Abstract
The ventral medial prefrontal cortex (vMPFC) facilitates the cardiac baroreflex response through N-methyl-d-aspartate (NMDA) receptor activation and nitric oxide (NO) formation by neuronal NO synthase (nNOS) and soluble guanylate cyclase (sGC) triggering. Glutamatergic transmission is modulated by the cannabinoid receptor type 1 (CB1) and transient receptor potential vanilloid type 1 (TRPV1) receptors, which may inhibit or stimulate glutamate release in the brain, respectively. Interestingly, vMPFC CB1 receptors decrease cardiac baroreflex responses, while TRPV1 channels facilitate them. Therefore, the hypothesis of the present study is that the vMPFC NMDA/NO pathway is regulated by both CB1 and TRPV1 receptors in the modulation of cardiac baroreflex activity. In order to test this assumption, we used male Wistar rats that had stainless steel guide cannulae bilaterally implanted in the vMPFC. Subsequently, a catheter was inserted into the femoral artery, for cardiovascular recordings, and into the femoral vein for assessing baroreflex activation. The increase in tachycardic and bradycardic responses observed after the microinjection of a CB1 receptors antagonist into the vMPFC was prevented by an NMDA antagonist as well as by the nNOS and sGC inhibition. NO extracellular scavenging also abolished these responses. These same pharmacological manipulations inhibited cardiac reflex enhancement induced by TRPV1 agonist injection into the area. Based on these results, we conclude that vMPFC CB1 and TRPV1 receptors inhibit or facilitate the cardiac baroreflex activity by stimulating or blocking the NMDA activation and NO synthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Baroreflex activity is a neural mechanism responsible for maintaining blood pressure (BP) in a narrow range of variation, by regulating cardiovascular parameters, such as heart rate (HR) and peripheral vascular resistance [9, 19]. The baroreceptors are nerve endings primarily located in both the carotid sinus and aortic arch [20]. They convert mechanical stimuli that arise from stretch of the aortic arch or carotid sinus into action potentials that are conveyed to the nucleus of the solitary tract (NTS), the primary site of termination of baroreceptor afferents in the central nervous system [35].
In this context, a number of studies have suggested that supramedullary areas of the brain, such as the prefrontal cortex, connect with the NTS and other medullary structures that take part in the baroreflex circuitry and so influence autonomic function [43, 44]. The prefrontal cortex is topographically divided into the lateral and medial prefrontal cortex [33]. The ventral portion of the medial prefrontal cortex (vMPFC) comprises the prelimbic (PL), infralimbic (IL), and dorsopenducular cortices [27]. Verberne and colleagues (1987) first demonstrated the involvement of the vMPFC, particularly the PL and IL, in baroreflex activity. Chemical lesions of the vMPFC reduced the cardiac responses of the baroreflex [56]. In addition, pharmacological ablation of this area with cobalt chloride, a non-selective presynaptic blocker, reduced the bradycardic reflex induced by BP increase, suggesting an involvement of vMPFC neurotransmission in the baroreceptor arc [49].
Nevertheless, chemical lesions and pharmacological ablation lead to unspecific interruption of vMPFC neurotransmission. Therefore, it is not possible to indicate which neurotransmitters in the vMPFC could be involved in the baroreflex pathway. Resstel and Corrêa (2006) showed that injection of glutamate into the vMPFC evoked increases in BP and HR. Such cardiovascular responses were dependent on NMDA receptors activation, nitric oxide (NO) production and release, as well as soluble guanylate cyclase (sGC) stimulation in awake rats [48]. Interestingly, it was demonstrated that this same neurotransmission works in the vMPFC to facilitate both the bradycardic and tachycardic components of the baroreflex [23].
In the vMPFC, glutamatergic neurotransmission can be regulated by other neurotransmitters, such as endocannabinoids, which are able to inhibit glutamate release by activating presynaptic CB1 receptors [3]. Activation of CB1 receptors leads to inhibition of adenylyl cyclase activity and decreased calcium entrance into neurons, in this way reducing glutamate release [46]. Ferreira-Junior and co-workers (2012) demonstrated that vMPFC CB1 receptors negatively modulate the bradycardic and tachycardic components of the baroreflex [22], raising the possibility that such cannabinoid receptors could inhibit glutamate release, decreasing baroreflex cardiac responses.
Anandamide (AEA) is one of the endogenous agonists for CB1 receptors [18]. Moreover, AEA was able to produce effects in the brain of CB1 receptor knockout mice, suggesting another site of action for AEA [8]. The best well-known non-cannabinoid receptor for AEA is the transient receptor potential vanilloid type 1 (TRPV1 receptors) [60]. These are calcium permeable ionotropic receptors, which facilitate glutamate release and NO production in several brain structures [41]. In addition, Lagatta and colleagues (2015) showed that vMPFC TRPV1 receptors are able to increase the tachycardic and bradycardic responses of the baroreflex [34]. Since they increase glutamate release, it is possible to assume that the facilitation of baroreflex activity mediated by vMPFC TRPV1 channels could happen through regulation of glutamatergic neurotransmission.
Therefore, the hypothesis of the present study was that vMPFC TRPV1 and CB1 receptors may either facilitate or inhibit cardiac baroreflex responses by increasing or decreasing activation of the NMDA/NO pathway, respectively.
Materials and methods
Ethical approval and animals
Experimental procedures were carried out following protocols approved by the Ethical Review Committee of the School of Medicine of Ribeirão Preto (Protocol number 019/2015), which complies with the Guiding Principles for Research Involving Animals and Human Beings of the American Physiological Society. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals [31]. Male Wistar rats weighing 230–270 g were used. Animals were housed in plastic cages in a temperature-controlled room at 25 °C in the Animal Care Unit of the Department of Pharmacology, School of Medicine of Ribeirão Preto, University of São Paulo. They were kept under a 12:00-h light–dark cycle (lights on between 6:00 h and 18:00 h) and had water and food ad libitum. The investigators understand the ethical principles under which the journal operates and their work complies with this animal ethics checklist.
Animal preparation
Four days before the experiment, rats were anesthetized with tribromoethanol (250 mg kg−1, i.p., Sigma, St. Louis, MO, USA). After local anesthesia with 2% lidocaine, the skull was surgically exposed, and stainless steel guide cannulae (26 G) were bilaterally implanted into the vMPFC using a stereotaxic apparatus (Stoelting, Wood Dale, IL, USA). Stereotaxic coordinates for cannulae implantation into the vMPFC were selected from The Rat Brain Atlas of Paxinos and Watson (1997) and were anteroposterior = + 3.4 mm, lateral = 2.6 mm from the medial suture, and vertical = − 3.3 mm from the skull, with a lateral inclination of 24°. Cannulae were fixed to the skull with dental cement and one metal screw. After surgery, animals were treated with a polyantibiotic preparation of streptomycins (30 mg/0.3 mL)/penicillins (72,000 UI/0.3 mL) (i.m., Pentabiotico®, Fort Dodge, Campinas, São Paulo, Brazil) to prevent infection and with the non-steroidal anti-inflammatory flunixin meglumine (0.02 mg/0.3 mL) (s.c., Banamine®, Schering Plow, Cotia, São Paulo, Brazil) for analgesia. One day before the experiment, rats were anesthetized with tribromoethanol (250 mg kg−1, i.p.), and a catheter (a 4-cm segment of PE-10 that was heat-bound to a 13 cm segment of PE-50, Clay Adams, Parsippany, NJ, USA) was inserted into the femoral artery, for recording BP. A second catheter was implanted into the femoral vein for the infusion of vasoactive substances. Both catheters were inserted under the skin and exteriorized on the animal’s dorsum. After surgery, treatment with anti-inflammatory drugs was repeated.
Measurement of cardiovascular responses
Pulsatile arterial pressure of freely moving animals was recorded using an ML870 preamplifier (LabChart, USA) and an acquisition board (PowerLab, AD Instruments, USA) connected to a computer. Mean arterial pressure (MAP) and heart rate (HR) values were derived from pulsatile recordings and processed on-line.
Drug injection
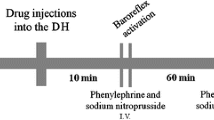
Needles (33 G, Small Parts, Miami Lakes, FL, USA) used for microinjection into the vMPFC were 1 mm longer than the guide cannulas and were connected to a 1-μL syringe (7002-H, Hamilton Co., Reno, NV, USA) through PE-10 tubing. The needle was carefully inserted into the guide cannula, and drugs were injected in a final volume of 200 nL over a 5-s period. After a 30-s period, the needle was removed and inserted into the second guide cannula for microinjection into the contralateral vMPFC.
Baroreflex assessment
The baroreflex was activated by phenylephrine (α1 adrenoceptor agonist 50 μg kg−1, 0.34 mL min−1) or sodium nitroprusside (SNP) (NO donor 50 μg kg−1, 0.8 mL min−1) infusion using an infusion pump (KD Scientific, Holliston, MA, USA). The phenylephrine or SNP infusion lasted 30–40 s and caused, respectively, an increase or decrease in BP.
Method used to evaluate baroreflex activity
Baroreflex curves were constructed, matching MAP variations with HR responses. Paired values for variations in MAP (ΔMAP) and HR (ΔHR) were plotted to create sigmoid curves for each rat, which were used to determine baroreflex activity [49]. To analyze bradycardic and tachycardic responses separately, HR values matching 10, 20, 30, and 40 mmHg of MAP changes were calculated [49]. Values were plotted to create linear regression curves for each rat, and their slopes were compared to determine changes in baroreflex gain.
Drugs
The following compounds were used: one TRPV1 receptor antagonist (6-iodo-nordihydrocapsaicin (6-IODO), Tocris, Westwoods Business Park Ellisville, MO, USA), dissolved in 100% DMSO; a TRPV1 receptor agonist (capsaicin; Tocris, Westwoods Business Park, Ellisville, MO, USA); and a CB1 receptor antagonist (AM251, Tocris, Westwoods Business Park Ellisville, MO, USA). Capsaicin and AM251 were dissolved in 10% DMSO in saline (0.9% NaCl). In addition, a NMDA receptor antagonist (AP7, Tocris, Westwoods Business Park Ellisville, MO, USA); a nNOS inhibitor, n-propyl (n-propyl-l-arginine, Tocris, Westwoods Business Park, Ellisville, MO, USA); a NO scavenger (c-PTIO Tocris, Westwoods Business Park, Ellisville, MO, USA); and a sGC inhibitor (ODQ, Tocris, Westwoods Business Park, Ellisville, MO, USA) were used. They were dissolved in sterile saline. Phenylephrine–HCl (Sigma, St. Louis, MO, USA) and SNP (Sigma, St. Louis, MO, USA) were dissolved in saline (0.9% NaCl). Tribromoethanol (Sigma, St. Louis, MO, USA) and urethane (Sigma, St. Louis, MO, USA) were dissolved in distilled water. The solutions were prepared immediately before use and were kept on ice and protected from light during the experimental sessions.
Experimental protocols
All groups of animals used in our study received three sets of phenylephrine or SNP infusion to determine control values of baroreflex activity. Each animal received two microinjections in both vMPFC hemispheres: the first group received microinjections of 200 nL of combined vehicles (10% DMSO and 100% DMSO or 10% DMSO and saline); the second group received microinjections of 200 nL of AM251 (100 pmol) and vehicle (100% DMSO) or AM251 (100 pmol) and 6-IODO (0.3 nmol); the third group received microinjections of 200 nL of 6-IODO (3 nmol) and vehicle (10% DMSO) or 6-IODO (3 nmol) and AM251 (10 pmol); the fourth group received microinjections of 200 nL of AM251 (100 pmol) combined with either AP7 (0.4 nmol) or n-propyl (8 pmol), or c-PTIO (0.2 nmol), or ODQ (0.2 nmol) or their respective vehicles; the fifth group received a bilateral microinjection of 200 nL of capsaicin (0.1 nmol) combined with either AP7 (0.4 nmol) or n-propyl (8 pmol), or c-PTIO (0.2 nmol or 0.4 nmol), or ODQ (0.2 nmol) or their respective vehicles. In all experimental groups, the interval between both microinjections was 5 min and phenylephrine and SNP infusion were repeated 10 and 60 min after the last bilateral vMPFC microinjection.
Histological procedure
At the end of the experiments, the rats were anesthetized with urethane (1.25 g kg−1, i.p.), and 200 nL of 1% Evan’s blue dye was bilaterally injected into the vMPFC as a marker of injection sites. The chest was surgically opened, the descending aorta occluded, the right atrium severed, and the brain perfused with 10% formalin through the left ventricle. Brains were post-fixed for 24 h at 4 °C, and 40-μm sections were cut with a cryostat (CM-1900, Leica, Wetzlar, Germany). The actual placement of the injection needles was verified in serial sections, according to the Rat Brain Atlas of Paxinos and Watson (1997).
Data analysis
Baseline cardiovascular values before and after pharmacological treatment in the vMPFC were compared using Student’s t test. Baroreflex activity was analyzed using sigmoid curves which were characterized with five parameters: (i) P1 (beats min−1) lower heart rate plateau and P2 (beats min−1) upper heart rate plateau; (ii) heart rate range (beats min−1), difference between upper and lower plateau levels (ΔP); and (iii) average baroreflex gain (G, beats min−1 mmHg−1), which is the average slopes of the non-linear curves. Another sigmoid parameter that is usually analyzed to assess the baroreflex activity is the medium blood pressure (BP50), which is the value of MAP when 50% of the HR is altered. However, it was already demonstrated that pharmacological manipulation of vMPFC neurotransmission assessed in the present study does not alter BP50 [22, 23, 34]. Therefore, BP50 analysis was not done. Furthermore, the NMDA/NO pathway inhibitors displace the X-intercept along the axis [23, 49]. Therefore, it was assessed in order to check the ineffectiveness of those compounds. Significant differences among sigmoid curves or linear regression parameters were analyzed using one-way ANOVA followed by the Dunnett’s post hoc test. The slope of linear regression curves (ΔHR vs. ΔMAP) before, 10 and 60 min after microinjection of each treatment was determined, and results were analyzed to detect alterations in cardiac baroreflex gain using one-way ANOVA followed by Dunnett’s post hoc test. Results of statistical tests where P < 0.05 were considered significant.
Results
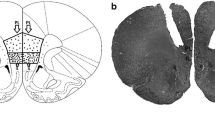
Figure 1 shows a representative photomicrography of a vMPFC coronal section of microinjection site of one animal used in this study. In addition, all animals had guide cannulae implanted in either PL and/or IL (data not shown), apart from the groups in which the cannulae were targeted to the vMPFC surrounding structures.
Effects of vMPFC CB1 and TRPV1 receptor antagonism on cardiac baroreflex activity in awake rats
In the control group, AM251 (100 pmol) was administered into the vMPFC, preceded by the injection of vehicle (DMSO 100%) (n = 6). This dose of AM251 was based on the study of Ferreira-Junior and colleagues (2012) [22]. We observed no alteration either in HR (before = 357 ± 11; after = 358 ± 12 bpm; t = 0.08; P > 0.05) or MAP (before = 97 ± 3.31; after = 99 ± 2.78 mmHg; t = 0.35; P > 0.05) basal levels. Blockade of vMPFC CB1 receptors increased the slope of the linear regression curves of the bradycardic (before = − 1.72 ± 0.18; 10 min = − 2.53 ± 0.11; F(2, 17) = 12.39; P < 0.05) and tachycardic (before = − 1.63 ± 0.14; 10 min = − 2.43 ± 0.11; F(2, 17) = 11.20; P < 0.05) baroreflex responses (Fig. 2a). The parameters of the non-linear regression curve (G, P1, P2, ΔP) were also enhanced (Fig. 2a and Table 1).
Linear regression and sigmoidal curves correlating the responses of ΔMAP and ΔHR before, 10 min, and 60 min after bilateral microinjection of the respective combinations (depicted in bold) into the vMPFC. (Top images; a, b; upper) Correlation r2 values for bradycardic linear regression curves were 0.81 and 0.78 (before); 0.78 and 0.57 (10 min after); and 0.85 and 0.78 (60 min). Correlation r2 values for tachycardic linear regression curves were 0.86 and 0.65 (before); 0.96 and 0.63 (10 min); and 0.92 and 0.77 (60 min). (a, b; lower) Sigmoid curve r2 correlation values were 0.95, 0.90 (before), 0.97, 0.86 (10 min), and 0.96; 0.87(60 min). (Bottom images; c, d; upper) Correlation r2 values for bradycardic linear regression curves were 0.91 and 0.56 (before); 0.93 and 0.74 (10 min); and 0.87 and 0.70 (60 min). Correlation r2 values for tachycardic linear regression curves were 0.85 and 0.76 (before); 0.86 and 0.84 (10 min); and 0.93 and 0.79 (60 min). (c, d; lower) Sigmoid curve r2 correlation values were 0.96, 0.88 (before), 0.97, 0.89 (10 min), and 0.96; 0.92 (60 min). Values are means ± SEM. bpm, beats min1
The slope of the linear regression curve of baroreflex cardiac responses (bradycardia: before = − 1.72 ± 0.18; 60 min = − 1.53 ± 0.16; F(2, 17) = 12.39; P > 0.05; tachycardia: before = − 1.63 ± 0.14; 60 min = − 1.73 ± 0.14; F(2, 17) = 11.20; P > 0.05), as well as the sigmoid parameters (G, P1, P2, ΔP) returned to basal values 60 min after microinjections into the vMPFC (Fig. 2a and Table 1).
Afterwards, the same dose of AM251 (100 pmol) was injected into the vMPFC, preceded by 6-IODO 0.3 nmol (n = 5). This dose was shown to be ineffective in the study of Lagatta and co-workers (2015) [34]. The basal levels of HR (before = 374 ± 14; after = 375 ± 13 bpm; t = 0,05; P > 0,05) and MAP (before = 101 ± 3.20; after = 103 ± 1.88 mmHg; t = 0.45; P > 0.05) were unaffected. The enhancement in the baroreflex bradycardic (before = − 1.51 ± 0.17; 10 min = − 1.84 ± 0.33; 60 min = − 1.58 ± 0,18; F(2, 17) = 0.53; P > 0.05) and tachycardic responses (before = − 2.08 ± 0.33; 10 min = − 2.20 ± 0.36; 60 min = − 1,59 ± 0,69; F(2, 17) = 0.74; P > 0.05) induced by AM251 was abolished by vMPFC TRPV1 receptor blockade (Fig. 2b). Sigmoid curve parameters (G, P1, P2, ΔP) also did not change (Fig. 2b and Table 1).
Analogously, microinjection of the TRPV1 antagonist, 6-IODO (3 nmol) preceded by the administration of vehicle (10% DMSO) (n = 6) into the vMPFC, was performed. This dose of 6-IODO was chosen based on the study of Lagatta and colleagues (2015) [34]. There was no alteration in the basal levels of HR (before = 360 ± 16; after = 359 ± 14 bpm; t = 0.04; P > 0.05) or MAP (before = 100 ± 3.39; after = 100 ± 2.65 mmHg; t = 0.43; P > 0.05). As expected, vMPFC TRPV1 receptor blockade reduced the slope of the regression line curve of the bradycardic (before = − 1.61 ± 0.11; after = − 1.17 ± 0.07; F(2, 17) = 5.11; P < 0.05) and tachycardic (before = − 1.72 ± 0.16; 10 min = − 1.16 ± 0.11; F(2, 17) = 3.83; P < 0.05) responses (Fig. 2c). Sigmoid curve parameters (G, P1, P2, ΔP) were also reduced (Fig. 2d and Table 1).
The slope of the linear regression curve 60 min after the injection of vehicle (10% DMSO) and 6-IODO (3 nmol) into the area (bradycardic response: before = − 1.61 ± 0.11; 60 min = − 1.34 ± 0.10; F(2, 17) = 5.11; P > 0.05; tachycardic response: before = − 1.72 ± 0.16; 60 min = − 1.65 ± 0.12; F(2, 17) = 3.83; P > 0.05) as well as the sigmoid curve values (G, P1, P2, ΔP) returned to the control values (Fig. 2c and Table 1).
In another experimental group (n = 6), injection of an ineffective dose of the CB1 receptor antagonist, AM251 (10 pmol) [22], previous to 6-IODO (3 nmol) was performed. Once again, the basal levels of HR (before = 363 ± 11; after = 361 ± 12 bpm; t = 0.10; P > 0.05) and MAP (before = 102 ± 2.74; after = 104 ± 2.23 mmHg; t = 0.62; P > 0.0 5) were unaffected. However, the reduction of the linear regression curves related to the bradycardic (before = − 1.51 ± 0.29, 10 min = − 1.88 ± 0.23; 60 min = − 1.56 ± 0.22; F(2, 17) = 0.75; P > 0.05) and tachycardic (before = − 2.24 ± 0.27 10 min = − 2.08 ± 0.19; 60 min = − 2.24 ± 0,15; F(2, 17) = 0.14; P > 0.05) responses was prevented by AM251 (Fig. 2d). Also, the sigmoid curve parameters (G, P1, P2, ΔP) were not altered (Fig. 2d and Table 1).
Characterization of ineffective doses of NMDA/NO pathway inhibitors and CB1 and TRPV1 antagonists administered into the vMPFC on cardiac baroreflex activity in awake rats
In order to determine the interactions of the vMPFC CB1 and TRPV1 with the glutamatergic and nitrergic neurotransmissions on the modulation of the baroreflex response, we sought ineffective doses of NMDA/NO pathway inhibitors. The ineffective doses for the NMDA/NO pathway inhibitors were found by reducing the concentrations used in the study of Ferriera-Junior and co-workers (2013) [23]. Injection of the NMDA antagonist (AP7; 0.4 nmol, n = 6) did not alter the baroreflex bradycardic (before = 1.60 ± 2.56; 10 min after = 4,29 ± 1,09; 60 min after = 0.16 ± 1.51; F(2, 17) = 1.27; P > 0.05) or tachycardic responses (before = 1.15 ± 4.49; 10 min after = 0.38 ± 3.23; 60 min after = − 0.58 ± 1.77; F(2, 17) = 0.06; P > 0.05) (data not shown). The inhibition of nNOS by n-propyl (8 pmol; n = 6) did not displace the bradycardic (before = 4.02 ± 1.50; 10 min after = 1.94 ± 2.96; 60 min after = 3.58 ± 0.81; F(2, 17) = 0.31; P > 0.05) or the tachycardic (before = − 0.21 ± 2.29; 10 min after = − 2.22 ± 0.88; 60 min after = − 1.27 ± 0.92; F(2, 17) = 0.44; P > 0.05) linear regression curves (data not shown). Moreover, administration of c-PTIO (0.2 nmol; n = 6) into the vMPFC did not affect the X-intercept of the bradycardic (before = 3.33 ± 0.49; 10 min after = − 1.62 ± 4.52; 60 min after = − 1.49 ± 0.96; F(2, 17) = 0.87; P > 0.05) or tachycardic (before = − 0.28 ± 1.34; 10 min after = − 4.51 ± 1.10; 60 min after = − 1.50 ± 1.43; F(2, 17) = 5.65; P > 0.05) baroreflex responses (data not shown). In another group of animals, a higher dose of c-PTIO (0.4 nmol; n = 5) was used. This also did not modify the X-intercept of the baroreflex bradycardic (before = 1.89 ± 1.84; 10 min after = − 1.31 ± 1.59; 60 min after = − 1.89 ± 0.76; F(2, 14) = 0.09; P > 0.05) or tachycardic (before = − 2.24 ± 1.75; 10 min after = − 3.36 ± 0.55; 60 min after = − 2.11 ± 1.11; F(2,14) = 0.31; P > 0.05) linear regression curves. Likewise, the sGC inhibitor ODQ (0.2 nmol; n = 6) did not affect the basal levels of either bradycardic (before = 2.86 ± 1.65; 10 min after = − 1.67 ± 2.25; 60 min after = 0.07 ± 1.34; F(2, 17) = 0.62; P > 0.05) or tachycardic (before = − 1.95 ± 1.62; 10 min after = − 0.90 ± 1.77; 60 min after = − 2.05 ± 0.65; F(2, 17) = 0.20; P > 0.05) responses (data not shown). Sigmoid curve parameters (G, P1, P2, ∆P) were not modified by either of these compounds administered at their respective doses (data not shown).
Effects of NMDA receptor antagonism in the vMPFC prior to the CB1 receptor blockade on cardiac baroreflex activity in awake rats
In the control group, microinjection of the CB1 receptor antagonist, AM251 (100 pmol), preceded by vehicle (saline) (n = 5) in the vMPFC did not affect the basal levels of HR (before = 353 ± 14; after = 358 ± 13 bpm; t = 0.28; P > 0.05) and MAP (before = 99 ± 2.71; after = 101 ± 2.40 mmHg; t = 0.50; P > 0.05). The slope of the linear regression related to the bradycardic (before = − 1.68 ± 0.18; 10 min = − 2.50 ± 0.12; F(2, 14) = 9.54; P < 0.01) and tachycardic (before = − 1.87 ± 0.16; 10 min = − 2.47 ± 0.11; F(2, 14) = 4.15; P < 0.05) responses of the baroreflex was increased (Fig. 3a). The non-linear regression values (G, P1, P2, ΔP) were enhanced by the CB1 receptor antagonist (Fig. 3a and Table 2).
Linear regression and sigmoidal curves correlating the responses of ΔMAP and ΔHR before, 10 min, and 60 min after bilateral microinjection of the respective combinations (depicted in bold) into the vMPFC. (Top images) (a, b; upper) Correlation r2 values for bradycardic linear regression curves were 0.83 and 0.51 (before); 0.96 and 0.67 (10 min); and 0.78 and 0.94 (60 min). Correlation r2 values for tachycardic linear regression curves were 0.87 and 0.88 (before); 0.97 and 0.70 (10 min); and 0.82 and 0.91 (60 min). (a, b; lower) Sigmoid curves r2 correlation values were 0.95 and 0.91 (before); 0.97 and 0.85 (10 min); and 0.94 and 0.97 (60 min). (Bottom images) (c, d; upper) Correlation r2 values for bradycardic linear regression curves were 0.81 and 0.75 (before); 0.92 and 0.71 (10 min); and 0.90 and 0.84 (60 min). Correlation r2 values for tachycardic linear regression curves were 0.71 and 0.60 (before); 0.83 and 0.65 (10 min); and 0.87 and 0.79 (60 min). (c, d; lower) Sigmoid curve r2 correlation values were 0.92 and 0.89 (before); 0.94 and 0.91 (10 min); and 0.96 and 0.94 (60 min). Values are means ± SEM. bpm, beats min1
The linear regression slope of both cardiac responses (bradycardia: before = − 1.68 ± 0.18; 60 min = − 1.52 ± 0.19; F(2, 14) = 9.52; P > 0.05; tachycardic response: before = − 1.87 ± 0.16; 60 min = − 1.90 ± 0.21; F(2, 14) = 4.15; P > 0.05) as well as the sigmoid curve parameters returned to basal levels 60 min after the microinjections (Fig. 3a and Table 2).
In a second group of animals, injection of the same dose of AM251 (100 pmol) into the vMPFC was preceded by the administration of an ineffective dose of the NMDA receptor antagonist, AP7 (0.4 nmol). The combination of these two compounds did not affect the basal values of HR (before = 371 ± 12; after = 372 ± 11 bpm; t = 0.09; P > 0.05) nor MAP (before = 101 ± 3.42; after = 99 ± 3.28 mmHg; t = 0.60; P > 0.05). Nevertheless, the NMDA blockade in the area prevented the effect of AM251 on both the baroreflex bradycardic (before = − 1.71 ± 0.36; 10 min = − 2.17 ± 0.32; 60 min = − 1.76 ± 0.10; F(2, 17) = 0.66; P > 0.05) and tachycardic responses (before = − 1.82 ± 0.14; after = − 2.23 ± 0.31; 60 min = − 1.84 ± 0.12; F(2, 17) = 1.21; P > 0.05) (Fig. 3b). Enhancement of sigmoid parameters (G, P1, P2, ΔP) was also prevented (Fig. 3b and Table 2).
Effects of nNOS inhibition in the vMPFC prior to CB1 receptor blockade on cardiac baroreflex activity of awake rats
Microinjection of the CB1 receptor antagonist, AM251 (100 pmol), preceded by vehicle (saline) (n = 6) in the vMPFC did not affect basal levels of HR (before = 372 ± 15; after = 373 ± 13 bpm; t = 0.02; P > 0.05) and MAP (before = 102 ± 2.85; after = 107 ± 2.46 mmHg; t = 1.32; P > 0.05). An increase in the slope of the linear regression corresponding to the bradycardic (before = − 1.94 ± 0.12; after = − 2.81 ± 0.38; F(2, 17) = 5.01; P < 0.05) and tachycardic (before = − 1.76 ± 0.17; after = − 2.73 ± 0.31; F(2, 17) = 5.27; P < 0.05) responses of the baroreflex was observed (Fig. 4a). Non-linear regression values (G, P1, P2, ΔP) were enhanced by CB1 receptor antagonist (Fig. 4a and Table 2).
Linear regression and sigmoidal curves correlating the responses of ΔMAP and ΔHR before, 10 min, and 60 min after bilateral microinjection of the respective combinations (depicted in bold) into the vMPFC. (Top images) (a, b; upper) Correlation r2 values for bradycardic linear regression curves were 0.92 and 0.68 (before); 0.71 and 0.90 (10 min); and 0.91 and 0.91 (60 min). Correlation r2 values for tachycardic linear regression curves were 0.83 and 0.85 (before); 0.78 and 0.79 (10 min); and 0.86 and 0.85 (60 min) (a, b; lower) Sigmoid curve r2 correlation values were 0.95 and 0.92 (before); 0.90 and 0.95 (10 min); and 0.9 and 0.96 (60 min). (Bottom images) (c, d; upper) Correlation r2 values for bradycardic linear regression curves were 0.63 and 0.65 (before); 0.90 and 0.59 (10 min); and 0.78 and 0.90 (60 min). Correlation r2 values for tachycardic linear regression curves were 0.64 and 0.73 (before); 0.82 and 0.78 (10 min); and 0.90 and 0.77 (60 min). (c, d; lower) Sigmoid curve r2 correlation values were 0.88 and 0.90 (before); 0.94 and 0.92 (10 min); and 0.95 and 0.94 (60 min). Values are means ± SEM. bpm, beats min1
The linear regression slope of both cardiac responses (bradycardia: before = − 1.94 ± 0.12; 60 min = − 1.83 ± 0.13; F(2, 17) = 5.01; P > 0.05; tachycardic response: before = − 1.76 ± 0.13; 60 min = − 1.93 ± 0.17; F(2, 17) = 5.27; P > 0.05) as well as sigmoid curve parameters returned to basal levels 60 min after the microinjections (Fig. 4a and Table 2).
In another group, injection of the same dose of AM251 (100 pmol) into the vMPFC was preceded by the administration of an ineffective dose of n-propyl (8 pmol). The administration of these two compounds did not affect the basal levels of HR (before = 354 ± 11; after = 358 ± 11 bpm; t = 0.29; P > 0.05) nor MAP (before = 101 ± 3.84; after = 101 ± 2.47 mmHg; t = 0.04; P > 0.05). Nevertheless, nNOS inhibition in the area prevented the effect of AM251 on both the baroreflex bradycardic (before = − 1.69 ± 0.25; 10 min = − 2.17 ± 0.32; 60 min = − 1.77 ± 0.13; F(2, 17) = 0.35; P > 0.05) and tachycardic responses (before = − 1.90 ± 0.17; after = − 1.73 ± 0.19; 60 min = − 1.87 ± 0.17; F(2, 17) = 0.26; P > 0.05) (Fig. 3b). The increase in the sigmoid parameters (G, P1, P2, ΔP) was also prevented (Fig. 4b and Table 2).
Effects of extracellular NO scavenging in the vMPFC prior to CB1 receptor blockade on cardiac baroreflex activity of awake rats
Microinjection of the CB1 receptor antagonist, AM251 (100 pmol), preceded by vehicle (saline) (n = 6) in the vMPFC did not affect basal levels of HR (before = 359 ± 11; after = 361 ± 13 bpm; t = 0.09; P > 0.05) and MAP (before = 102 ± 2.72; after = 105 ± 2.64 mmHg; t = 1.32; P > 0.05). An increase in the slope of the linear regression corresponding to the bradycardic (before = − 1.96 ± 0.19; after = − 2.92 ± 0.18; F(2, 17) = 8.98; P < 0.01) and tachycardic (before = − 2.07 ± 0.20; after = − 3.07 ± 0.26; F(2, 17) = 5.43; P < 0.05) responses of the baroreflex was observed (Fig. 5a). Non-linear regression values (G, P1, P2, ΔP) were enhanced by the CB1 receptor antagonist (Fig. 5a and Table 2).
Linear regression and sigmoidal curves correlating the responses of ΔMAP and ΔHR before, 10 min, and 60 min after bilateral microinjection of the respective combinations (depicted in bold) into the vMPFC. (Top images) (a, b; upper) Correlation values for bradycardic linear regression curves were 0.84 and 0.77 (before); 0.93 and 0.66 (10 min); and 0.87 and 0.94 (60 min). Correlation values for tachycardic linear regression curves were 0.83 and 0.71 (before); 0.86 and 0.75 (10 min); and 0.78 and 0.89 (60 min). (a, b; lower) Sigmoid curve correlation values were 0.94 and 0.93; 0.96 and 0.91 (10 min); and 0.95 and 0.96 (60 min). (Bottom images) (c, d; upper) Correlation r2 values for bradycardic linear regression curves were 0.87 and 0.91 (before); 0.92 and 0.76 (10 min); and 0.71 and 0.85 (60 min). Correlation values for tachycardic regression curves were 0.72 and 0.70 (before); 0.94 and 0.80 (10 min); and 0.88 and 0.74 (60 min). (c, d; lower) Sigmoid curve correlation values were 0.93 and 0.92 (before); 0.96 and 0.91 (10 min); and 0.93 and 0.93 (60 min) Values are means ± SEM. bpm, beats min1
The linear regression slope of both cardiac responses (bradycardia: before = − 1.94 ± 0.12; 60 min = − 1.82 ± 0.13; F(2, 17) = 5.01; P > 0.05; tachycardic response: before = − 1.76 ± 0.17; 60 min = − 1.93 ± 0.17; F(2, 17) = 5.27; P > 0.05) as well as sigmoid curve parameters returned to basal levels 60 min after the microinjections (Fig. 5a and Table 2).
The injection of c-PTIO, an NO scavenger (0.2 nmol), before to the same dose of AM251 (100 pmol) into the vMPFC did not change basal levels of HR (before = 380 ± 13; after = 376 ± 12 bpm; t = 0.19; P > 0.05) nor MAP (before = 108 ± 2.06; after = 109 ± 1.98 mmHg; t = 0.04; P > 0.05). In addition, NO scavenging in the area abolished the effect of AM251 on both the baroreflex bradycardic (before = − 1.87 ± 0.22; after = − 1.66 ± 0.27; 60 min = − 1.90 ± 0.10; F(2, 17) = 0.40; P > 0.05) and tachycardic responses (before = − 1.96 ± 0.27; after = − 2.03 ± 0.25; 60 min = − 2.45 ± 0.18; F(2, 17) = 0.35; P > 0.05) (Fig. 5b). The increase in the sigmoid parameters (G, P1, P2, ΔP) was also prevented (Fig. 5b and Table 2).
Effects of guanylate cyclase inhibition in the vMPFC prior to CB1 receptor blockade on cardiac baroreflex activity of awake rats
Microinjection of CB1 receptor antagonist, AM251 (100 pmol), preceded by vehicle (saline) (n = 6) in the vMPFC did not affect basal levels of HR (before = 363 ± 12; after = 367 ± 12 bpm; t = 0.22; P > 0.05) and MAP (before = 105 ± 2.18; after = 108 ± 1.67 mmHg; t = 0.92; P > 0.05). However, there was an increase in the slope of the linear regression corresponding to the bradycardic (before = − 1.85 ± 0.20; after = − 2.71 ± 0.28; F(2, 17) = 5.87; P < 0.05) and tachycardic (before = − 2.00 ± 0.14; after = − 2.64 ± 0.11; F(2, 17) = 4.70; P < 0.05) responses of the baroreflex (Fig. 6a). Non-linear regression values (G, P1, P2, ΔP) were enhanced by the CB1 receptors antagonist (Fig. 6a and Table 2).
(Top) Linear regression and sigmoidal curves correlating the responses of ΔMAP and ΔHR before, 10 min, and 60 min after bilateral microinjection of the respective combinations (depicted in bold) into the vMPFC. (Top images) (a, b; upper) Correlation values for bradycardic linear regression curves were 0.79 and 0.71 (before); 0.81 and 0.75 (10 min); and 0.81 and 0.90 (60 min). Correlation r2 values for tachycardic linear regression curves were 0.91 and 0.62 (before); 0.96 and 0.70 (10 min); and 0.86 and 0.87 (60 min). (a, b; lower) Sigmoid curve correlation values were 0.94 and 0.90 (before); 0.92 and 0.91 (10 min); and 0.95 and 0.91 (60 min). (Bottom images) (c, d; upper) Correlation values for bradycardic regression curves were 0.80 and 0.76 (before); 0.86 and 0.59 (10 min); and 0.81 and 0.78 (60 min). Correlation r2 values for tachycardic regression curves were 0.93 and 0.69 for data recorded before; 0.97 and 0.74 for data recorded 10 min after; and 0.85 and 0.40 for data recorded 60 min after the microinjections into the vMPFC. (c, d; lower) Sigmoid curve correlation values were 0.95 and 0.90 (before); 0.96 and 0.90 10 min); and 0.94 and 0.91 (60 min). Values are means ± SEM. bpm, beats min1
The linear regression slope of both cardiac responses (bradycardic response: before = − 1.85 ± 0.20; 60 min = − 1.71 ± 0.18; F(2, 17) = 5.87; P > 0.05; tachycardic response: before = − 2.00 ± 0.14; 60 min = − 2.22 ± 0.19; F(2, 17) = 4.70; P > 0.05) as well as sigmoid curve parameters (G, P1, P2, ΔP) returned to basal levels 60 min after the microinjections (Fig. 6a and Table 2).
The injection of ODQ, a guanylate cyclase inhibitor (0.2 nmol) (n = 6), before the same dose of AM251 (100 pmol) into the vMPFC did not change basal levels of HR (before = 370 ± 14; after = 373 ± 13 bpm; t = 0.17; P > 0.05) nor MAP (before = 104 ± 3.71; after = 106 ± 2.29 mmHg; t = 0.46; P > 0.05). In addition, sGC inhibition in the vMPFC abolished the effect of AM251 on both the baroreflex bradycardic (before = − 1.83 ± 0.25;10 min = − 1.95 ± 0.24; 60 min = − 1.95 ± 0.14; F(2, 17) = 0.11; P > 0.05) and tachycardic responses (before = − 1.88 ± 0.31; 10 min = − 2.13 ± 0.30; 60 min = − 2.12 ± 0.17; F(2, 17) = 0.28; P > 0.05) (Fig. 5b). The increase in the sigmoid parameters (G, P1, P2, ΔP) was also prevented (Fig. 6b and Table 2).
Effects of NMDA receptor blockade in the vMPFC prior to TRPV1 receptor activation on cardiac baroreflex activity of awake rats
The TRPV1 receptor agonist, capsaicin (0.1 nmol), preceded by vehicle (saline) (n = 6) was injected into the vMPFC. This dose of capsaicin was based on the study of Lagatta and co-workers (2015) [34]. There was no alteration in the basal levels of HR (before = 372 ± 13; after = 373 ± 13 bpm; t = 0.05; P > 0.05) and MAP (before = 104 ± 2.56; after = 101 ± 2.44 mmHg; t = 0.75; P > 0.05). However, there was an increase in the slope of the linear regression corresponding to the bradycardic (before = − 1.98 ± 0.21 and after = − 2.82 ± 0.17; F(2, 17) = 6.55; P < 0.01) and tachycardic (before = − 1.56 ± 0.21 and after = − 2.68 ± 0.26; F(2, 17) = 9.04; P < 0.01) responses of the baroreflex (Fig. 3c). Non-linear regression values (G, P1, P2, ΔP) were enhanced by the TRPV1 receptor agonist (Fig. 3c and Table 3).
The linear regression slope of both cardiac responses (bradycardic response: before = − 1.98 ± 0.21; 60 min = − 2.11 ± 0.15; F(2, 17) = 6.55; P > 0.05; tachycardic response: before = − 1.56 ± 0.21; 60 min = − 1.64 ± 0.13; F(2, 17) = 9.03; P > 0.05) as well as sigmoid curve parameters (G, P1, P2, ΔP) returned to basal levels 60 min after the microinjections (Fig. 3c and Table 3).
The injection of AP7, an NMDA receptor antagonist (0.4 nmol) (n = 6), prior to the same dose of capsaicin (0.1 nmol) into the vMPFC did not change basal levels of HR (before = 373 ± 13; after = 373 ± 11 bpm; t = 0.01; P > 0.05) nor MAP (before = 103 ± 2.67; after = 102 ± 2.81 mmHg; t = 0.26; P > 0.05). In addition, NMDA receptor blockade in the vMPFC abolished the effect of capsaicin on both the baroreflex bradycardic (before = − 1.65 ± 0.20;10 min = − 2.19 ± 0.30; 60 min = − 1.56 ± 0.14; F(2, 17) = 2.35; P > 0.05) and tachycardic responses (before = − 1.91 ± 0.33 and 10 min = − 1.69 ± 0.27; 60 min = − 2.10 ± 0.23; F(2, 17) = 0.54; P > 0.05) (Fig. 3d). The increase in the sigmoid parameters (G, P1, P2, ΔP) was also prevented (Fig. 3d and Table 3).
Effects of nNOS inhibition in the vMPFC prior to TRPV1 receptor activation on cardiac baroreflex activity of awake rats
Microinjection of the TRPV1 receptor agonist, capsaicin (0.1 nmol), preceded by vehicle (saline) (n = 6) in the vMPFC did not affect basal levels of HR (before = 375 ± 14; after = 376 ± 14 bpm; t = 0.05; P > 0.05) and MAP (before = 102 ± 2.98; after = 100 ± 2.75 mmHg; t = 0.37; P > 0.05). However, there was an increase in the slope of the linear regression corresponding to the bradycardic (before = − 1.77 ± 0.29 and after = − 2.75 ± 0.20; F(2, 17) = 4.59; P < 0.01) and tachycardic (before = − 1.83 ± 0.29 and after = − 2.87 ± 0.29; F(2, 17) = 7.45; P < 0.01) responses of the baroreflex (Fig. 4c). Non-linear regression values (G, P1, P2, ΔP) were enhanced by the TRPV1 receptor agonist (Fig. 4c and Table 3).
The linear regression slope of both cardiac responses (bradycardic response: before = − 1.77 ± 0.29; 60 min = − 1.99 ± 0.22; F(2, 17) = 4.59; P > 0.05; tachycardic response: before = − 1.83 ± 0.29; 60 min = − 1.62 ± 0.11; F(2, 17) = 7.45; P > 0.05) as well as sigmoid curve parameters (G, P1, P2, ΔP) returned to basal levels 60 min after the microinjections (Fig. 4c and Table 3).
The injection of n-propyl, nNOS inhibitor (8 pmol) (n = 6), before the same dose of capsaicin (0.1 nmol) in the vMPFC did not change the basal levels of HR (before = 365 ± 12; after = 369 ± 10 bpm; t = 0.24; P > 0.05) nor MAP (before = 100 ± 2.12; after = 101 ± 2.12 mmHg; t = 0.28; P > 0.05). In addition, nNOS inhibition into the vMPFC abolished the effect of capsaicin on both the baroreflex bradycardic (before = − 1.54 ± 0.24;10 min = − 1.98 ± 0.35; 60 min = − 1.73 ± 0.13; F(2, 17) = 0.76; P > 0.05) and tachycardic responses (before = − 2.75 ± 0.35; 10 min = − 2.65 ± 0.30; 60 min = − 2.23 ± 0.26; F(2, 17) = 0.71; P > 0.05) (Fig. 3d). The increase in the sigmoid parameters (G, P1, P2, ΔP) was also prevented (Fig. 4d and Table 3).
Effects of extracellular NO scavenging in the vMPFC prior to TRPV1 receptor activation on cardiac baroreflex activity of awake rats
Microinjection of the TRPV1 receptor agonist, capsaicin (0.1 nmol), preceded by vehicle (saline) (n = 6) in the vMPFC did not affect basal levels of HR (before = 362 ± 13; after = 363 ± 12 bpm; t = 0.06; P > 0.05) and MAP (before = 103 ± 2.74; after = 104 ± 2.40 mmHg; t = 0.32; P > 0.05). However, there was an increase in the slope of the linear regression corresponding to the bradycardic (before = − 1.68 ± 0.29 and after = − 2.59 ± 0.24; F(2, 17) = 25.76; P < 0.0001) and tachycardic (before = − 1.83 ± 0.29 and after = − 2.87 ± 0.19; F(2, 17) = 19.47; P < 0.001) responses of the baroreflex (data not shown). Non-linear regression values (G, P1, P2, ΔP) were enhanced by the TRPV1 receptors agonist (Table 3).
The linear regression slope of both cardiac responses (bradycardic response: before = − 1.68 ± 0.29; 60 min = − 1.99 ± 0.20; F(2, 17) = 25.76; P > 0.05; tachycardic response: before = − 1.83 ± 0.29; 60 min = − 1.62 ± 0.10; F(2, 17) = 19.47; P > 0.05) (data not shown) as well as sigmoid curve parameters (G, P1, P2, ΔP) returned to basal levels 60 min after the microinjections (Table 3).
The injection of c-PTIO (0.2 nmol) (n = 6), a NO scavenger, before capsaicin (0.1 nmol) into the vMPFC did not change basal levels of HR (before = 372 ± 11; after = 375 ± 10 bpm; t = 0.20; P > 0.05) nor MAP (before = 102 ± 2.86; after = 101 ± 1.34 mmHg; t = 0.01; P > 0.05). However, the NO scavenger did not abolish the effect of capsaicin on both the baroreflex bradycardic (before = − 1.51 ± 0.23; 10 min = − 2.33 ± 0.15; F(2, 17) = 15.26; P < 0.001) and tachycardic responses (before = − 1.78 ± 0.16; 10 min = − 2.76 ± 0.24; F(2, 17) = 16.00; P < 0.001) (data not shown). Sigmoid parameters were also increased (Table 3).
The linear regression slope of both cardiac responses (bradycardic response: before = − 1.51 ± 0.23; 60 min = − 1.70 ± 0.14; F(2, 17) = 15.26; P > 0.05; tachycardic response: before = − 1.78 ± 0.16; 60 min = − 1.73 ± 0.06; F(2, 17) = 16.00; P > 0.05) (data not shown), as well as sigmoid curve parameters (G, P1, P2, ΔP) (Table 3), returned to basal levels 60 min after the microinjections.
In another group of animals, c-PTIO was injected into the vMPFC at a higher dose (0.4 nmol), prior to the same dose of capsaicin (0.1 nmol; n = 6). Neither microinjections altered the basal levels of HR (before = 359 ± 15; after = 365 ± 13 bpm; t = 0.29; P > 0.05) or MAP (before = 103 ± 3.14; after = 102 ± 4.00 mmHg; t = 0.31; P > 0.05). Different from the previous protocol, this higher dose of c-PTIO was able to prevent the effects of capsaicin on the bradycardic (before = − 2.23 ± 0.19; 10 min = − 2.22 ± 0.25; F(2, 14) = 0.02; P > 0.05) and tachycardic (before = − 2.05 ± 0.29; 10 min = − 2.11 ± 0.21; F(2, 14) = 0.06; P > 0.05) responses of the baroreflex (Fig. 5d). The values of the sigmoid curve (G, P1, P2, ∆P) were also not affected (Fig. 4d and Table 3). For this experiment, a control group was used, in which the injection of capsaicin was preceded by vehicle (saline) (n = 6). Basal levels of HR (before = 357 ± 11; after = 362 ± 9 bpm; t = 0.32; P > 0.05) and MAP (before = 101 ± 2.55; after = 104 ± 2.99 mmHg; t = 0.59; P > 0.05) were not modified by the injections. The slope of the bradycardic (before = − 1.91 ± 0.15; 10 min = − 2.89 ± 0.21; F(2, 17) = 10.46; P < 0.01) and tachycardic (before = − 1.80 ± 0.24; 10 min = − 2.62 ± 0.16; F(2, 17) = 16.16; P < 0.001) reflexes was enhanced after the microinjections into the area (Fig. 5c). The values of the sigmoid curve (G, P1, P2, ∆P) were also increased (Fig. 5c and Table 3).
The linear regression slope of both cardiac responses (bradycardic response: before = − 1.91 ± 0.15; 60 min = − 2.14 ± 0.27; F(2, 17) = 10.46; P > 0.05; tachycardic response: before = − 1.80 ± 0.24; 60 min = − 1.84 ± 0.08; F(2, 17) = 16.16; P > 0.05) as well as sigmoid curve parameters (G, P1, P2, ΔP) returned to the basal levels 60 min after microinjections (Fig. 5c and Table 3).
Effects of sGC inhibition in the vMPFC prior to TRPV1 receptor activation on cardiac baroreflex activity of awake rats
Microinjection of the TRPV1 receptor agonist, capsaicin (0.1 nmol), preceded by vehicle (saline) (n = 6) in the vMPFC did not affect basal levels of HR (before = 350 ± 12; after = 351 ± 10 bpm; t = 0.05; P > 0.05) and MAP (before = 102 ± 2.59; after = 104 ± 1.93 mmHg; t = 0.36; P > 0.05). However, there was an increase in the slope of the linear regression corresponding to the bradycardic (before = − 1.82 ± 0.20; after = − 2.69 ± 0.23; F(2, 17) = 7.35; P < 0.01) and tachycardic (before = − 1.94 ± 0.11; after = − 2.61 ± 0.10; F(2, 17) = 5.46; P < 0.05) responses of the baroreflex (Fig. 6c). Non-linear regression values (G, P1, P2, ΔP) were enhanced by the TRPV1 receptor agonist (Fig. 6c and Table 3).
The linear regression slope of both cardiac responses (bradycardic response: before = − 1.82 ± 0.20; 60 min = − 1.68 ± 0.17; F(2, 17) = 7.36; P > 0.05; tachycardic response: before = − 1.82 ± 0.20; 60 min = − 2.19 ± 0.20; F(2, 17) = 5.46; P > 0.05) as well as sigmoid curve parameters (G, P1, P2, ΔP) returned to the basal levels 60 min after the microinjections (Fig. 6c and Table 3).
The injection of ODQ, a sGC inhibitor (0.2 nmol) (n = 6), before the same dose of capsaicin (0.1 nmol) into the vMPFC did not change the basal levels of HR (before = 372 ± 13; after = 373 ± 12 bpm; t = 0.06; P > 0.05) nor MAP (before = 98 ± 2.15; after = 98 ± 2.12 mmHg; t = 0.17; P > 0.05). In addition, sGC inhibition in the vMPFC abolished the effect of capsaicin on both the baroreflex bradycardic (before = − 1.39 ± 0.17; 10 min = − 1.47 ± 0.26; 60 min = − 1.49 ± 0.17; F(2, 17) = 0.06; P > 0.05) and tachycardic responses (before = − 2.11 ± 0.30; 10 min = − 2.24 ± 0.28; 60 min = − 1.64 ± 0.42; F(2, 17) = 0.86; P > 0.05) (Fig. 6d). The increase in the sigmoid parameters (G, P1, P2, ΔP) was also prevented (Fig. 6d and Table 3).
Effects of either 6-IODO (3 nmol), AM251 (100 pmol), or capsaicin (0.1 nmol) in the vMPFC surrounding structures combined with the respective vehicles on cardiac baroreflex activity
Microinjection of 6-IODO (3 nmol) preceded by vehicle (DMSO 10%) (n = 4) into the vMPFC surrounding areas did not modify basal levels of HR (before = 362 ± 15; after = 364 ± 17 bpm; t = 0.09; P > 0.05) or MAP (before = 103 ± 3.49; after = 107 ± 2.96 mmHg; t = 0.77; P > 0.05). In addition, there was no alteration in the bradycardic (before = − 1.84 ± 0.11; 10 min = − 1.57 ± 0.12; 60 min after = − 1.60 ± 0.12; F(2, 11) = 3.93; P > 0.05) or tachycardic response (before = − 1.72 ± 0.07; 10 min = − 1.73 ± 0.04; 60 min after = − 1.91 ± 0.07; F(2, 11) = 3.39; P > 0.05) (data not shown). Sigmoid curve parameters (G, P1, P2, ∆P) were also not altered (data not shown).
Injection of AM251 (100 pmol) preceded by vehicle (saline) (n = 5) into the vMPFC surrounding structures did not change basal levels of HR (before = 379 ± 10; after = 372 ± 10 bpm; t = 0.47; P > 0.05) or MAP (before = 103 ± 2.80; after =106 ± 2.71 mmHg; t = 0.51; P > 0.05). We observed no alteration in either the bradycardic (before = − 1.82 ± 0.15; 10 min = − 1.83 ± 0.19; 60 min after = − 1.74 ± 0.08; F(2, 14) = 0.16; P > 0.05) or tachycardic reflexes (before = − 2.09 ± 0.08; 10 min = − 2.10 ± 0.11; 60 min after = − 2.06 ± 0.07; F(2, 14) = 0.04; P > 0.05) (data not shown). There was also no alteration in the non-linear regression parameters (G, P1, P2, ∆P) (data not shown).
Administration of capsaicin (0.1 nmol) preceded by vehicle (saline) in the vMPFC surrounding structures did not alter basal levels of HR (before = 371 ± 15; after = 374 ± 13 bpm; t = 0.11; P > 0.05) or MAP (before = 104 ± 3.48; after = 101 ± 2.94 mmHg; t = 0.61; P > 0.05). Moreover, regression line curves related to the bradycardic (before = − 1.99 ± 0.20; 10 min = − 1.68 ± 0.08; 60 min after = − 1.75 ± 0.16; F(2, 14) = 1.15; P > 0.05) or tachycardic responses (before = − 1.97 ± 0.08; 10 min = − 2.08 ± 0.13; 60 min after = − 2.00 ± 0.07; F(2, 14) = 0.32; P > 0.05) were not changed (data not shown). Parameters of the sigmoid curve (G, P1, P2, ∆P) were also not affected (data not shown).
Effects of bilateral microinjections of combined vehicles (10% DMSO and 100% DMSO) into the vMPFC on cardiac baroreflex activity
In order to exclude the possible effects of the vehicles used, we performed the injection of the combination of vehicles into the vMPFC, as a control group. Microinjection of 10% DMSO preceded by 100% DMSO into the vMPFC did not change basal levels of HR (before = 370 ± 16; after = 368 ± 15 bpm; t = 0.09; P > 0.05) and MAP (before = 104 ± 2.06; after = 104 ± 3.16 mmHg; t = 0.05; P > 0.05). Moreover, there was no alteration in the slope of the regression line curve of both the bradycardic (before = − 1.48 ± 0.07; 10 min = − 1.49 ± 0.09; 60 min = − 1.47 ± 0.04; 60 min = 1,47 ± 0.04; F(2, 14) = 0.01; P > 0.05) and tachycardic (before = − 1.76 ± 0.07; 10 min = − 1.66 ± 0.05; 60 min = − 1.67 ± 0.08; F(2, 14) = 3.83; P < 0.05) baroreflex responses. Sigmoid curve parameters (G, P1, P2, ∆P) were also not affected (data not shown).
Discussion
One of our hypotheses is that vMPFC CB1 and TRPV1 act jointly in cardiac baroreflex modulation. They are co-expressed in several brain areas, including the vMPFC, and can be activated by their endogenous agonist, the endocannabinoid AEA [18, 60]. Despite being stimulated by the same ligand, they are capable of inducing opposite effects in the brain [37]. Rubino and colleagues (2008) showed that vMPFC injection with methanandamide induced a CB1 receptor-dependent anxiolytic-like effect at lower doses. On the other hand, an anxiogenic-like effect through TRPV1 receptor stimulation was observed at a higher concentration of this compound [51]. Furthermore, there is an interaction between these two receptors in the PL in the modulation of anxiety behaviors [24], suggesting an opposing physiological relationship of both systems in the vMPFC. Corroborating these results, data from our group demonstrate that vMPFC CB1 and TRPV1 may either reduce or enhance the cardiac responses of the baroreflex, respectively [22, 34]. Therefore, we sought to investigate the interaction between these two receptors into vMPFC in baroreflex modulation. Interestingly, we found that there is a balance in the activation of both receptors in vMPFC. We strongly suggest that AEA is responsible for such balance, once that it is the only endogenous mediator, known so far, able to bind to both receptors [18, 60]. We cannot exclude the participation of other neuromodulators in this pathway, such as 2-arachidonoylglycerol and N-arachidonoyldopamine [18, 52]. However, we do not believe they are engaged in this balance, once they are selective endogenous agonists for CB1 or TRPV1 receptors, respectively [18, 52]. In addition, it was demonstrated that inhibitors of AEA degradation or uptake induced a CB1 receptor-dependent reduction on baroreflex activity, when injected into the vMPFC [22]. Therefore, we believe that when CB1 receptors are blocked, the action of AEA on TRPV1 becomes predominant, leading to an enhancement of the baroreflex response. In the opposite situation, TRPV1 receptor antagonism favors the stimulation of CB1 receptors by AEA, reducing baroreflex activity. Although authors have found difficult it to compare the affinities of AEA to such different molecular targets, it has been suggested that this compound has a bigger affinity to CB1 receptors, when compared to TRPV1. Also, AEA displays a greater intrinsic activity to CB1 receptors then to TRPV1 channels [40, 45, 50]. Such pharmacological profile suggests that TRPV1 receptors are activated when a higher concentration of AEA in the synaptic cleft is reached. However, our data indicates that AEA concentration is already enough to activate both receptors, since that when one of them is antagonized the effect of the other one predominates. Therefore, we emphasize that there is a balance in the activation of CB1 and TRPV1 receptors by AEA in vMPFC on the modulation of baroreflex response, despite of the different pharmacological profile of this compound for both receptors.
The converse effects of these two receptors are based on their particular intracellular cascades. TRPV1 receptors are coupled to calcium channels, which facilitate ion influx to neurons, being found in either presynaptic or postsynaptic membranes [12, 55]. Meanwhile, CB1 receptors are mainly located at presynaptic neurons and are coupled to a Gi protein, which activates potassium channels and inhibits calcium entrance [59]. Therefore, both receptors influence the intracellular concentration of calcium, being able to control the release of neurotransmitters in the brain, such as glutamate [3, 37]. Glutamate may activate NMDA, non-NMDA receptors, and metabotropic receptors. NMDA receptors increase calcium concentration inside postsynaptic neurons, stimulating AEA synthesis, which in turn binds to CB1 and TRPV1 [60]. Auclair and colleagues (2000) showed that CB1 receptors have an inhibitory role on postsynaptic excitatory currents in the vMPFC [3], suggesting that these receptors are capable of reducing l-glu release in this structure. Moreover, vMPFC glutamatergic transmission facilitates bradycardic and tachycardic responses through NMDA receptors [23]. Since vMPFC CB1 receptors negatively modulate both glutamate release and the baroreflex response, we assumed that there is a physiological interaction between these cannabinoid receptors and NMDA channels in the modulation of the baroreflex activity. In fact, when the NMDA receptors were antagonized, prior to CB1 blockade in the vMPFC, this effect was prevented.
Additionally, the baroreflex response mediated by vMPFC NMDA receptors depends on NO synthesis, through nNOS activation [23]. Because enhancement of baroreflex activity induced by CB1 receptor blockade involves the NMDA receptor activation, we aimed to verify whether this effect would engage nNOS. vMPFC nNOS inhibition was able to prevent the baroreflex increase induced by CB1 receptor antagonism.
After its synthesis, NO may be released from the postsynaptic neuron, acting on the presynaptic terminal as a retrograde messenger [42]. Thus, we administered the NO scavenger, c-PTIO, prior to CB1 antagonist injection in the vMPFC in order to confirm this possibility. This compound cannot cross the cell membrane, binding to extracellular NO molecules, which precludes NO diffusion through the presynaptic membrane [32]. Consequently, we conclude that CB1 receptors may reduce NO release to the cleft, buffering baroreflex response. Moreover, NO seems to activate soluble guanylate cyclase (sGC) in this pathway, since its inhibition also forecloses the effect of CB1 antagonism on the cardiac baroreflex.
On the other hand, the predominant effect of AEA on TRPV1 receptors in the vMPFC due to CB1 blockade could result in NMDA activation and NO synthesis, once they are able to facilitate glutamate release and NO production in the brain [41]. Conversely, both vMPFC TRPV1 receptors have been implicated in augmentation of cardiac baroreflex responses [34]. Based on our results, these receptors exert their function on baroreflex response by acting on the same pathway in which CB1 receptor does. Nevertheless, c-PTIO (0.2 nmol) which had blocked the effects of CB1 receptor antagonism in the vMPFC was unable to inhibit the effects of capsaicin on baroreflex response (data not shown). Consequently, a higher dose of c-PTIO (0.4 nmol) was used. This new dose inhibited the effects of vMPFC TRPV1 activation on the baroreflex. Such result may reflect a larger amount of NO released in the synaptic cleft due to TRPV1 stimulation on account of stimulation of TRPV1 receptors located in cells that do not take part in the neurotransmission proposed, promoting non-physiological effects. One of the possibilities is the involvement astrocytes and microglia in this effect. Such cells are capable of releasing not only glutamate but also NO as a consequence of TRPV1 activation [4]. The higher concentration of NO in the cleft would, in turn, impair the effect at the lower dose of c-PTIO. Raising the concentration of the NO scavenger, a lower amount of NO molecules would reach the presynaptic neuron, precluding the baroreflex enhancement promoted by TRPV1 activation.
Based on these results, we believe that there is, indeed, a balance between vMPFC CB1 and TRPV1 receptor activation by AEA for baroreflex modulation. Furthermore, they are both linked to inhibition or facilitation of NMDA/NO/sGC pathway, in order to either decrease or increase the baroreflex response, respectively. Moreover, the effects of vMPFC microinjection are specific to the compounds used, since the combination of vehicles induced no effect on baroreflex parameters. Despite of the high concentration of DMSO used in this study, this result is in line with other observations found in the literature, which demonstrates no effect of DMSO 100% injected in the vMPFC on behavioral responses, in which cardiovascular changes are elicited [24] (Fig. 7).
Theoretical model of the vMPFC neurotransmission involved in the modulation of the cardiac baroreflex response: (1) during baroreflex stimulation, glutamate is released in the vMPFC on account of calcium influx into neurons. (2) Glutamate activates NMDA receptors, engaging calcium to enter in the postsynaptic neuron. (3) Calcium ions stimulate NO synthesis through nNOS. (4) NO is then released into the synaptic cleft and diffuses across the presynaptic membrane, as a retrograde neurotransmitter. (5) Once in the presynaptic neuron, NO activates sGC, producing cGMP. (6) This intracellular messenger is involved in the activation of protein kinases, facilitating calcium entrance and (7) glutamate release. The facilitation of this process enhances the tachycardic and bradycardic baroreflex responses (3′) NMDA receptor incoming calcium ions are also involved in the AEA synthetic metabolic pathway. (4′) AEA acts as retrograde neuromodulator and activates presynaptic CB1 receptors. (5′) CB1 receptors, in turn, inhibit calcium influx through the presynaptic membrane and glutamate release. By negatively modulating NMDA/NO pathway, the CB1 receptors are able to decrease baroreflex cardiac responses. (6′) AEA can also activate the TRPV1 receptors, which triggers calcium entrance into postsynaptic membrane, (7′) stimulating NO production by nNOS activation and consequently, glutamate release. Because of this, TRPV1 channels are able to counteract the effect of CB1 receptors on baroreflex response
In addition, GABAergic neurotransmission is also present in vMPFC. Importantly, CB1 receptors were found in parvalbumin GABAergic neurons in the area [58]. Furthermore, administration of a GABAA receptor antagonist in the vMPFC reduced pressor and tachycardic responses evoked by restraint stress in rats [21]. Such result suggests it could be involved in the modulation of short-term cardiovascular responses, such as the baroreflex. However, this idea has not been tested yet. Thus, this matter deserves further investigation.
In this context, it is well described that cardiovascular adaptations during stressful conditions occur to redistribute blood flow throughout the body. Both cardiac parasympathetic and sympathetic branches are activated during these situations in order to regulate HR and MAP increases [17, 25]. So that these alterations may happen the baroreflex set point must be displaced towards higher BP values. In addition, the tachycardic reflex is potentiated while the bradycardic response is reduced by aversive stimuli [17]. Interestingly, vMPFC glutamatergic transmission facilitates the behavioral and cardiovascular alterations elicited during the conditioned emotional response (CER) through NMDA receptors and NO production [47]. Also, TRPV1 receptors located in the vMPFC are also able to increase HR and MAP responses during CER, while CB1 receptor decrease such responses [36, 54]. Taking these results together with our data, glutamatergic and nitrergic transmissions of the vMPFC may be controlled by both TRPV1 and CB1 receptors in order to regulate the modifications in the baroreflex sympathetic and parasympathetic responses during CER, allowing cardiovascular adjustments discriminative of stressful situations [17].
Another eliciting factor for cardiovascular changes is physical exercise (PE) [53]. The vMPFC nitrergic system is activated in rats during the treadmill running test, since an increased concentration of NO metabolites (NOx) in the vMPFC was observed. In addition, intraperitoneal injection of a nNOS inhibitor attenuated the HR increase at the moment of the test. Therefore, NO transmission into the vMPFC may be involved in cardiac baroreflex modulation during PE. However, pretreatment of those animals with a NMDA antagonist did not block the elevation in NOx concentration in the vMPFC, which implies that such receptors may not be a source of NO for the baroreflex regulation in this case [11]. Nevertheless, TRPV1 receptors in turn could lead to NO synthesis in the area during the treadmill running, since they are also engaged in nNOS activation through calcium influx [5]. Therefore, we suggest from the present study that the proposed vMPFC neurotransmission may regulate the cardiac baroreflex response not only in aversive situations but also during physiological stress, such as PE.
The autonomic activity may be impaired in neurodegenerative conditions. For instance, in Alzheimer’s disease (AD), it has been observed that the cardiac sympathetic drive is considerably raised, while the parasympathetic tone is reduced, pointing to a sympatho-vagal imbalance in AD [1]. Furthermore, baroreflex sensitivity is decreased in patients with this disorder and the impairment is more pronounced in advanced cases [38]. Another example of a disease that may alter the cardiovascular and baroreflex activities is multiple sclerosis (MS), since lower levels of HR and postural hypotension were observed in MS patients [2]. Interestingly, both diseases affect the MPFC. In AD, there is an aberrant glutamate and NO release as well as an increase in the expression of CB1 receptors in the structure [6, 39]. Moreover, TRPV1 channels have been suggested to exert a neurotoxic effect in AD [7]. Meanwhile, in MS glutamate release and AEA content are decreased in the MPFC [10, 13]. The reduction in AEA concentration could cause imbalance of CB1 and TRPV1 activation in the MPFC and, therefore, NMDA/NO signaling. In this way, we suggest that functional alterations in the vMPFC neurotransmission found in these infirmities could induce modifications in baroreflex activity, leading to autonomic symptoms observed in patients. Furthermore, the baroreflex response is also impaired in some important neurological and mental diseases, such as neuropathic pain, posttraumatic stress disorder, and major depressive disorder. At the same time, vMPFC neurotransmission described in the present study is affected by those pathologies [26, 28,29,30]. However, it has not yet been clarified whether alteration in cortical neurotransmission is indeed involved in baroreflex dysfunction in such diseases and this deserves further investigation. Revealing such mechanisms would be the first step in preventing the cardiac events described in patients that suffer from these disorders [14,15,16, 57].
In conclusion, the present study has shown opposing physiological roles for vMPFC TRPV1 and CB1 receptors in modulation of cardiac baroreflex activity. Furthermore, TRPV1 facilitates baroreflex activity by inducing NMDA channel activation and NO production. On the other hand, this same neuronal pathway in the vMPFC is inhibited by CB1 receptors, which can, therefore, reduce baroreflex cardiac responses.
References
Aharon-Peretz J, Harel T, Revach M, Ben-Haim SA (1992) Increased sympathetic and decreased parasympathetic cardiac innervation in patients with Alzheimer’s disease. Arch Neurol 49:919–922
Arata M, Sternberg Z (2014) Transvascular autonomic modulation: a modified balloon angioplasty technique for the treatment of autonomic dysfunction in multiple sclerosis patients. J Endovasc Ther Off J Int Soc Endovasc Spec 21:417–428. https://doi.org/10.1583/13-4605MR.1
Auclair N, Otani S, Soubrie P, Crepel F (2000) Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol 83:3287–3293
Bari M, Bonifacino T, Milanese M, Spagnuolo P, Zappettini S, Battista N, Giribaldi F, Usai C, Bonanno G, Maccarrone M (2011) The endocannabinoid system in rat gliosomes and its role in the modulation of glutamate release. Cell Mol Life Sci 68:833–845. https://doi.org/10.1007/s00018-010-0494-4
Batista PA, Fogaça MV, Guimarães FS (2015) The endocannabinoid, endovanilloid and nitrergic systems could interact in the rat dorsolateral periaqueductal gray matter to control anxiety-like behaviors. Behav Brain Res 293:182–188. https://doi.org/10.1016/j.bbr.2015.07.019
Bedse G, Romano A, Cianci S, Lavecchia AM, Lorenzo P, Elphick MR, Laferla FM, Vendemiale G, Grillo C, Altieri F, Cassano T, Gaetani S (2014) Altered expression of the CB1 cannabinoid receptor in the triple transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis JAD 40:701–712. https://doi.org/10.3233/JAD-131910
Benito C, Tolón RM, Castillo AI, Ruiz-Valdepeñas L, Martínez-Orgado JA, Fernández-Sánchez FJ, Vázquez C, Cravatt BF, Romero J (2012) β-amyloid exacerbates inflammation in astrocytes lacking fatty acid amide hydrolase through a mechanism involving PPAR-α, PPAR-γ and TRPV1, but not CB1 or CB2 receptors. Br J Pharmacol 166:1474–1489. https://doi.org/10.1111/j.1476-5381.2012.01889.x
Breivogel CS, Griffin G, Di Marzo V, Martin BR (2001) Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol 60:155–163
Bristow JD, Honour AJ, Pickering GW, Sleight P, Smyth HS (1969) Diminished baroreflex sensitivity in high blood pressure. Circulation 39:48–54
Cabranes A, Venderova K, de Lago E, Fezza F, Sánchez A, Mestre L, Valenti M, García-Merino A, Ramos JA, Di Marzo V, Fernández-Ruiz J (2005) Decreased endocannabinoid levels in the brain and beneficial effects of agents activating cannabinoid and/or vanilloid receptors in a rat model of multiple sclerosis. Neurobiol Dis 20:207–217. https://doi.org/10.1016/j.nbd.2005.03.002
Camargo LHA, Alves FHF, Biojone C, Correa FMA, Resstel LBM, Crestani CC (2013) Involvement of N-methyl-D-aspartate glutamate receptor and nitric oxide in cardiovascular responses to dynamic exercise in rats. Eur J Pharmacol 713:16–24. https://doi.org/10.1016/j.ejphar.2013.04.046
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824. https://doi.org/10.1038/39807
Chanaday NL, Vilcaes AA, de Paul AL, Torres AI, Degano AL, Roth GA (2015) Glutamate release machinery is altered in the frontal cortex of rats with experimental autoimmune encephalomyelitis. Mol Neurobiol 51:1353–1367. https://doi.org/10.1007/s12035-014-8814-6
Cohen BE, Edmondson D, Kronish IM (2015) State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens 28:1295–1302. https://doi.org/10.1093/ajh/hpv047
Cohen H, Kotler M, Matar MA, Kaplan Z, Loewenthal U, Miodownik H, Cassuto Y (1998) Analysis of heart rate variability in posttraumatic stress disorder patients in response to a trauma-related reminder. Biol Psychiatry 44:1054–1059
Cohen H, Neumann L, Shore M, Amir M, Cassuto Y, Buskila D (2000) Autonomic dysfunction in patients with fibromyalgia: application of power spectral analysis of heart rate variability. Semin Arthritis Rheum 29:217–227
Crestani CC, Tavares RF, Alves FHF, Resstel LBM, Correa FMA (2010) Effect of acute restraint stress on the tachycardiac and bradycardiac responses of the baroreflex in rats. Stress Amst Neth 13:61–72. https://doi.org/10.3109/10253890902927950
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
Di Rienzo M, Parati G, Mancia G, Pedotti A, Castiglioni P (1997) Investigating baroreflex control of circulation using signal processing techniques. IEEE Eng Med Biol Mag Q Mag Eng Med Biol Soc 16:86–95
Donoghue S, Felder RB, Jordan D, Spyer KM (1984) The central projections of carotid baroreceptors and chemoreceptors in the cat: a neurophysiological study. J Physiol 347:397–409
Fassini A, Resstel LBM, Corrêa FMA (2016) Prelimbic cortex GABAA receptors are involved in the mediation of restraint stress-evoked cardiovascular responses. Stress Amst Neth 19:576–584. https://doi.org/10.1080/10253890.2016.1231177
Ferreira-Junior NC, Fedoce AG, Alves FHF, Corrêa FMA, Resstel LBM (2012) Medial prefrontal cortex endocannabinoid system modulates baroreflex activity through CB(1) receptors. Am J Physiol Regul Integr Comp Physiol 302:R876–R885. https://doi.org/10.1152/ajpregu.00330.2011
Ferreira-Junior NC, Fedoce AG, Alves FHF, Resstel LBM (2013) Medial prefrontal cortex N-methyl-D-aspartate receptor/nitric oxide/cyclic guanosine monophosphate pathway modulates both tachycardic and bradycardic baroreflex responses. J Neurosci Res 91:1338–1348. https://doi.org/10.1002/jnr.23248
Fogaça MV, Aguiar DC, Moreira FA, Guimarães FS (2012) The endocannabinoid and endovanilloid systems interact in the rat prelimbic medial prefrontal cortex to control anxiety-like behavior. Neuropharmacology 63:202–210. https://doi.org/10.1016/j.neuropharm.2012.03.007
Frysztak RJ, Neafsey EJ (1994) The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Res 643:181–193
Gemes G, Rigaud M, Dean C, Hopp FA, Hogan QH, Seagard J (2009) Baroreceptor reflex is suppressed in rats that develop hyperalgesia behavior after nerve injury. Pain 146:293–300. https://doi.org/10.1016/j.pain.2009.07.040
Groenewegen HJ (1988) Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience 24:379–431
Hughes JW, Dennis MF, Beckham JC (2007) Baroreceptor sensitivity at rest and during stress in women with posttraumatic stress disorder or major depressive disorder. J Trauma Stress 20:667–676. https://doi.org/10.1002/jts.20285
Kemp AH, Quintana DS, Felmingham KL, Matthews S, Jelinek HF (2012) Depression, comorbid anxiety disorders, and heart rate variability in physically healthy, unmedicated patients: implications for cardiovascular risk. PLoS One 7:e30777. https://doi.org/10.1371/journal.pone.0030777
Kemp AH, Quintana DS, Quinn CR, Hopkinson P, Harris AWF (2014) Major depressive disorder with melancholia displays robust alterations in resting state heart rate and its variability: implications for future morbidity and mortality. Front Psychol 5:1387. https://doi.org/10.3389/fpsyg.2014.01387
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother 1:94–99. https://doi.org/10.4103/0976-500X.72351
Ko GY, Kelly PT (1999) Nitric oxide acts as a postsynaptic signaling molecule in calcium/calmodulin-induced synaptic potentiation in hippocampal CA1 pyramidal neurons. J Neurosci 19:6784–6794
Krettek JE, Price JL (1977) The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol 171:157–191. https://doi.org/10.1002/cne.901710204
Lagatta DC, Ferreira-Junior NC, Resstel LBM (2015) Medial prefrontal cortex TRPV1 channels modulate the baroreflex cardiac activity in rats. Br J Pharmacol 172:5377–5389. https://doi.org/10.1111/bph.13327
Lipski J, McAllen RM, Spyer KM (1975) The sinus nerve and baroreceptor input to the medulla of the cat. J Physiol 251:61–78
Lisboa SF, Reis DG, da Silva AL, Corrêa FMA, Guimarães FS, Resstel LBM (2010) Cannabinoid CB1 receptors in the medial prefrontal cortex modulate the expression of contextual fear conditioning. Int J Neuropsychopharmacol Off Sci J Coll Int Neuropsychopharmacol CINP 13:1163–1173. https://doi.org/10.1017/S1461145710000684
Marinelli S, Vaughan CW, Christie MJ, Connor M (2002) Capsaicin activation of glutamatergic synaptic transmission in the rat locus coeruleus in vitro. J Physiol 543:531–540
Meel-van den Abeelen ASS, Lagro J, Gommer ED, Reulen JPH, Claassen JAHR (2013) Baroreflex function is reduced in Alzheimer’s disease: a candidate biomarker? Neurobiol Aging 34:1170–1176. https://doi.org/10.1016/j.neurobiolaging.2012.10.010
Molokanova E, Akhtar MW, Sanz-Blasco S, Tu S, Piña-Crespo JC, McKercher SR, Lipton SA (2014) Differential effects of synaptic and extrasynaptic NMDA receptors on Aβ-induced nitric oxide production in cerebrocortical neurons. J Neurosci 34:5023–5028. https://doi.org/10.1523/JNEUROSCI.2907-13.2014
Moreira FA, Aguiar DC, Terzian ALB, Guimarães FS, Wotjak CT (2012) Cannabinoid type 1 receptors and transient receptor potential vanilloid type 1 channels in fear and anxiety-two sides of one coin? Neuroscience 204:186–192. https://doi.org/10.1016/j.neuroscience.2011.08.046
Musella A, De Chiara V, Rossi S, Prosperetti C, Bernardi G, Maccarrone M, Centonze D (2009) TRPV1 channels facilitate glutamate transmission in the striatum. Mol Cell Neurosci 40:89–97. https://doi.org/10.1016/j.mcn.2008.09.001
Musleh WY, Shahi K, Baudry M (1993) Further studies concerning the role of nitric oxide in LTP induction and maintenance. Synap N Y N 13:370–375. https://doi.org/10.1002/syn.890130409
Owens NC, Sartor DM, Verberne AJ (1999) Medial prefrontal cortex depressor response: role of the solitary tract nucleus in the rat. Neuroscience 89:1331–1346
Owens NC, Verberne AJ (2001) Regional haemodynamic responses to activation of the medial prefrontal cortex depressor region. Brain Res 919:221–231
Pertwee RG, Howlett AC, Abood ME, Alexander SPH, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA (2010) International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev 62:588–631. https://doi.org/10.1124/pr.110.003004
Piomelli D (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4:873–884. https://doi.org/10.1038/nrn1247
Resstel LBM, Corrêa FM de A, Guimarães FS (2008) The expression of contextual fear conditioning involves activation of an NMDA receptor-nitric oxide pathway in the medial prefrontal cortex. Cereb Cortex N Y N 1991 18:2027–2035. https://doi.org/10.1093/cercor/bhm232
Resstel LBM, Corrêa FMA (2006) Injection of l-glutamate into medial prefrontal cortex induces cardiovascular responses through NMDA receptor-nitric oxide in rat. Neuropharmacology 51:160–167. https://doi.org/10.1016/j.neuropharm.2006.03.010
Resstel LBM, Fernandes KBP, Corrêa FMA (2004) Medial prefrontal cortex modulation of the baroreflex parasympathetic component in the rat. Brain Res 1015:136–144. https://doi.org/10.1016/j.brainres.2004.04.065
Ross RA (2003) Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol 140:790–801. https://doi.org/10.1038/sj.bjp.0705467
Rubino T, Realini N, Castiglioni C, Guidali C, Viganó D, Marras E, Petrosino S, Perletti G, Maccarrone M, Di Marzo V, Parolaro D (2008) Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex N Y N 1991 18:1292–1301. https://doi.org/10.1093/cercor/bhm161
van der Stelt M, Trevisani M, Vellani V, De Petrocellis L, Schiano Moriello A, Campi B, McNaughton P, Geppetti P, Di Marzo V (2005) Anandamide acts as an intracellular messenger amplifying Ca2+ influx via TRPV1 channels. EMBO J 24:3026–3037. https://doi.org/10.1038/sj.emboj.7600784
Tarasova OS, Borzykh AA, Kuz’min IV, Borovik AS, Lukoshkova EV, Sharova AP, Vinogradova OL, Grigor’ev AI (2012) Dynamics of heart rate changes in rats following stepwise change of treadmill running speed. Ross Fiziol Zh Im I M Sechenova 98:1372–1379
Terzian ALB, dos Reis DG, Guimarães FS, Corrêa FMA, Resstel LBM (2014) Medial prefrontal cortex transient receptor potential vanilloid type 1 (TRPV1) in the expression of contextual fear conditioning in Wistar rats. Psychopharmacology 231:149–157. https://doi.org/10.1007/s00213-013-3211-9
Tóth A, Boczán J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Edes I, Csiba L, Blumberg PM (2005) Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res 135:162–168. https://doi.org/10.1016/j.molbrainres.2004.12.003
Verberne AJ, Lewis SJ, Worland PJ, Beart PM, Jarrott B, Christie MJ, Louis WJ (1987) Medial prefrontal cortical lesions modulate baroreflex sensitivity in the rat. Brain Res 426:243–249
Wang M-Y, Chiu C-H, Lee H-C, Su C-T, Tsai P-S (2016) Cardiovascular reactivity in patients with major depressive disorder with high- or low-level depressive symptoms: a cross-sectional comparison of cardiovascular reactivity to laboratory-induced mental stress. Biol Res Nurs 18:221–229. https://doi.org/10.1177/1099800415596227
Wedzony K, Chocyk A (2009) Cannabinoid CB1 receptors in rat medial prefrontal cortex are colocalized with calbindin―but not parvalbumin―and calretinin-positive GABA-ergic neurons. Pharmacol Rep PR 61:1000–1007
Wilson RI, Nicoll RA (2002) Endocannabinoid signaling in the brain. Science 296:678–682. https://doi.org/10.1126/science.1063545
Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400:452–457. https://doi.org/10.1038/22761
Acknowledgments
The authors wish to thank Camargo, L.H.A. and Mesquita O. for technical help. This study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Protocol number 461/2009); the Conselho Nacional para o Desenvolvimento Científico e Tecnológico (CNPq) (Protocol number 156718/2012-0); the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) fellowship (Protocol number 2011/19494-8), and the Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da FMRP-USP (FAEPA).
Author information
Authors and Affiliations
Contributions
The experiments were all performed in the Laboratory of Neuropharmacology, School of Medicine of Ribeirão Preto, University of São Paulo. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. D. C. L., N. C. F-J., L. B. K., and L. R. conceived the study and designed the research; D. C. L., N. C. F-J., and L. B. K. performed the experiments; D. C. L. and L. R. analyzed the data; D. C. L., N. C. F-J., L. B. K., and L. R. interpreted results of experiments; D. C. L. prepared figures; D. C. L. drafted the manuscript; D. C. L. and L. R. edited and revised the manuscript; all the authors approved the final version of the manuscript and agree to be accountable for all aspects of the work. In addition, all people designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest, financial or otherwise.
Rights and permissions
About this article
Cite this article
Lagatta, D.C., Kuntze, L.B., Ferreira-Junior, N.C. et al. Medial prefrontal cortex TRPV1 and CB1 receptors modulate cardiac baroreflex activity by regulating the NMDA receptor/nitric oxide pathway. Pflugers Arch - Eur J Physiol 470, 1521–1542 (2018). https://doi.org/10.1007/s00424-018-2149-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2149-5