followed by the secondary antibody for 1 h at room temperature. The PVDF membranes were then developed using the ECL detection systems (Imaging Technology, Ontario, Canada).The following antibodies used in this study were purchased from Santa Cruz Biotechnology (America): anti-ROCK2 (1:500), anti-PCNA (1:1000), anti-GAPDH (1:1000), anti-cyclin D1 (1:500), anti-β-catenin (1:500), anti-MMP2 (1:500), anti-MMP9 (1:500), anti-Flag (1:500) and anti-Ki-67 (1:500).
Immunohistochemical analyses
Serial sections measuring 5 μm thick were mounted on glass slides coated with 10% polylysine. Tissue sections were deparaffinized in xylene and rehydrated using a series of graded washes with ethanol. After inhibition of endogenous peroxidase and antigen retrieval (high temperature and pressure in an autoclave and incubating the tissue sections for 3 min in 10 mM citrate buffer at pH 6.0), sections were incubated with anti-ROCK2 (1:400, Santa Cruz Biotechnology) [13] and anti-Ki-67 (1:100, Santa Cruz Biotechnology) antibody at 4 °C overnight. All slides were processed using the peroxidase–antiperoxidase method (Dako, Hamburg, Germany), and the peroxidase reaction was visualized by incubating the sections with DAB, counterstained with hematoxylin to distinguish the nucleus from the cytoplasm, and evaluated under a microscope, then dehydrated and mounted in resin mount. All the immunostained sections were evaluated in a blinded manner without knowledge of the clinical and pathological parameters of the gastric cancer patients. Five visual fields were randomly selected from each pathological section to determine the staining results, and at least 300 cells were counted each field. Expression score was determined by staining intensity and immunoreactive cell percentage. The cells were scored as follows: 1 (25% tumor cells stained), 2 (26–50% tumor cells stained), 3 (51–75% tumor cells stained) and 4 (76–100% tumor cells stained). For intensity evaluation: 0 no staining, 1 weak staining, 2 moderate staining and 3 strong staining. Then, we multiplied the two scores and divided into the ROCK2 low-expression (<6) and ROCK2 high-expression (6–12) groups [14]. A cutoff value of 50% or more positively stained nuclei in 5 high-power fields was used to identify Ki-67 staining: high-expression group (≥50%) and low-expression group (<50%).
Negative controls were processed by replacing the primary antibody with non-immune IgG (1:100, Santa Cruz Biotechnology).
Staining was repeated three times to avoid possible technical errors, and similar results were obtained in these samples.
Cell proliferation assays
MGC803 cells (normal and transfection of ROCK2-siRNA) and GES-1 cells (normal and transfection of ROCK2-Flag) were seeded onto 96-well cell culture cluster plates (Corning inc, Corning NY) at a density of 2 × 104 cells/well in 100 μl culture and grown overnight. According to the manufacturer’s protocol, cells were measured using a commercial CCK-8 (Dojindo, Kumamoto, Japan). During 2–5-day culture periods, 10 μl of the CCK-8 solution was added to the cell culture every day and incubated for 2 h. The absorbance was read at the wavelength of 490 nm in an automated plate reader. The experiments must repeat at least three times.
Colony formation assays
MGC803 cells (normal and transfection of ROCK2-siRNA) and GES-1 cells (normal and transfection of ROCK2-Flag) were cultured in 6-cm plate at a density of 200 cells/well for 10–14 days. When the clone was observed, the cells were washed twice with PBS, after that fixed with methanol for 30 min and stained with crystal violet for 30 min. Clearly visible colonies (>50 cells/colony) were counted as positive for growth.
Flow cytometric analysis
MGC803 cells and GES-1 cells were seeded overnight in six-well plates (Corning inc, Corning NY). Cells were transfected with ROCK2-siRNA (MGC803 cells) or ROCK2-Flag (GES-1 cells). After 48 h, the cells were collected and fixed in 70% ethanol for at least 24 h at −20 °C. After that, the cells incubated in PBS containing 1 mg/ml RNase A and stained with propidium iodide (PI, 50 μg/mL) in PBS-Triton × 100 for an additional 20 min at 4 °C. The cell cycle was assessed by Becton–Dickinson flow cytometer BD FACS can (San Jose, CA) as well as CellQuest acquisition and analysis programs. Results are representatives of three independent experiments with triplicate samples for each condition.
Trans-well migration assay
Cell migration assay was performed using a 24-well trans-well unit (pore size, 8 μm; Costar, Cambridge, NY, USA). About 1 × 105 cells/ml MGC803 cells (normal and transfection of ROCK2-siRNA) were seeded in the upper chambers in medium, and RPMI-1640 was added to the bottom chambers. After 24 h, the top cells which were not migrated were removed and the bottom cells which were migrated were fixed with paraformaldehyde and stained with crystal violet. The number of migrating cells in five fields was counted under 200× magnification, and the means for each chamber were determined. All experiments were repeated three times.
Trans-well invasion assay
Cell migration assay was performed using a 24-well trans-well unit (pore size, 8 μm; Costar, Cambridge, NY, USA). BD Matrigel TM Basement Membrane Matrix was added to the upper chamber for 1 h at 37 °C. Then, 500 μl medium containing chemotactic factor was placed in the lower chamber. About 1 × 105 cells/ml MGC803 cells (normal and transfection of ROCK2-siRNA) were seeded in the upper chambers at 37 °C for 24 h. Cells were then fixed with paraformaldehyde and stained with crystal violet. All experiments were repeated three times.
Statistical analysis
All statistical analysis was carried out using the SPSS statistics 19 software package. The ROCK2 and Ki-67 expression and the clinicopathologic features were analyzed by using the Chi-square test. Overall survival curves were calculated by the Kaplan–Meier method and compared by the log-rank test. The univariate and multivariate analysis was analyzed by Cox’s proportional hazards regression model, and the risk ratio and its 95% confidence interval were recorded for every marker. The values were expressed as mean ± SE, and P < 0.05 was considered statistically significant.
Result
ROCK2 was overexpressed in gastric cancer tissues
To explore the expression of ROCK2 in GC tissues, we examined the expression of ROCK2 and PCNA, as well as GAPDH as a loading control, by Western blot analysis in eight paired fresh GC tissues and the adjacent normal tissues. Results showed that ROCK2 was consistent with the expression of PCNA which was higher in GC tissues compared with the adjacent normal tissues (Fig. 1).
ROCK2 and PCNA expression in human gastric cancer by Western blot analysis. a The protein level of ROCK2 in gastric cancer tissue (tumor) and adjacent non-tumorous tissues (Normal). GAPDH was used as a loading control; b the bar chart showed the ratio of ROCK2 protein to GAPDH. Mean ± SD of three independent experiments. (*p < 0.05 compared with control non-tumorous adjacent tissues)
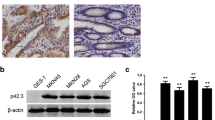
Next we observed the expression of ROCK2 and Ki-67 in 135 GC tissue samples by immunohistochemistry (IHC) analyses. We can see that in Fig. 2 ROCK2 had a significantly higher expression in GC tissue than adjacent normal tissue, which was consistent with Ki-67. ROCK2 was expressed in cell nucleus, while cytoplasm Ki-67 mainly in the nucleus.
Representative photographs of ROCK2 and Ki-67 expressions in gastric cancer tissues by IHC. Paraffin-embedded tissue sections were stained with antibodies for ROCK2 and Ki-67 and counterstained with hematoxylin. a, b Negative staining of Ki-67 and ROCK2 in adjacent normal tissues; c, d strong staining of Ki-67 and ROCK2 in gastric cancer tissues; e, f staining of IgG in gastric cancer tissues and adjacent normal tissues as negative controls; amplification (× 200)
ROCK2 expression was correlated with Ki-67 in GC and its relationship to clinicopathologic variables and clinical prognosis
In order to confirm the clinical significance of ROCK2 in GC progression, we investigated the correlation between ROCK2 and Ki-67 by Pearson correlation test. Results showed that ROCK2 was positively associated with Ki-67 (r = 0.764, p < 0.001, Fig. 3a).
The relation between ROCK2 and clinical characteristics. a Correlation between ROCK2 and Ki-67 expression in gastric cancer tissues. The correlation between ROCK2 and Ki-67 expression was evaluated by Pearson correlation test (r = 0.764, p < 0.001). b Kaplan–Meier survival analysis of 135 gastric cancer patients based on ROCK2 expression level. According to the ROCK2 percentages, patients were divided into high ROCK2 expressers and low ROCK2 expressers. Patients in the high-expression ROCK2 group had a significantly shorter overall survival than low-expression ROCK2 group (p < 0.001)
In addition, we further evaluated the association of ROCK2 expression with clinicopathologic variables including Ki-67 by Pearson Chi-square test. The level of ROCK2 and Ki-67 expression was divided into high-expression group (n = 97) and low-expression group (n = 38) according to the IHC scores. The correlation between ROCK2 expression and clinicopathological characteristics is summarized in Table 1. The results showed that consistent with previous Pearson correlation test, significant positive correlations were found between the expression level of ROCK2 and Ki-67 (P = 0.035; Table 1). Moreover, high expression of ROCK2 was positively associated with tumor grade (p < 0.05), TNM stage (p < 0.05), infiltration depth (p < 0.05) and lymph node invasion (p < 0.05). However, there was no correlation between ROCK2 expression and other clinical variables, such as age, gender and nerve invasion.
Kaplan–Meier survival curves showed that high expression of ROCK2 was associated with poor overall survival and hazard ratio (Fig. 3b). Univariate analysis proved that tumor grade (p < 0.05), TNM stage (p < 0.05), infiltration depth (p < 0.05) and lymph node invasion (p < 0.05) were associated with overall survival (Table 2). Cox’s proportional hazards model using for multivariate analysis showed that ROCK2 was an independent prognostic factor for GC patients’ overall survival (HR 2.383, 95% CI: 1.278–4.442, p = 0.006, Table 3). These data indicated that the expression of ROCK2 was significantly associated with clinical characteristics and poor overall survival.
ROCK2 promoted cells proliferation, metastasis and invasive in vitro
To further investigate the role of ROCK2 in GC cells proliferation, metastasis and invasive, we first analyzed the expression of ROCK2 in normal gastric epithelial cell line GES-1 and GC cell line-MGC803. As expected, ROCK2 expression is lower in the normal gastric epithelial cell line GES-1 than it was in GC cell BGC823, MGC803 and MKN45 (Fig. 4a). GES-1 cells transfected with Flag-ROCK2 and MGC803 cells transfected with ROCK2-siRNA were used for following experiments. Transfection effects were verificated by Western blot as shown in Fig. 4b.
ROCK2 promotes the proliferation ability of gastric cancer cells. a The expression of ROCK2 in GES-1, BGC823, MGC803 and MKN45 cell lines. b GES-1 cells were transiently transfected with ROCK2-Flag plasmid as described above for 48 h, Western blot analysis the expression of ROCK2-Flag. MGC803 cells were transiently transfected with ROCK2-siRNA for 48 h, Western blot analysis knockdown expression of ROCK2. c In vitro Cell Counting Kit (CCK)-8 assay was used for GES-1 cells transfected with control or ROCK2-Flag and MGC803 cells transfected with control or ROCK2-siRNA then examine cells growth by absorbance at 490 nm at the indicated time. d Colony formation analysis was used for GES-1 cells transfected with control or ROCK2-Flag and MGC803 cells transfected with control or ROCK2-siRNA. e Cell cycle analysis was shown after GES-1 cells transfected with control or ROCK2-Flag and MGC803 cells transfected with control or ROCK2-siRNA by flow cytometry (mean ± SD from three independent experiments *p < 0.05)
Based on the data listed above, ROCK2 was positively correlated with cells proliferation markers, PCNA and Ki-67. We explored the role of ROCK2 in cell proliferation ability by Cell Counting Kit-8 (CCK-8) assay, colony formation assays and flow cytometric analysis. Cell Counting Kit-8 (CCK-8) assay proved that the proliferation ability was cut down in ROCK2-siRNA-transfected MGC803 cells compared with the cells transfected with the vector control, while GES-1 cells transfected with ROCK2-Flag showed the opposite result (P < 0.05, Fig. 4c). The same results were also seen in colony formation assays (P < 0.05, Fig. 4d) and flow cytometric analysis (P < 0.05, Fig. 4e). All results show that ROCK2 promoted GC cell proliferation.
As ROCK2 could increase cells metastasis and invasion in other cancers, we further researched the function of ROCK2 in cell metastasis and invasion by trans-well assays. Trans-well migration assays showed that the metastasis significantly decreased in ROCK2-siRNA-transfected MGC803 cells compared with the cells transfected with the vector control (P < 0.05 Fig. 5a). Further, Matrigel invasion assays demonstrated that downregulation of ROCK2 could repressed the invasive ability of MGC803 cells (P < 0.05, Fig. 5b).
ROCK2 promotes the metastasis and invasive ability of gastric cancer cells and the effects of ROCK2 on the related signal pathway. a MGC803 cell migration transfected with control or ROCK2-siRNA was examined by trans-well assays. b The cell invasive ability transfected with control or ROCK2-siRNA was examined with Matrigel cell culture chambers. c GES-1 cells were transiently transfected with ROCK2-Flag plasmid, and MGC803 cells were transiently transfected with ROCK2-siRNA. Western blot analysis showed the protein expression of cyclin D1, β-catenin, MMP2 and MMP9 (mean ± SD from three independent experiments. *, p < 0.05)
Overall, we could come to a conclusion that ROCK2 promoted cells proliferation, metastasis and invasion in vitro.
The signal pathway of ROCK2 in GC cells
To further study the mechanism of which ROCK2 regulated GC cells proliferation, metastasis and invasion, we decided to detect the related protein changed by Western blot. As shown in Fig. 5c, the expression of cyclin D1, β-catenin, MMP2 and MMP9 was upregulated when ROCK2 was overexpressed in gastric epithelial GES-1 cells by transfected with ROCK2-Flag. The expression of cyclin D1, β-catenin, MMP2 and MMP9 was downregulated in MGC803 cells when ROCK2 was knockdown by ROCK2-siRNA.
Discussion
Gastric cancer (GC) is difficult to cure unless it is found at an early stage [15]. Owing to the lack of specificity, early diagnosis rate is low and the majority of gastric cancer patients are at middle-late stage when diagnosis; 40–60% patients with gastric cancer received gastric cancer radical operation will often have postoperative recurrence and metastasis, and these characteristics seriously affect the long-term survival in patients with gastric cancer [16, 17]. Despite great advancement of new diagnosis and treatment strategies of gastric cancer, the exact molecular mechanisms of gastric cancer remains poorly understand. Thus, the identification of the molecular mechanism during gastric cancer progression and metastasis may provide patients with novel diagnostic and therapeutic strategies.
Rho-associated kinase (ROCK) is an primary downstream effector of the Rho small GTPase, which acts as a molecular switch that binds GTP and GDP to regulate cell survival, proliferation and cytoskeleton organization, inducing alterations in cell shape/morphology, adhesion and movement [18–20]. Increased expression of ROCK is well documented in tumors and related to cancer progression, metastasis and poor prognosis [10, 21–23]. Conversely, inhibition of ROCK induces cell death, disrupts angiogenesis and reduces metastasis in several tumor types [9, 24]. Therefore, ROCK proteins are currently under consideration as therapeutic targets for cancer. Two existing isoforms, ROCK1 and ROCK2, are known.
In this study, we found that ROCK2 protein was frequently overexpressed in human GC and promoted cells proliferation, metastasis and invasion in GC. At first, we found that the expression of ROCK2 was higher in GC tissues than adjacent normal tissues. So, we analyzed the relationship between expression of ROCK2, clinicopathologic variables and clinical prognosis. Just as expected, ROCK2 was associated with tumor grade, TNM stage, infiltration depth and lymph node invasion. Importantly, multivariate analysis showed that ROCK2 was an independent prognostic factor for GC patients’ overall survival.
Furthermore, we found that overexpression of ROCK2 promoted GC cells proliferation,metastasis and invasion of GC cells in vitro. This mirrored the findings of ROCK2 overexpression in human GC samples and its association with more frequent tumor clinicopathologic variables. Conversely, when we knocked down the endogenous ROCK2 specifically using ROCK2-siRNA approach in GC cells, the proliferative ability, mobility and invasiveness of MGC803 cells were significantly reduced, indicating that ROCK2 was closely involved in GC proliferation, metastasis and invasion. We also detected the signal-path relative protein of ROCK to further prove the role of ROCK2.
The expression and physiological significance of ROCK2 in GC have never been addressed. Reduction in ROCK2 expression might be a feasible target for antagonizing tumor progress in GC.
References
Hironaka S, Sugimoto N, Yamaguchi K, Moriwaki T, Komatsu Y, Nishina T, et al. S-1 plus leucovorin versus S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin in patients with advanced gastric cancer: a randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(1):99–108. doi:10.1016/S1470-2045(15)00410-6.
Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, et al. Association between helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 2016;. doi:10.1053/j.gastro.2016.01.028.
Wang L, Yin J, Wang X, Shao M, Duan F, Wu W, et al. C-Type lectin-Like receptor 2 suppresses AKT signaling and invasive activities of gastric cancer cells by blocking expression of phosphoinositide 3-Kinase subunits. Gastroenterology. 2016;. doi:10.1053/j.gastro.2016.01.034.
Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17(3):309–18. doi:10.1016/S1470-2045(15)00553-7.
Van Cutsem E, Ducreux M. Colorectal and gastric cancer in 2015: the development of new agents and molecular classifications. Nat Rev Clin Oncol. 2016;13(2):69–70. doi:10.1038/nrclinonc.2015.217.
Kalaji R, Wheeler AP, Erasmus JC, Lee SY, Endres RG, Cramer LP, et al. ROCK1 and ROCK2 regulate epithelial polarisation and geometric cell shape. Biol Cell. 2012;104(8):435–51. doi:10.1111/boc.201100093.
Goetsch KP, Snyman C, Myburgh KH, Niesler CU. ROCK-2 is associated with focal adhesion maturation during myoblast migration. J Cell Biochem. 2014;115(7):1299–307. doi:10.1002/jcb.24784.
Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases. 2014;5:e29846. doi:10.4161/sgtp.29846.
Vigil D, Kim TY, Plachco A, Garton AJ, Castaldo L, Pachter JA, et al. ROCK1 and ROCK2 are required for non-small cell lung cancer anchorage-independent growth and invasion. Cancer Res. 2012;72(20):5338–47. doi:10.1158/0008-5472.CAN-11-2373.
Wong CC, Wong CM, Tung EK, Man K, Ng IO. Rho-kinase 2 is frequently overexpressed in hepatocellular carcinoma and involved in tumor invasion. Hepatology. 2009;49(5):1583–94. doi:10.1002/hep.22836.
Qiu Y, Yuan R, Zhang S, Chen L, Huang D, Hao H, et al. Rock2 stabilizes beta-catenin to promote tumor invasion and metastasis in colorectal cancer. Biochem Biophys Res Commun. 2015;467(4):629–37. doi:10.1016/j.bbrc.2015.10.103.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics. CA Cancer J Clin. 2014;64(1):9–29. doi:10.3322/caac.21208.
Zhang J, He X, Ma Y, Liu Y, Shi H, Guo W, et al. Overexpression of ROCK1 and ROCK2 inhibits human laryngeal squamous cell carcinoma. Int J Clin Exp Pathol. 2015;8(1):244–51.
Wan C, Gong C, Zhang H, Hua L, Li X, Chen X, et al. beta2-adrenergic receptor signaling promotes pancreatic ductal adenocarcinoma (PDAC) progression through facilitating PCBP2-dependent c-myc expression. Cancer Lett. 2016;373(1):67–76. doi:10.1016/j.canlet.2016.01.026.
Wang J, Yu JC, Kang WM, Ma ZQ. Treatment strategy for early gastric cancer. Surg Oncol. 2012;21(2):119–23. doi:10.1016/j.suronc.2010.12.004.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi:10.3322/caac.20107.
Cao Y, DePinho RA, Ernst M, Vousden K. Cancer research: past, present and future. Nat Rev Cancer. 2011;11(10):749–54. doi:10.1038/nrc3138.
Zhang C, Zhang S, Zhang Z, He J, Xu Y, Liu S. ROCK has a crucial role in regulating prostate tumor growth through interaction with c-Myc. Oncogene. 2014;33(49):5582–91. doi:10.1038/onc.2013.505.
Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6(2):167–80. doi:10.1038/nrm1587.
Patel RA, Forinash KD, Pireddu R, Sun Y, Sun N, Martin MP, et al. RKI-1447 is a potent inhibitor of the Rho-associated ROCK kinases with anti-invasive and antitumor activities in breast cancer. Cancer Res. 2012;72(19):5025–34. doi:10.1158/0008-5472.CAN-12-0954.
Oellers P, Schroer U, Senner V, Paulus W, Thanos S. ROCKs are expressed in brain tumors and are required for glioma-cell migration on myelinated axons. Glia. 2009;57(5):499–509. doi:10.1002/glia.20777.
Lochhead PA, Wickman G, Mezna M, Olson MF. Activating ROCK1 somatic mutations in human cancer. Oncogene. 2010;29(17):2591–8. doi:10.1038/onc.2010.3.
Liu X, Choy E, Hornicek FJ, Yang S, Yang C, Harmon D, et al. ROCK1 as a potential therapeutic target in osteosarcoma. J Orthop Res. 2011;29(8):1259-66. doi:10.1002/jor.21403
Montalvo J, Spencer C, Hackathorn A, Masterjohn K, Perkins A, Doty C, et al. ROCK1 & 2 perform overlapping and unique roles in angiogenesis and angiosarcoma tumor progression. Curr Mol Med. 2013;13(1):205–19.
Acknowledgements
This work was supported by Nantong University innovation project (No.YKC15077, No.YKC15076); the National Natural Science Foundation of China (No. 81541052); the Science and Technology Project Foundation of Nantong City (No. MS22015092, No. BK2014084, No. MS22015005); the Medical Scientific Research Projects of Jiangsu Provincial Bureau of Health (No. 201455); Six Talent Peaks Project in Jiangsu Province; and Health Bureau Youth Science Foundation of Nantong Grant (No. WQ2015056).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Manhua Li and Jing Ke have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, M., Ke, J., Wang, Q. et al. Upregulation of ROCK2 in gastric cancer cell promotes tumor cell proliferation, metastasis and invasion. Clin Exp Med 17, 519–529 (2017). https://doi.org/10.1007/s10238-016-0444-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-016-0444-z