Abstract
The concentration of inorganic phosphate (Pi) in plasma is under hormonal control, with deviations from normal values promptly corrected to avoid hyper- or hypophosphatemia. Major regulators include parathyroid hormone (PTH), fibroblast growth factor 23 (FGF-23), and active vitamin D3 (calcitriol). This control is achieved by mechanisms largely dependent on regulating intestinal absorption and renal excretion, whose combined actions stabilise plasma Pi levels at around 1–2 mM. Instead, Pi concentrations up to 13 and 40 mM have been measured in saliva from humans and ruminants, respectively, suggesting that salivary glands have the capacity to concentrate Pi. Here we analysed the transcriptome of parotid glands, ileum, and kidneys of mice, to investigate their potential differences regarding the expression of genes responsible for epithelial transport of Pi as well as their known regulators. Given that Pi and Ca2+ homeostasis are tightly connected, the expression of genes involved in Ca2+ homeostasis was also included. In addition, we studied the effect of vitamin D3 treatment on the expression of Pi and Ca2+ regulating genes in the three major salivary glands. We found that parotid glands are equipped preferentially with Slc20 rather than with Slc34 Na+/Pi cotransporters, are suited to transport Ca2+ through the transcellular and paracellular route and are potential targets for PTH and vitamin D3 regulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salivation plays several roles in the gastrointestinal tract, including hydration of epithelia, initiation of digestion, and defence against microbes (for review, see [51]). Most of the daily salivary output is produced in response to eating, whereas salivation rate is slow between meals and virtually absent during sleep. Mammals have three major pairs of salivary glands, namely submandibular, sublingual and parotid. As other secretory tissues, salivary glands contain two types of epithelial cells: acinar cells that are responsible for secretion of the primary saliva, and ductal cells accountable for the secretion/reabsorption processes that determine the composition of the final saliva. In acinar cells, transepithelial Cl− secretion together with paracellular Na+ transport generates the osmotic gradient required for water secretion into the acinar lumen. Acinar Cl− secretion is supported by basolateral uptake of Cl−, mediated by Na+/K+/Cl− cotransporters and Cl−/HCO3− exchangers, followed by apical efflux via a Ca2+-dependent Cl−-channel [17, 44, 57], whereas Na+ and water transport are mediated by claudin-2 and aquaporin-5, respectively [35]. As this plasma-like isotonic primary secretion travels along the duct, ductal cells reabsorb most of the NaCl by mechanisms involving the apical Na+ and Cl− channels ENaC and CFTR, respectively, as well as a Cl−/HCO3− exchanger, whereas they secrete K+ probably via apical Slo K+ channels [48, 55, 62, 64]. Since ductal cells are rather impermeable to water, these changes in electrolyte composition result in an alkaline hypotonic final saliva (for review, see [10, 51]).
The reported concentration of Pi in saliva ranges from 1.3 to 13 mM in humans [60] whereas up to 40 mM have been reported in ruminants [38], suggesting that salivary glands have the capacity to concentrate Pi. Furthermore, salivary Pi was recently reported to be increased in patients undergoing hemodialysis [61] and to correlate with carotid intima media thickness, a marker of atherosclerosis [47]. The mechanisms responsible for Pi transport in salivary glands have been studied mostly in ruminants. Unlike in other mammals, handling of Pi in ruminants involves a so-called endogenous Pi recycling, consisting of a large delivery of Pi into the gastrointestinal tract via salivary secretion followed by downstream Pi absorption mostly in the jejunum (for review, see [54]). This recycling mechanism provides high amounts of Pi to the forestomach which are required to support microbial growth as well as to increase the intestinal buffering capacity, since ruminal microbial fermentation produces large quantities of short-chain fatty acids. In ovine, the acinar cells of the parotid glands are known to secrete large amounts of Pi, a process that is initiated by uptake of Pi across the basolateral membrane by a Na+-dependent and phosphonoformic acid-inhibitable mechanism attributed to NaPi-IIb/Slc34a2 [38, 66, 72], followed by apical extrusion mediated by a mechanism which molecular identity remains unknown (for review, see [54]). Transport of Pi across basolateral membrane vesicles isolated from ovine parotid glands is not regulated by dietary Pi [37, 66]. This is unlike transport of Pi into intestinal brush border membranes vesicles (BBMV), a process also mediated by NaPi-IIb whose intestinal expression is regulated by the dietary content of Pi [27, 30].
Expression of NaPi-IIb was confirmed in adult human parotid and submandibular glands obtained from resection material of patients undergoing maxillofacial surgery [34]. In both glands, NaPi-IIb was detected in acinar and ductal cells, with the expression in acinar cells restricted to the basolateral membrane whereas apical expression was found along ducts. More recently, the expression of NaPi-IIb at the apical membrane of submandibular ductal cells was also described in mice [40]. However, unlike previous reports in ruminants, the salivary Pi concentration in mice reflects the dietary content of Pi, with higher salivary Pi levels in mice fed for a week with high Pi diets than those fed on low Pi [40]. Moreover, a high Pi diet (as well as an intragastric Pi bolus) reduces the expression of NaPi-IIb at the apical membrane of sublingual and/or submandibular ductal cells in mice. However, no NaPi-IIb/Slc34a2 transcripts were detected in a recently published single-cell RNA-seq analysis in which different cell populations of mice submandibular glands were investigated [67]. Unlike Pi, the salivary concentration of Ca2+ is slightly lower than its plasma concentration [60].
Here, we performed RNA-seq to compare the expression of Pi transporters and regulatory genes in mouse parotid glands with their expression in ileum and kidney, the two epithelial tissues in which Pi transport is better studied. Because many of the regulators of Pi homeostasis also affect intestinal and renal Ca2+ handling, the expression of Ca2+ transporters was also investigated. In addition, the effect of 3 days vitamin D3 treatment was examined since this treatment is known to regulate Pi and Ca2+ transport in kidney and intestine.
Material and methods
Animal handling and sample collection
Wild type male mice (C57BL/6J) 10–12 weeks old were purchased from Janvier (Genest Saint Isle; France) and let to adapt for 1 week, during which they were fed ad libitum with standard chow (maintenance rodent diet 3436, Promivi Kliba AG with 0.8% phosphate and 1% calcium, 1000 IU vitamin D3) and held in individually ventilated cages. Then, tissue samples of 5 mice were extracted and used for RNA quantification whereas 5 mice were fixed with paraformaldehyde (PFA) for immunofluorescence studies. Additionally, 12 mice were injected intraperitoneally during two consecutive days (once per day, in the morning) with either vehicle (corn oil/ethanol/PBS) or 4 μg kg−1 BW of 1,25(OH)2 vitamin D3 (6 animals per group) and tissue samples were extracted for RNA quantification.
Mice were anaesthetized with isoflurane (Piramal Critical Care Deutschland GmbH. Hallbergmoos, Germany) and killed by cervical dislocation prior to collection of kidneys, ileum, and salivary glands (submandibular, parotid and sublingual). Kidneys were decapsulated whereas the ileum was rinsed with 0.9% NaCl and inverted in order to scrape off the epithelial layer. All samples were snap-frozen in liquid nitrogen and stored at – 80 °C. For fixation, mice were perfused through the left ventricle with 15 ml of a solution containing 5000 U/ml heparin, 1% lidocaine, 16% CaCl2, and 0.9% NaCl in distilled H2O, followed by 40 ml of 4% PFA in PBS. Tissues were extracted and kept overnight at 4 °C in 4% PFA and then sequentially incubated in 10%, 20%, and 30% sucrose/PBS during the following 2 days. Upon embedding in OCT media (Sakura) samples were frozen in liquid propane and stored at − 80 °C. Animal experiments were performed according to the Swiss law of animal welfare and were approved by the local veterinary authorities (Kantonales Veterinäramt Zürich) under the license number ZH240/19.
RNA extraction, reverse transcription, and real-time quantitative PCR (qPCR)
One of each of the sublingual, submandibular, and parotid glands as well as half a kidney and a piece of the ileum epithelial scrapping were lysed in RT buffer containing 1% β-mercaptoethanol, using MagNA Lyser Green Beads and the Precellys 24 tissue homogeniser (Bertin Corp., Rockville, MD, USA) at 5500 rpm 2 × 20 s. RNA was purified with NucleoSpin RNA Column (Macherey–Nagel) and eluted in RNase free H20. Aliquots of 300 ng were reverse transcribed into cDNA using the TaqMan Reverse Transcription kit (ThermoScientific, USA). The incubation protocol consisted of 10 min at 25 °C, 30 min at 48 °C and 5 min at 95 °C. The expression of the genes of interest was analysed by qPCR using the KAPA Probe Fast qPCR Universal Master Mix (2 ×) Kit (Kapa Biosystems. Cape Town, South Africa) in the presence of gene-specific FAM/TAMRA-labelled probe (0.1 µM) and primers (1 µM) (Supplementary Table 1). In few cases, SYBR green, instead of labelled probes, was used for quantification. The qPCR protocol consisted of 20 s incubation at 95 °C followed by 40 cycles of 3 s at 95 °C and 30 s at 60 °C, using the 7500 Fast Real-Time PCR System (Applied Biosystems, Switzerland). Cycle threshold (Ct) were manually set at the exponential phase of the amplification curve. Ct values of the analysed genes were normalised to that of the hypoxanthine–guanine phosphoribosyltransferase (Hprt) gene, and relative mRNA expression levels were calculated as 2(Ct_Hprt –Ct_gene). Primers/probes were designed with the Primer BLAST tool of the National Center of Biotechnical Information (NCBI) and ordered from Microsynth (Balgach, Switzerland).
RNA-seq
Transcriptome sequence analysis of RNA samples from parotid glands, kidney and ileum was done at the Functional Genomics Centre Zürich (FGCZ). Libraries were prepared using the Illumina TruSeq mRNA method and sequenced with the Illumina NovaSeq6000 system. Original reads were cleaned by removing adapter sequences, trimming low quality ends, and filtering reads with low quality (phred quality < 20) using Fastp (Version 0.20) [11]. Then, cleaned reads were aligned to the Mouse reference genome (build GRCm38.p5) and mRNA expression was quantified using Kallisto (Version 0.46.1) [9].
Immunohistochemistry
Cryopreserved salivary glands and ileum samples were cut into 2–3 μm thin slices at – 25 °C using a Cryostat CM1850 (Leica) and mounted on pre-cooled SuperFrost Plus Slides (Mezel GmbH). Samples were rehydrated in PBS and incubated with 50 mM NH4Cl/PBS for 20 min at room temperature. For antigen retrieval, sections were then treated with either Tris–EDTA, pH 9 (10 mM Tris base, 1 mM EDTA, 0.05% Tween20) for 10 min at 95 °C or with 1% SDS/PBS for 5 min at room temperature. Bovine serum albumin (BSA) at 1% in PBS was used for blocking as well as for dilution of antibodies. Sections were incubated at 4 °C overnight with 1: 100 dilutions of a commercial (NPT2b11-A, Alpha Diagnostic International, San Antonio, TX, USA) or a homemade antibody against NaPi-IIb [30]. After washing the slides sequentially with hypertonic and isotonic PBS, samples were incubated for 1 h at room temperature in the dark with secondary anti-rabbit antibodies (1:1000, Alexa Fluor 488, A21202, Molecular Probes). All samples were co-stained with DAPI (1:500, D3571, Invitrogen). After washing, sections were embedded with DAKO-Glycergel mounting media (DAKO) and stored at 4 °C. Images were taken with a fluorescence microscope (Leica) and processed in ImageJ.
Statistical analysis
Statistical analysis of gene expression in salivary glands from mice injected with vehicle versus vitamin D3-treated mice was done with GraphPad Prism8 (GraphPad Software, San Diego, USA). For each gland, differences between both groups were analysed with the unpaired Student’s t test. p ≤ 0.05 was considered significant. All data are presented as individual values with mean ± SD.
Results
Comparative mRNA expression of Na+/Pi cotransporters in salivary glands, ileum, and kidney
The mRNA abundance of Slc34 and Slc20 paralogues, the Na+/Pi cotransporters identified at the BBM of intestinal and renal epithelial cells (for review, see [18, 49]) was compared among the 3 types of salivary glands, ileum and kidney. Semi-quantitative measurements were performed by qPCR using primers with similar amplification efficiencies [53]. As previously reported [53], Slc34a1/NaPi-IIa and Slc34a3/NaPi-IIc mRNAs were detected in renal samples but not in ileum, and within kidney the abundance of Slc34a1 was about 1 order of magnitude higher than Slc34a3 (Fig. 1a, c); none of the 2 transcripts was detected in the different salivary glands. Also as anticipated, Slc34a2/NaPi-IIb mRNA was highly expressed in ileum, with 2.5 orders of magnitude lower levels also detected in kidney as recently reported [53] (Fig. 1b); low Slc34a2 expression was observed in all salivary glands as well. As expected, Slc20a1/Pit-1 mRNA was expressed both in ileum and kidney (Fig. 1d), though at far lower levels than Slc34a1 and Slc34a2; Slc20a1 transcripts were also detected in salivary glands. The expression of Slc20a2/Pit-2 mRNA was also comparable in ileum and kidney (Fig. 1e), but in both tissues was well below the Slc34a1 and Slc34a2 paralogues levels; Slc20a2 transcripts were detected in the 3 salivary glands as well.
Expression of Na/Pi cotransporters mRNAs in salivary glands, ileum, and kidney. The mRNA expression of a Slc34a1, b Slc34a2, c Slc34a3, d Slc20a1, and e Slc20a2 was quantified by qPCR in samples of ileum, kidney as well as from submandibular, sublingual, and parotid glands of mice (n = 5). Data was normalised to the expression of Hprt. Protein expression of NaPi-IIb/Slc34a2 was analysed by immunofluorescence in sections of f submandibular, g sublingual, and h parotid glands. Scale = 25 µm
The expression of NaPi-IIb at the protein level was analysed in salivary glands by staining tissue sections with a home-made antibody that detects the cotransporter in mouse intestinal epithelia [30] (Supplementary Fig. 1). However, this antibody failed to provide reliable signals in any of the salivary glands (data not shown). Instead, a commercial antibody previously reported to stain NaPi-IIb in submandibular glands of mice [40] labelled ductal structures in all 3 glands (Fig. 1f-h), with the signal concentrated on the luminal side. We did not observe any basolateral staining. The specificity of this antibody was confirmed by performing immunofluorescence of intestinal samples, since it reacted with a luminal protein in sections of ileum of wild type but not of intestinal-specific NaPi-IIb-deficient mice (Supplementary Fig. 1).
Transcriptome analysis of Na+/Pi cotransporters in parotid glands, ileum, and kidney
RNA-seq was performed in samples of parotid glands, ileum and kidney (Supplementary Table 2). From all salivary glands, parotids were chosen as they tended to have higher expression of Na+/Pi transporters than the other 2 glands (Fig. 1b, d and e). RNA-seq detected the presence of 15,210 transcripts in ileum, 16,008 in kidney and 15,609 in parotid glands (Supplementary Table 2). This analysis confirmed the relative mRNA expression of Slc34 transporters in ileum and kidney provided by qPCR, i.e. the abundance of Slc34a1/NaPi-IIa (Fig. 2a) and Slc34a3/NaPi-IIc (Fig. 2c) transcripts was about 4 and 1.5 orders of magnitude higher in kidney than in ileum, respectively, whereas Slc34a2/NaPi-IIb mRNA expression was 2.5 orders of magnitude higher in intestine than in kidney (Fig. 2b). This analysis also confirmed the near absence of Slc34 paralogues in parotid glands except for some low expression of Slc34a2, as expected based on the qPCR and immunofluorescence data described above (Fig. 1b, f-h). In contrast to Slc34 paralogues, both Slc20 transcripts were expressed in all analysed tissues, in agreement with their ubiquitous distribution, and differences between organs never reached 1 order of magnitude (Fig. 2d, e). Xpr1, the gene encoding a putative basolateral transporter for Pi [23], was expressed at comparably high levels in ileum and kidney, whereas its abundance was about 1 order of magnitude lower in parotid glands (Fig. 2f). The expression of Cldn3, a component of tight junctions suggested to seal the paracellular pathway for Pi in the gut [26], was almost 2 orders of magnitude higher in intestine than in kidney, with only low levels detected in parotid glands (Fig. 2g).
Transcriptome quantification of Na/Pi cotransporters, Xpr1 and Cldn3 in parotid glands, ileum, and kidney. Abundance of a Slc34a1, b Slc34a2, c Slc34a3, d Slc20a1, e Slc20a2 as well as f Xpr1, the putative basolateral transporter and g Cldn3, a claudin suggested to tighten the intestinal epithelia for paracellular transport of Pi, in the RNAseq-based transcriptome analysis of ileum, kidney, and parotid glands (n = 4). Data is expressed as transcripts per kilobase million (tpm)
Transcriptome analysis of genes involved in transport of Ca2+ in parotid glands, ileum, and kidney
As expected, transcriptome data showed that the apical Ca2+ channel Trpv5 (transient receptor potential cation channel vanilloid) is highly abundant in kidney but absent from ileum (Fig. 3a), since it is well known that extra-renal expression of the channel is limited to duodenum/jejunum and placenta [32]. Trpv6 was also expressed in kidney (though at lower levels than Trpv5) but was absent in ileum (Fig. 3b), also in agreement with previous reports showing intestinal expression of Trpv6 in mouse duodenum/jejunum and colon but not in ileum [58]. Trpv6 (Fig. 3b) but not Trpv5 (Fig. 3a) was found in parotid glands, fitting well with data obtained from human salivary glands [33].
Transcriptome quantification of genes involved in Ca2+ transport in parotid glands, ileum, and kidney. Abundance of apical channels a Trpv5 and b Trpv6, of intracellular Ca2+-binding proteins, c Calb1, d S100g, and e Cabp1, of sarcoplasmic reticulum ATPases f Atp2a1, g Atp2a2, and h Atp2a3, of basolateral Ca2+ ATPases i Atp2b1, j Atp2b2, and k Atp2b4, of basolateral Na+/Ca.2+ exchangers, l Slc8a1, and of claudins, m Cldn2, n Cldn12, o Cldn16, and p Cldn19, in the RNA-seq-based transcriptome analysis of ileum, kidney, and parotid glands (n = 4). Data is expressed as transcripts per kilobase million (tpm)
The Ca2+-binding protein Calb1/calbindin-D28K was highly expressed in kidney whereas it was absent from ileum (Fig. 3c). Calb2/calbindin-D29K mRNA was not detected in any of the analysed tissues (data not shown), whereas S100g/Calb3/calbindin-D9K expression was about 2 orders of magnitude higher in kidney than ileum (Fig. 3d), in agreement with duodenum being the intestinal segment with highest expression of Calb3 in humans [4]. None of the 3 Calb transcripts was detected in parotids, partially fitting a previous study reporting the absence of Calb1 and Calb3, though Calb2 expression, in human parotid glands [33]. Low mRNA levels of Cabp1/Ca2+-binding protein 1 were measured in all samples (Fig. 3e).
From the 3 Serca paralogues, the Ca2+ ATPases that transport Ca2+ from the cytoplasm into the sarco (SR)/endoplasmic reticulum (ER), Atp2a2/Serca2 and Atp2a3/Serca3 but not Atp2a1/Serca1 have been shown to be widely distributed [75]. Here, we found that Atp2a1/Serca1 was detected only in the transcriptome of parotid glands (Fig. 3f), whereas Atp2a2/Serca2 was more abundant in ileum and kidney than in parotid glands (Fig. 3g). Instead, Atp2a3/Serca3 expression was about 1 order of magnitude higher in ileum and parotid glands than in kidney (Fig. 3h). This pattern of expression fits partially with published data, as Atp2a2 and Atp2a3 (though not Atp2a1), were found in human parotid glands [33] and Atp2a3 is known to be particularly abundant in intestine [75].
From the 4 paralogues of Pmca, the plasma membrane Ca2+ ATPase that mediates basolateral efflux of Ca2+, Atp2b1/Pmca1, and Atp2b4/Pmca4 are widely expressed whereas expression of Atp2b2/Pmca2 and Atp2b3/Pmca3 is restricted to few tissues [68]. Atp2b1/Pmca1 was the most abundant in the 3 analysed transcriptomes, and its expression was higher in ileum than in kidney and parotid glands (Fig. 3i). The levels of Atp2b2/Pmca2 (Fig. 3j) and Atp2b4/Pmca4 (Fig. 3k) were about 1 order of magnitude higher in kidney than in the other tissues, whereas Atp2b3/Pmca3 was not detected in any of the 3 organs (data not shown). Our data fits well with their general pattern of expression and with their expression in salivary glands of humans and rats [8, 33]. From the 3 Ncx2 paralogues, the basolateral Na+/Ca2+ exchanger, Slc8a1/Ncx1 is virtually ubiquitously expressed whereas Slc8a2/Ncx2 and Slc8a3/Ncx3 are restricted to lung and excitable tissues [42]. Accordingly, Slc8a1/Ncx1 mRNA was detected in all analysed tissues, though it was 2 orders of magnitude more abundant in the renal transcriptome than in ileum and parotid glands (Fig. 3i). The expression of Slc8a2/Ncx2 was below 0.2 tpm in all tissues, whereas Slc8a3/Ncx3 mRNA was not detected in any of the investigated samples, in agreement with their restricted tissue expression and their reported absence from human salivary glands [33].
From the claudins involved in paracellular transport of Ca2+, Cldn2 (Fig. 3m) and Cldn12 (Fig. 3n) were the ones with higher expression in ileum and kidney where their levels were 1 to 2 orders of magnitude higher than in parotid glands. This data is in agreement with the known role of both Cldns in intestinal and renal (re)absorption of Ca2+ [6]. Expression of Clnd2 and Cldn12 mRNA was recently reported in parotid glands of pigs [77], though a previous report failed to detect Cldn2 immunosignal in any of the salivary gland in rats [59]. Very low levels of Cldn14 were observed in all analysed tissues (data not shown), consistent with the low expression of this negative regulator of paracellular Ca2+ permeability in the absence of hypercalcemia [16, 25]. In agreement with previous reports [39], mRNAs of Cldn16 (Fig. 3o) and Cldn19 (Fig. 3p), both responsible for familial hypomagnesemia with hypercalciuria and nephrocalcinosis [7, 73], were mostly restricted to kidney, though Cldn16 immunoreactivity has been reported at the basal membrane of acinar cells in human submandibular glands [45].
Transcriptome analysis of genes involved in hormonal regulation of Pi and Ca2+ homeostasis in parotid glands, ileum, and kidney
Next, we compared the expression of the receptors of the main endocrine factors involved in hormonal regulation of Pi and Ca2+ transport, namely parathyroid hormone (PTH), fibroblast growth factor 23 (Fgf-23) and vitamin D3 (for review, see [36, 49, 52]). As expected, the mRNA expression of the PTH receptor Pth1r was more than 2 orders of magnitude higher in kidney than in ileum, with only low levels detected in parotid glands (Fig. 4a). Pth2r was hardly detected in any of the studied samples (data not shown).
Transcriptome quantification of genes involved in regulation of Pi and Ca.2+ homeostasis in parotid glands, ileum, and kidney. Abundance of a the PTH receptor Pthr1, of Fgf receptors b Fgfr1, c Fgfr2, d Fgfr3, e Fgfr4, and f Kl, the klotho co-receptor, of vitamin D3 metabolising enzymes and receptors g Cyp2r1, h Cyp27b1, i Cyp24a1, j Vdr, and of k Casr, in the RNA-seq-based transcriptome analysis of ileum, kidney and parotid glands (n = 4). Data is expressed as transcripts per kilobase million (tpm)
Though extra-osseous expression of Fgf-23 mRNA has been reported [14, 56], Fgf-23 transcripts were not detected in any of the analysed tissues (data not shown). The kidney was the organ with the highest expression of FGF receptors (Fgfrs) mRNA, with similarly high levels of Fgfr1 (Fig. 4b), Fgfr3 (Fig. 4d) and Fgfr4 (Fig. 4e), and slightly lower Fgfr2 (Fig. 4c), a pattern that fits well with previously published data [2, 22]. Fgfr2 and Fgfr3 were the most abundant transcripts in ileum, whereas Fgfr1 and Fgfr2 were the mRNAs with higher expression in parotid glands (Fig. 4b-e). Protein expression of all 4 receptors has been reported in human ileum as well as along the whole intestine in mice, though Fgfr3 seems particularly enriched in crypts whereas Fgfr4 was detected in the embryonic gut [1] (for review, see [13]). The presence of Pth1r and Fgfr1 and absence of Fgfr3 and Fgfr4 transcripts was recently reported in salivary glands of mice, but the expression of Fgfr2 was not analysed [40]. As expected based on previous reports [46], while mRNA of the Fgf-23 coreceptor αklotho (Kl) was highly expressed in kidney, it was not detected in ileum (Fig. 4f); moreover, the coreceptor was barely expressed in parotid glands (Fig. 4f), in agreement with a recent report [40].
A number of hydroxylases are involved in the activation (Cyp2r1, Cyp27b1) and inactivation (Cyp24a1) of vitamin D3 (for review see [52]). As expected, all of them were highly or moderately expressed in kidney (Fig. 4g-i). Instead, they were either not detected or detected at very low levels in ileum and parotid glands, except for Cyp2r1 transcripts the levels of which were comparable in all tissues. As expected, the vitamin D receptor Vdr mRNA was highly expressed in ileum and kidney, and it was also detected in parotid glands though at about 2 orders of magnitude lower levels than in the other organs (Fig. 4j). The expression of the Ca2+ sensing receptor (Casr) was more than 2 orders of magnitude higher in kidney than in the other tissues where it was hardly detectable (Fig. 4k) in agreement with a recent report [40].
Short-term treatment with vitamin D3 does not alter the mRNA expression of Pi and Ca2+ transporters in salivary glands
We next analysed the effect of vitamin D3 on the expression of selected genes in submandibular, sublingual and parotid glands. To confirm that the treatment had produced the expected systemic effect, the expression of the already mentioned hydroxylases involved in vitamin D3 metabolism was first analysed. As expected, the renal expression of the catabolic Cyp24a1 was higher in mice injected with vitamin D3 as compared with vehicle-injected mice (1.45 ± 0.46 vs 0.21 ± 0.24, t test p = 0.0002; n = 6), whereas the expression of the anabolic Cyp27b1 was lower in the vitamin D3-injected group (0.10 ± 0.07 vs 0.0022 ± 0.001, t test p = 0.005; n = 6), thus confirming the effectiveness of the treatment. Administration of vitamin D3 did not alter the mRNA expression of any of the apical Pi-transporters shown to be expressed in parotid glands (Slc34a2, Slc20a1 and Slc20a2), neither that of the putative transporter mediating basolateral efflux (Xpr1) (Fig. 5a-d). Finally, vitamin D3 treatment did not alter either the mRNA levels of the apical (Trpv6) or basolateral (Atpb1/Pmca1) transporters of Ca2+ that were detected at higher levels in parotid glands transcriptome (Fig. 5e-f).
Effect of short-term vitamin D3 treatment on the expression of selected genes involved in regulation of Pi and Ca2+ homeostasis in submandibular, sublingual, and parotid glands. The expression of a Slc34a2, b Slc20a1, c Slc20a2, d Xpr1, e Trpv6, and f Atp2b1 was quantified by qPCR in submandibular (SM), sublingual (SL), and parotid glands (PT) of mice injected once a day during two consecutive days with either vehicle or 4 μg kg−1 BW of 1,25(OH)2 vitamin D3 (VD3) (n = 6/group). Data was normalised to the expression of Hprt. Statistical analysis was done with Student’s t test; P ≤ 0.05 was considered as statistically significant
Discussion
Transport of Pi across intestinal and renal epithelia has been analysed in detail, whereas handling by salivary glands is poorly understood. Intestinal Pi absorption depends upon passive/paracellular as well as active/transcellular processes, whereas Pi transport across the tight renal proximal epithelia relies only on the transcellular pathway ([5, 12, 43, 50], for review, see [29, 49]). In both tissues, active uptake of Pi across the BBM is mostly mediated by the Slc34 family of Na+/Pi cotransporters (Slc34a2/NaPi-IIb in intestine and Slc34a1/NaPi-IIa and Slc34a3/NaPi-IIc in kidney) with some potential minor contribution from Slc20 paralogues (for review, see [18, 49]). The identity of the molecule(s) responsible for paracellular transport in the intestine and for basolateral efflux in both epithelia remains to be fully elucidated, though Cldn3 [26] and Xpr1 [23] have been proposed to tighten the intestinal epithelia for Pi and to mediate basolateral efflux, respectively. The high concentration of Pi in the saliva suggests that salivary glands are able to concentrate Pi, and expression of NaPi-IIb has been reported in human parotid and submandibular glands, with the cotransporter localizing to either the basolateral (acinar cells) or the apical (ductal cells) membrane [34]. Though single-cell RNA-seq failed to detect Slc34a2/NaPi-IIb transcripts in mouse submandibular glands [67], immunofluorescence studies confirmed its apical expression in ductal submandibular cells of mice [40]. Here, we compared the transcriptome of parotid glands with those of ileum and kidney, to address their similarities/differences regarding the expression of genes involved in Pi homeostasis. Given the interconnections between Pi and Ca2+ metabolism, the expression of genes involved in Ca2+ homeostasis was also analysed.
Paracellular intestinal absorption of Pi has been reported along the whole intestine in mice [43], whereas active/transcellular absorption is restricted to particular segments in a species-specific manner, namely duodenum/jejunum in rabbits, rats, and humans and ileum in mice (for review, see [29]). Instead, renal reabsorption of Pi depends only on the transcellular route and transport takes place along the proximal tubule (for review, see [18, 49]). In addition to the well documented specific distribution of Slc34 paralogues and ubiquitous expression of Slc20 paralogues, the transcriptome analysis revealed high expression of Cldn3 and Xpr1 mRNA in ileum whereas Xpr1 but not Cldn3 was highly expressed in kidney. The low levels of Cldn3 in kidney may indicate that this claudin is required to tighten a per se leaky epithelia such as the intestine. Unlike in intestine and kidney, Slc20 paralogues seem the most abundant apical Pi transporters in parotid glands, though low mRNA expression of Slc34a2/NaPi-IIb was also observed and confirmed at the protein level. Moreover, parotid glands had a low expression of Xpr1 and Cldn3 mRNAs. Assuming that indeed Cldn3 tightens a leaky epithelium, its low levels in parotid glands may indicate that this tissue does not express the molecular components of the paracellular route. The absence of a paracellular Pi pore in salivary glands is physiologically coherent, since particularly in the more distal parts of the ducts such pore would result in passive transport of Pi back from the lumen (where its concentration may reach up to 13 mM in humans [60]) into the blood. On the other hand, the low expression of Xpr1 in the salivary gland transcriptome may suggest the presence of additional transporters mediating basolateral efflux of Pi in ductal cells as well as apical efflux in acinar cells. Expression of Slc34a2/NaPi-IIb has been proposed to occur at basolateral sides in human acinar cells and at luminal sides in ductal cells [34]. We (this report) and others [40] confirm the luminal localization of NaPi-IIb in ductal cells but both failed to detect basolateral staining in murine glands. It remains to be clarified whether this represents a species difference or basolateral staining depends on the specificity of the antibody used. We verified the specificity of the antibody in ileum sections of intestinal-specific NaPi-IIb KO mice. Of note, in all healthy tissues examined to date, NaPi-IIb has been exclusively localized to apical domains but not to basolateral membranes [19, 70].
Transepithelial transport of Ca2+ in intestine and kidney involves active and passive components. The active transport depends upon the presence of apical channels that mediate luminal uptake (Trpv), intracellular Ca2+-binding proteins and pumps that prevent changes in cytoplasmic Ca2+ concentration (Calb, Cabp, and Serca), and basolateral pumps and exchangers that extrude Ca2+ across the basolateral membrane (Pmca and Ncx), whereas the passive route relies on claudins (for review, see [15, 31]). In the intestine, active Ca2+ transport predominates in duodenum and jejunum whereas passive absorption takes place along most of the intestinal tract. Accordingly, genes coding apical Ca2+ channels were not detected in the transcriptome analysis of ileum, whereas this tissue expressed high levels of Cldn2 and Cldn12. Both Cldns are known to contribute to intestinal paracellular transport of Ca2+ and to be upregulated by vitamin D3 in vitro and in vivo [20]. The high expression of Atp2a/Serca (paralogues a2 and a3) and Atp2b/Pmca (paralogue b1) mRNAs detected in the transcriptome of ileum may be related to intracellular Ca2+ signalling rather than to transcellular transport. On the other hand, active transport of Ca2+ in the kidney occurs in the distal convoluted tubule and connecting duct whereas passive reabsorption takes place along the proximal tubule and thick ascending limb. Reflecting the presence of all nephron segments, the renal transcriptome analysis revealed the expression of all gene families involved in transcellular and paracellular transport of Ca2+, with particularly high expression of Trpv5, Calb3, Atp2a2, Atp2b1, Slc8a1, and Cldn2/Cln12. Similarly, apical channels (Trpv6) as well as Cldn (Cldn2 and Cldn12) were detected in the transcriptome of parotid glands, suggesting the presence of both transport pathways in this salivary tissue. Interestingly, intracellular Ca2+ buffering in parotid cells seems to depend not on the Calb family but on the Cabp1, whereas transport into ER does not seem to rely only on the ubiquitous Serca paralogues (Atp2a2 and Atp2a3) but also on the Serca1/Atp2a1, a pump whose expression was suggested to be restricted to fast-twitch cardiac striated muscles [75].
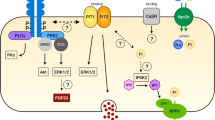
To study whether parotid glands can be a target for hormones involved in Pi (and Ca2+) metabolism, we analysed the expression of receptors for their main endocrine regulators, namely PTH, FGF-23 and vitamin D3 (for review, see [36, 49, 52]. PTH regulates plasma levels of Pi and Ca2+ by acting on bones, intestine and kidneys. Its effects are mediated by the Pthr1 receptor, a member of the G protein-coupled receptor superfamily that also binds PTH-related protein (PTHrP); a second receptor (Pthr2) was identify by homology-cloning, but later experiments showed that it had a very low affinity for PTH or PTHrP (for review, see [21]). Although at low level compared with kidney, parotid glands also expressed mRNA for the PTH receptor Pth1r. Indeed, PTH has been reported to have a direct effect in parotid glands by increasing the concentration of Pi in parotid saliva in vascularly isolated parotid glands of sheep as well as in parotid cell aggregates of rats [65, 74], thus indirectly implying the presence of the hormonal receptor in the tissue. Instead, Pth1r mRNA was hardly detected in ileum, suggesting this segment is not a direct target of PTH, though the PTH status indirectly influences intestinal transport of Pi and Ca2+ by regulating the circulating levels of vitamin D3. Fgf-23 also controls Pi and Ca2+ homeostasis by modulating their renal reabsorption. Its effects depend upon binding to receptors of the tyrosine kinase family, and in most cases require the presence of αklotho as a co-receptor (for review, see [36]). Parotid glands expressed moderate levels of Fgfr1 and Fgfr2 transcripts; however, the near absence of αklotho mRNA makes it unlikely for these salivary glands to be responsive to FGF-23 unless circulating soluble αklotho is available to bind to FGF receptors. A similar argument can be made for ileum since, despite moderate gene expression of some Fgfr, klotho was absent. Vitamin D3 regulates renal and/or intestinal transport of Pi and Ca2+ by stimulating the expression of particular transporters [20, 27, 32]. The transcriptional effects of vitamin D3 are mediated by the Vdr, a receptor from the nuclear receptor family of transcription factors. The transcriptome data revealed low but consistent mRNA expression of the Vdr in parotid glands. Figure 6 shows a hypothetical model of parotid cells showing the expression of transporters and regulators involved in Pi (left) and Ca2+ (right) homeostasis based on the transcriptomic data.
Although the transcriptome analysis suggested that parotid glands can be a target for vitamin D3, we found that short-term administration of vitamin D3 to mice had no effect on the gene expression of the analysed Pi and Ca2+ transporters. In this regard, vitamin D3-induced upregulation of intestinal NaPi-IIb is proposed to associate with transcriptional changes in young but not adult rodents [76], and Vdr-deficient mice have low levels of NaPi-IIb protein without changes at the mRNA level [63]. The responsiveness of Slc20 paralogues to vitamin D3 administration is probably tissue-specific, whereas a single report suggests that Xpr1 is not responsive [28, 41, 69]. On the other hand, the mRNA expression of intestinal Trpv5 but not of Pmca1 is reduced in Vdr-deficient mice [71], with both transcriptional stimulation [3] and unchanged mRNA levels of Pmca1 [28] reported in vitamin D3 treated mice. Nevertheless, it should be noticed that the vitamin D3 status was previously shown to affect parotid gland function in rats, with the rate of parotid salivation as well as the content of Ca2+ and Pi in parotid saliva reduced in rats with chronic vitamin D deficiency [24].
Current models of saliva production state that formation of primary saliva is driven by ion secretory processes in acinar cells leading to the secondary water movement into the luminal space. Once primary saliva is formed, reabsorption of Na+ and Cl− and secretion of HCO3− and K+ in the absence of water permeability along the salivary ducts forms hypotonic, bicarbonate-rich secondary saliva. This saliva contains higher concentrations of Pi than plasma. At this stage, it remains unclear how Pi is first translocated across the epithelium into the primary saliva and then enriched in the final saliva. The latter is likely not due to the reabsorption of water as the ductal epithelium is considered to be highly water-impermeable. Our data confirm and expand previous observations that salivary glands express a series of proteins that are involved in other epithelia in Pi (re)absorption. However, our findings provide no explanation for the high concentration of Pi in final saliva as all transport proteins described here would favour reabsorption due to electrochemical gradients. This would suggest that unknown mechanisms must exist that mediate the secretion of Pi into saliva against its chemical gradient.
In summary, our data suggest that (a) parotid glands are equipped preferentially with Slc20 rather than with Slc34 Na+/Pi cotransporters and express very low levels of Cldn3 and Xpr1 mRNAs, the current candidates for regulation of paracellular permeability and basolateral transport of Pi; (b) parotid glands are susceptible to transport Ca2+ through the transcellular and paracellular route; (c) parotid glands are potential targets for PTH and vitamin D3 regulation.
Data availability
All supporting data are included in the manuscript and supplements.
References
Al Alam D, Danopoulos S, Schall K, Sala FG, Almohazey D, Fernandez GE, Georgia S, Frey MR, Ford HR, Grikscheit T, Bellusci S (2015) Fibroblast growth factor 10 alters the balance between goblet and Paneth cells in the adult mouse small intestine. Am J Physiol-Gastr L 308:G678–G690
Andrukhova O, Zeitz U, Goetz R, Mohammadi M, Lanske B, Erben RG (2012) FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2-SGK1 signaling pathway. Bone 51:621–628
Armbrecht HJ, Boltz MA, Wongsurawat N (1994) Expression of plasma membrane calcium pump mRNA in rat intestine: effect of age and 1,25-dihydroxyvitamin D. Biochim Biophys Acta 1195:110–114. https://doi.org/10.1016/0005-2736(94)90016-7
Barley NF, Prathalingam SR, Zhi P, Legon S, Howard A, Walters JR (1999) Factors involved in the duodenal expression of the human calbindin-D9k gene. Biochem J 341(Pt 3):491–500
Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS (1998) Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci U S A 95:5372–5377. https://doi.org/10.1073/pnas.95.9.5372
Beggs MR, Young K, Pan WL, O'Neill DD, Saurette M, Plain A, Rievaj J, Doschak MR, Cordat E, Dimke H, Alexander RT (2021) Claudin-2 and claudin-12 form independent, complementary pores required to maintain calcium homeostasis. P Natl Acad Sci USA 118. ARTN e2111247118 https://doi.org/10.1073/pnas.2111247118
Blanchard A, Jeunemaitre X, Coudol P, Dechaux M, Froissart M, May A, Demontis R, Fournier A, Paillard M, Houillier P (2001) Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int 59:2206–2215. https://doi.org/10.1046/j.1523-1755.2001.00736.x
Borke JL, Zaki AE, Eisenmann DR, Ashrafi SH, Sharawy MM, Rahman SS (1995) In situ hybridization and monoclonal antibody analysis of plasma membrane Ca-pump mRNA and protein in submandibular glands of rabbit, rat and man. Scanning Microsc 9:817–823; discussion 723–814
Bray NL, Pimentel H, Melsted P, Pachter L (2016) Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34:525–527. https://doi.org/10.1038/nbt.3519
Catalan MA, Nakamoto T, Melvin JE (2009) The salivary gland fluid secretion mechanism. J Med Invest 56(Suppl):192–196. https://doi.org/10.2152/jmi.56.192
Chen S, Zhou Y, Chen Y, Gu J (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34:i884–i890. https://doi.org/10.1093/bioinformatics/bty560
Danisi G, Straub RW (1980) Unidirectional Influx of Phosphate across the Mucosal Membrane of Rabbit Small-Intestine. Pflug Arch Eur J Phy 385:117–122. https://doi.org/10.1007/Bf00588690
Danopoulos S, Schlieve CR, Grikscheit TC, Al Alam D (2017) Fibroblast Growth Factors in the Gastrointestinal Tract: Twists and Turns. Dev Dynam 246:344–352
Daryadel A, Ruiz PA, Gehring N, Stojanovic D, Ugrica M, Bettoni C, Sabrautzki S, Pastor-Arroyo EM, Frey-Wagner I, Lorenz-Depiereux B, Strom TM, de Angelis MH, Rogler G, Wagner CA, Rubio-Aliaga I (2021) Systemic Jak1 activation provokes hepatic inflammation and imbalanced FGF23 production and cleavage. FASEB J 35:e21302. https://doi.org/10.1096/fj.202002113R
Diaz de Barboza G, Guizzardi S, Tolosa de Talamoni N (2015) Molecular aspects of intestinal calcium absorption. World J Gastroenterol 21:7142–7154. https://doi.org/10.3748/wjg.v21.i23.7142
Dimke H, Desai P, Borovac J, Lau A, Pan WL, Alexander RT (2013) Activation of the Ca2+-sensing receptor increases renal claudin-14 expression and urinary Ca2+ excretion. Am J Physiol-Renal 304:F761–F769. https://doi.org/10.1152/ajprenal.00263.2012
Evans RL, Park K, Turner RJ, Watson GE, Nguyen HV, Dennett MR, Hand AR, Flagella M, Shull GE, Melvin JE (2000) Severe impairment of salivation in Na+/K+/2Cl- cotransporter (NKCC1)-deficient mice. J Biol Chem 275:26720–26726. https://doi.org/10.1074/jbc.M003753200
Forster IC, Hernando N, Biber J, Murer H (2013) Phosphate transporters of the SLC20 and SLC34 families. Mol Aspects Med 34:386–395. S0098–2997(12)00090–8 [pii] https://doi.org/10.1016/j.mam.2012.07.007
Frei P, Gao B, Hagenbuch B, Mate A, Biber J, Murer H, Meier PJ, Stieger B (2005) Identification and localization of sodium-phosphate cotransporters in hepatocytes and cholangiocytes of rat liver. American Journal of Physiology - Gastrointestinal & Liver Physiology 288:G771-778
Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H (2008) Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell 19:1912–1921
Gardella TJ, Vilardaga JP (2015) International Union of Basic and Clinical Pharmacology. XCIII. The parathyroid hormone receptors-family B G protein-coupled receptors. Pharmacol Rev 67:310–337. https://doi.org/10.1124/pr.114.009464
Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, Mohammadi M, Baum M (2009) FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol-Renal 297:F282–F291
Giovannini D, Touhami J, Charnet P, Sitbon M, Battini JL (2013) Inorganic phosphate export by the retrovirus receptor XPR1 in metazoans. Cell Rep 3:1866–1873. S2211–1247(13)00268–4 [pii] https://doi.org/10.1016/j.celrep.2013.05.035
Glijer B, Peterfy C, Tenenhouse A (1985) The effect of vitamin D deficiency on secretion of saliva by rat parotid gland in vivo. J Physiol 363:323–334. https://doi.org/10.1113/jphysiol.1985.sp015713
Gong YF, Renigunta V, Himmerkus N, Zhang JQ, Renigunta A, Bleich M, Hou JH (2012) Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. Embo J 31:1999–2012. https://doi.org/10.1038/emboj.2012.49
Hashimoto N, Matsui I, Ishizuka S, Inoue K, Matsumoto A, Shimada K, Hori S, Lee DG, Yasuda S, Katsuma Y, Kajimoto S, Dol YH, Yamaguchi S, Kubota K, Oka T, Sakaguchi Y, Takabatake Y, Hamano T, Lsaka Y (2020) Lithocholic acid increases intestinal phosphate and calcium absorption in a vitamin D receptor dependent but transcellular pathway independent manner see commentary. Kidney Int 97:1164–1180. https://doi.org/10.1016/j.kint.2020.01.032
Hattenhauer O, Traebert M, Murer H, Biber J (1999) Regulation of small intestinal Na-P-i type IIb cotransporter by dietary phosphate intake. Am J Physiol-Gastr L 277:G756–G762. https://doi.org/10.1152/ajpgi.1999.277.4.G756
Hernando N, Pastor-Arroyo EM, Marks J, Schnitzbauer U, Knopfel T, Burki M, Bettoni C, Wagner CA (2021) 1,25(OH)(2) vitamin D-3 stimulates active phosphate transport but not paracellular phosphate absorption in mouse intestine. J Physiol-London 599:1131–1150
Hernando N, Wagner CA (2018) Mechanisms and Regulation of Intestinal Phosphate Absorption. Compr Physiol 8:1065–1090. https://doi.org/10.1002/cphy.c170024
Hilfiker H, Hattenhauer O, Traebert M, Forster I, Murer H, Biber J (1998) Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc Natl Acad Sci U S A 95:14564–14569. https://doi.org/10.1073/pnas.95.24.14564
Hoenderop JG, Nilius B, Bindels RJ (2005) Calcium absorption across epithelia. Physiol Rev 85:373–422. https://doi.org/10.1152/physrev.00003.2004
Hoenderop JG, van der Kemp AW, Hartog A, van de Graaf SF, van Os CH, Willems PH, Bindels RJ (1999) Molecular identification of the apical Ca2+ channel in 1, 25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem 274:8375–8378. https://doi.org/10.1074/jbc.274.13.8375
Homann V, Kinne-Saffran E, Arnold WH, Gaengler P, Kinne RK (2006) Calcium transport in human salivary glands: a proposed model of calcium secretion into saliva. Histochem Cell Biol 125:583–591
Homann V, Rosin-Steiner S, Stratmann T, Arnold WH, Gaengler P, Kinne RK (2005) Sodium-phosphate cotransporter in human salivary glands: molecular evidence for the involvement of NPT2b in acinar phosphate secretion and ductal phosphate reabsorption. Arch Oral Biol 50:759–768. https://doi.org/10.1016/j.archoralbio.2005.01.009
Hosoi K (2016) Physiological role of aquaporin 5 in salivary glands. Pflugers Arch 468:519–539. https://doi.org/10.1007/s00424-015-1749-6
Hu MC, Shiizaki K, Kuro-o M, Moe OW (2013) Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75:503–533. https://doi.org/10.1146/annurev-physiol-030212-183727
Huber K, Roesler U, Holthausen A, Pfeffer E, Breves G (2007) Influence of dietary calcium and phosphorus supply on epithelial phosphate transport in preruminant goats. J Comp Physiol B 177:193–203. https://doi.org/10.1007/s00360-006-0121-8
Huber K, Roesler U, Muscher A, Hansen K, Widiyono I, Pfeffer E, Breves G (2003) Ontogenesis of epithelial phosphate transport systems in goats. Am J Physiol Regul Integr Comp Physiol 284:R413-421
Hwang I, Yang H, Kang HS, Ahn CH, Lee GS, Hong EJ, An BS, Jeung EB (2014) Spatial expression of claudin family members in various organs of mice. Mol Med Rep 9:1806–1812. https://doi.org/10.3892/mmr.2014.2031
Ikuta K, Segawa H, Hanazaki A, Fujii T, Kaneko I, Shiozaki Y, Tatsumi S, Ishikawa Y, Miyamoto K (2019) Systemic network for dietary inorganic phosphate adaptation among three organs. Pflug Arch Eur J Phy 471:123–136. https://doi.org/10.1007/s00424-018-2242-9
Keasey MP, Lemos RR, Hagg T, Oliveira JRM (2016) Vitamin-D receptor agonist calcitriol reduces calcification in vitro through selective upregulation of SLC20A2 but not SLC20A1 or XPR1. Sci Rep-Uk 6
Khananshvili D (2013) The SLC8 gene family of sodium-calcium exchangers (NCX) - Structure, function, and regulation in health and disease. Mol Aspects Med 34:220–235. https://doi.org/10.1016/j.mam.2012.07.003
Knopfel T, Himmerkus N, Gunzel D, Bleich M, Hernando N, Wagner CA (2019) Paracellular transport of phosphate along the intestine. Am J Physiol Gastrointest Liver Physiol 317:G233–G241. https://doi.org/10.1152/ajpgi.00032.2019
Kondo Y, Nakamoto T, Jaramillo Y, Choi S, Catalan MA, Melvin JE (2015) Functional differences in the acinar cells of the murine major salivary glands. J Dent Res 94:715–721. https://doi.org/10.1177/0022034515570943
Kriegs JO, Homann V, Kinne-Saffran E, Kinne RKH (2007) Identification and subcellular localization of paracellin-1 (claudin-16) in human salivary glands. Histochem Cell Biol 128:45–53. https://doi.org/10.1007/s00418-007-0291-9
Kuroo M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, ShirakiIida T, Nishikawa S, Nagai R, Nabeshima Y (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51. https://doi.org/10.1038/36285
Labat C, Thul S, Pirault J, Temmar M, Thornton SN, Benetos A, Back M (2018) Differential associations for salivary sodium, potassium, calcium, and phosphate levels with carotid intima media thickness, heart rate, and arterial stiffness. Dis Markers 2018:3152146. https://doi.org/10.1155/2018/3152146
Lee MG, Choi JY, Luo X, Strickland E, Thomas PJ, Muallem S (1999) Cystic fibrosis transmembrane conductance regulator regulates luminal Cl-/HCO3- exchange in mouse submandibular and pancreatic ducts. J Biol Chem 274:14670–14677
Levi M, Gratton E, Forster IC, Hernando N, Wagner CA, Biber J, Sorribas V, Murer H (2019) Mechanisms of phosphate transport. Nat Rev Nephrol 15:482–500. https://doi.org/10.1038/s41581-019-0159-y
Marks J, Lee GJ, Nadaraja SP, Debnam ES, Unwin RJ (2015) Experimental and regional variations in Na+-dependent and Na+-independent phosphate transport along the rat small intestine and colon. Physiol Rep 3. https://doi.org/10.14814/phy2.12281
Melvin JE, Yule D, Shuttleworth T, Begenisich T (2005) Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67:445–469. https://doi.org/10.1146/annurev.physiol.67.041703.084745
Meyer MB, Pike JW (2020) Mechanistic homeostasis of vitamin D metabolism in the kidney through reciprocal modulation of Cyp27b1 and Cyp24a1 expression. J Steroid Biochem 196. ARTN 105500 https://doi.org/10.1016/j.jsbmb.2019.105500
Motta SE, Imenez Silva PH, Daryadel A, Haykir B, Pastor-Arroyo EM, Bettoni C, Hernando N, Wagner CA (2020) Expression of NaPi-IIb in rodent and human kidney and upregulation in a model of chronic kidney disease. Pflugers Arch 472:449–460. https://doi.org/10.1007/s00424-020-02370-9
Muscher-Banse AS, Breves G (2019) Mechanisms and regulation of epithelial phosphate transport in ruminants: approaches in comparative physiology. Pflugers Arch 471:185–191
Nakamoto T, Romanenko VG, Takahashi A, Begenisich T, Melvin JE (2008) Apical maxi-K (KCa1.1) channels mediate K+ secretion by the mouse submandibular exocrine gland. Am J Physiol Cell Physiol 294:C810-819. https://doi.org/10.1152/ajpcell.00511.2007
Nakashima Y, Mima T, Yashiro M, Sonou T, Ohya M, Masumoto A, Yamanaka S, Koreeda D, Tatsuta K, Hanba Y, Moribata M, Negi S, Shigematsu T (2016) Expression and localization of fibroblast growth factor (FGF)23 and Klotho in the spleen: its physiological and functional implications. Growth Factors 34:196–202. https://doi.org/10.1080/08977194.2016.1273222
Nguyen HV, Stuart-Tilley A, Alper SL, Melvin JE (2004) Cl(-)/HCO(3)(-) exchange is acetazolamide sensitive and activated by a muscarinic receptor-induced [Ca(2+)](i) increase in salivary acinar cells. Am J Physiol Gastrointest Liver Physiol 286:G312-320. https://doi.org/10.1152/ajpgi.00158.2003
Nijenhuis T, Hoenderop JG, van der Kemp AW, Bindels RJ (2003) Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J Am Soc Nephrol 14:2731–2740. https://doi.org/10.1097/01.asn.0000094081.78893.e8
Peppi M, Ghabriel MN (2004) Tissue-specific expression of the tight junction proteins claudins and occludin in the rat salivary glands. J Anat 205:257–266. https://doi.org/10.1111/j.0021-8782.2004.00332.x
Rehak NN, Cecco SA, Csako G (2000) Biochemical composition and electrolyte balance of “unstimulated” whole human saliva. Clin Chem Lab Med 38:335–343. https://doi.org/10.1515/CCLM.2000.049
Rodrigues R, Vidigal MTC, Vieira WA, Nascimento GG, Sabino-Silva R, Blumenberg C, Siqueira MF, Siqueira WL, Paranhos LR (2022) Salivary changes in chronic kidney disease and in patients undergoing hemodialysis: a systematic review and meta-analysis. J Nephrol. https://doi.org/10.1007/s40620-022-01274-4
Schneyer LH (1970) Amiloride inhibition of ion transport in perfused excretory duct of rat submaxillary gland. Am J Physiol 219:1050–1055. https://doi.org/10.1152/ajplegacy.1970.219.4.1050
Segawa H, Kaneko I, Yamanaka S, Ito M, Kuwahata M, Inoue Y, Kato S, Miyamoto K (2004) Intestinal Na-P(i) cotransporter adaptation to dietary P(i) content in vitamin D receptor null mice. Am J Physiol Renal Physiol 287:F39-47. https://doi.org/10.1152/ajprenal.00375.200300375.2003[pii]
Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, Wall SM, Muallem S (2008) The Slc26a4 transporter functions as an electroneutral Cl-/I-/HCO3- exchanger: role of Slc26a4 and Slc26a6 in I- and HCO3- secretion and in regulation of CFTR in the parotid duct. J Physiol (Lond) 586:3813–3824
Shiraki M, Gee MV, Baum BJ, Roth GS (1986) Parathyroid-Hormone Stimulates Phosphate Efflux through an Apparently Adenosine 3’,5’-Monophosphate-Independent Process in Rat Parotid Cell Aggregates. Endocrinology 118:2009–2015
Shirazibeechey SP, Beechey RB, Penny J, Vayro S, Buchan W, Scott D (1991) Mechanisms of Phosphate-Transport in Sheep Intestine and Parotid-Gland - Response to Variation in Dietary Phosphate Supply. Exp Physiol 76:231–241. https://doi.org/10.1113/expphysiol.1991.sp003489
Song EAC, Min S, Oyelakin A, Smalley K, Bard JE, Liao L, Xu JM, Romano RA (2018) Genetic and scRNA-seq analysis reveals distinct cell populations that contribute to salivary gland development and maintenance. Sci Rep-Uk 8. ARTN 14043 https://doi.org/10.1038/s41598-018-32343-z
Stauffer TP, Guerini D, Carafoli E (1995) Tissue distribution of the four gene products of the plasma membrane Ca2+ pump. A study using specific antibodies. J Biol Chem 270:12184–12190. https://doi.org/10.1074/jbc.270.20.12184
Tatsumi S, Segawa H, Morita K, Haga H, Kouda T, Yamamoto H, Inoue Y, Nii T, Katai K, Taketani Y, Miyamoto KI, Takeda E (1998) Molecular cloning and hormonal regulation of PiT-1, a sodium-dependent phosphate cotransporter from rat parathyroid glands. Endocrinology 139:1692–1699
Traebert M, Hattenhauer O, Murer H, Kaissling B, Biber J (1999) Expression of type II Na-P(i) cotransporter in alveolar type II cells. Am J Physiol 277:L868-873
Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G (2001) Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci U S A 98:13324–13329. https://doi.org/10.1073/pnas.231474698
Vayro S, Kemp R, Beechey RB, Shirazi-Beechey S (1991) Preparation and characterization of basolateral plasma-membrane vesicles from sheep parotid glands. Mechanisms of phosphate and D-glucose transport. Biochem J 279:843–848
Weber S, Schneider L, Peters M, Misselwitz J, Ronnefarth G, Boswald M, Bonzel KE, Seeman T, Sulakova T, Kuwertz-Broking E, Gregoric A, Palcoux JB, Tasic V, Manz F, Scharer K, Seyberth HW, Konrad M (2001) Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol 12:1872–1881
Wright RD, Blairwest JR, Nelson JF, Tregear GW (1982) Local-Action of Parathyroid-Hormone (1–34) and (1–84) at the Parotid-Gland of Sheep. J Endocrinol 94:37–41
Wu KD, Lee WS, Wey J, Bungard D, Lytton J (1995) Localization and quantification of endoplasmic reticulum Ca(2+)-ATPase isoform transcripts. Am J Physiol 269:C775-784. https://doi.org/10.1152/ajpcell.1995.269.3.C775
Xu H, Bai LQ, Collins JF, Ghishan FK (2002) Age-dependent regulation of rat intestinal type IIb sodium-phosphate cotransporter by 1,25-(OH)(2) vitamin D-3. Am J Physiol-Cell Ph 282:C487–C493
Zhang XM, Huang Y, Zhang K, Qu LH, Cong X, Su JZ, Wu LL, Yu GY, Zhang Y (2018) Expression patterns of tight junction proteins in porcine major salivary glands: a comparison study with human and murine glands. J Anat 233:167–176. https://doi.org/10.1111/joa.12833
Funding
Open access funding provided by University of Zurich. This study was supported by grants from the Swiss National Science Foundation (SNSF) to C.A.W (31003A-176125 and 310030–212303).
Author information
Authors and Affiliations
Contributions
Study conception and design: NH and CAW. Material preparation, data collection and analysis SOM, BH, CJK, and CB. The first draft of the manuscript was written by NH and CAW. All authors commented on previous versions of the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
n/a.
Consent for publication
All authors have seen and approved the manuscript.
Competing interests
CAW received honoraria from Ardelyx, Advicenne, Medice, Salmon Pharma, and Chugai outside of the work presented here. The rest of authors declare that they have no competing interests.
Human and ethical ethics
Animal experiments were performed according to the Swiss law of animal welfare and were approved by the local veterinary authorities (Kantonales Veterinäramt Zürich) under the license number ZH240/19.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moser, S.O., Haykir, B., Küng, C.J. et al. Expression of phosphate and calcium transporters and their regulators in parotid glands of mice. Pflugers Arch - Eur J Physiol 475, 203–216 (2023). https://doi.org/10.1007/s00424-022-02764-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-022-02764-x