Abstract
The dorsal hippocampus (DH) is involved in the modulation of the cardiac baroreflex function. There is a wide expression of the NMDA and AMPA/Kainate receptors within the DH. Glutamate administration into the DH triggers both tachycardia and pressor responses. Moreover, GABAergic interneurons and endocannabinoid system play an important role in modulation of the activity of glutamatergic neurons within the DH. Therefore, the present work aimed to evaluate the involvement of the glutamatergic, GABAergic, and endocannabinoid neurotransmissions within the DH in cardiac baroreflex function in rats. We have used the technique of vasoactive drugs infusion to build both sigmoidal curves and linear regressions to analyze the cardiac baroreflex function. Bilateral injection into the DH of DL-AP7, a NMDA receptor antagonist (10 or 50 nmol/500 nL), or NBQX, an AMPA/Kainate antagonist (100 nmol/ 500 nL), reduced the cardiac baroreflex function. On the other hand, bilateral injection of Bicuculline, a GABAA receptor antagonist (1 nmol/500 nL), or AM251, a CB1 receptor antagonist (10 or 100 pmol/500 nL), increased the cardiac baroreflex function. Furthermore, 1 nmol/500 nL of the NMDA receptor antagonist, when administrated alone, was ineffective to change baroreflex function, but it was able to inhibit the alteration in the cardiac baroreflex function elicited by the dose of 100 pmol/500 nL of the CB1 receptor antagonist. Taken together, these findings suggest that glutamatergic, GABAergic, and endocannabinoid neurotransmissions interact each other within the DH to modulate the cardiac baroreflex function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early anatomical and functional studies have proposed a subdivision of the hippocampus in dorsal (DH) and ventral (VH) hippocampus [5, 79]. The hippocampus is a structure of the limbic system [60]. The limbic system and visceral brain were terms coined by MacLean in 1949 to refer a group of cortical and subcortical structures forming an integrated neural system related to the integration of autonomic and behavioral responses [49, 50].
Hippocampus stimulation may cause behavioral changes such as escape, defensive, attack, and attention responses [43, 51, 74] as well as signs of autonomic nervous system activation such as salivation, pupillary changes, inhibition of pyloric antral peristalsis, and respiratory inhibition [3, 9, 42]. Likewise, electrical stimulation of the hippocampus can evoke decreases in heart rate (HR) and increases in pulse pressure [76], decreases in blood pressure (BP) with either increases or decreases in HR [3], and sympathetic activation as indicated by pupillary dilatation along with the increase in the BP [4].
Moreover, previous work from our group has demonstrated the involvement of the hippocampus in the modulation of cardiovascular reflex responses. Administration of cobalt chloride (CoCl2), a calcium-dependent synaptic neurotransmission blocker, into the DH increased the heart rate reflex responses promoted by vasoactive drugs (phenylephrine or sodium nitroprusside) intravenously injected. Thus, it suggests that the DH has an inhibitory influence on cardiac baroreflex function [28].
Glutamate (glut) is the primary excitatory neurotransmitter in the central nervous system (CNS). Glut administration into several areas of the CNS promotes changes in both BP and HR [7, 8, 77, 84]. N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/Kainate receptors are extensively expressed in the hippocampus [10, 34, 38, 83] and unilateral administration of glut into the DH of awake rats elicit marked decrease in BP, HR, and respiratory rate [34, 68].
In contrast, gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the CNS. GABA is highly expressed within the DH [20, 39], but GABA content is reduced in the hippocampus of spontaneous hypertensive rats (SHR) [36, 41]. Moreover, intracerebroventricular injection of GABA reduces the sympathetic nerve activity, BP, and HR in a dose-dependent manner in both normotensive rats and SHR, but the magnitude of these effects is larger in SHR than normotensive animals [71].
Furthermore, the endocannabinoid system is implicated in several hippocampal functions [34, 35, 44, 48, 85]. The enzymes, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), responsible for degradation of endocannabinoids anandamide and 2-arachidonoylglycerol, respectively, are found in the hippocampus [18, 64] as well as the cannabinoid receptors (subtype 1 and 2) are widely expressed in the hippocampus [45, 64]. The cannabinoid receptor subtype 1 (CB1) may be found in presynaptic glutamatergic axon terminals [45, 64], and the binding of endocannabinoids in the CB1 receptors initiates different signaling pathways that hyperpolarize the cell membrane triggering the decrease in the exocytose of glut from presynaptic neurons in the hippocampus [35, 44, 48, 85].
Therefore, considering that the hippocampus can modulate the cardiac baroreflex function, administration of glut in the DH promotes cardiovascular responses, GABAergic neurotransmission is impaired in hippocampus of SHR, and activation of CB1 receptors in the hippocampus may reduce glutamate exocytose; we tested the hypothesis that glutamatergic, GABAergic, and endocannabinoid neurotransmissions interact with each other within the DH to modulate the cardiac baroreflex function in rats.
Materials and methods
Ethical approval
Experimental procedures were carried out according to the protocols approved by the Ethical Review Committee of the School of Medicine of Ribeirao Preto (Protocol number 128/2010) that follows the rules of the European Communities Council Directive of 24 November 1986 (86/609/EEC) and the Guiding Principles for Research Involving Animals and Human Beings of the American Physiological Society.
Drugs
The following drugs were used: DL-AP7 (Tocris Bioscience, Bristol, UK; NMDA receptor antagonist), NBQX (Tocris Bioscience, Bristol, UK; AMPA/Kainate receptor antagonist), AM251 (Tocris Bioscience, Bristol, UK; CB1 receptor antagonist), Bicuculline (Tocris Bioscience, Bristol, UK; GABAA receptor antagonist), lidocaine 2% (S.C., S.S. White, Rio de Janeiro, RJ, Brazil), ketamine (I.P., 100 mg/kg, Sespo, Ind e Comércio, Paulínia, SP, Brazil), xylazine (I.P., 20 mg/kg, Ind e Comércio, Paulínia, SP, Brazil), urethane (I.P., 1.25 g/kg, Sigma-Aldrich, St. Louis, MO, USA), flunixin meglumine (S.C., 5 mg/kg, Banamine®, Schering-Plough, Cotia, São Paulo, Brazil), and polyantibiotic preparation of streptomycin and penicillins (I.M., Pentabiotico®, Fort Dodge, Campinas, São Paulo, Brazil).
Animal surgeries
We utilized in this work male Wistar rats weighing 230–270 g. Animals were obtained from the animal breeding facility of the University of Sao Paulo (Ribeirao Preto, SP). They were housed individually in plastic cages in a temperature-controlled room at 25 °C and kept under a 12- and 12-h light–dark cycle (lights on between 06:00 and 18:00 h) with food and water ad libitum in the Animal Facility of the Department of Pharmacology, School of Medicine of Ribeirao Preto.
Before starting the surgical procedures, rats were anesthetized with ketamine (100 mg/kg, I.P., Sespo, Ind e Comércio, Paulínia, SP, Brazil) and xylazine (20 mg/kg, I.P., Sespo, Ind e Comércio, Paulínia, SP, Brazil). To assess the level of consciousness by the degree of antinociception (lack of response to noxious stimuli) after the anesthesia procedure, we gently pinched the back paw of the animals and observed the musculoskeletal response and cardiovascular and respiratory function.
Six days before cardiovascular recordings, rats were anesthetized with ketamine and xylazine. After local anesthesia with 2% lidocaine, the skull was surgically exposed and stainless-steel guide cannulae (26 G) were bilaterally implanted into the DH using a stereotaxic apparatus (Stoelting, Wood Dale, IL, USA). Stereotaxic coordinates for cannula implantation (AP = − 3.8 mm, L = +2.8 mm from the medial suture and V = − 2.2 mm from the skull) were selected from the Rat Brain Atlas of Paxinos and Watson (2007). Cannulae were fixed to the skull with dental cement and one metal screw. After the surgery, animals were treated with the polyantibiotic preparation (to prevent infection) and nonsteroidal anti-inflammatory (for postoperative analgesia).
One day before cardiovascular recordings, rats were again anesthetized with ketamine and xylazine and a catheter (4-cm segment of PE-10 heat-bound to a 13-cm segment of PE-50, Clay Adams, Parsippany, NJ, USA) was inserted into the abdominal aorta through the femoral artery for blood pressure recording. A second catheter was implanted into the femoral vein, and it was used to infuse vasoactive drugs to evoke arterial BP alterations. Both catheters were tunneled under the skin and exteriorized on the animal’s dorsum. The treatment with nonsteroidal anti-inflammatory was repeated after the surgery.
Drug injection
We have diluted the drugs in appropriate vehicles according to the datasheet of the manufacturer. DL-AP7 has been diluted in NaOH 100 nM and Bicuculline in DMSO 2%, while the NBQX and the AM251 were diluted in DMSO 10%. The vehicles and concentrations used in this work did not produce any effects on the baroreflex function as well as they did not generate toxic effects when they were used in previously published works [13, 26, 47, 59, 69]. Needles (33 G, Small Parts, Miami Lakes, FL, USA) used for microinjection into the DH were 1 mm longer than guide cannulas. After the syringe was filled with the drug, the needle was connected to a 2-μL syringe (7002-H, Hamilton Co., Reno, NV, USA) through the PE-10 tubing. The needle was carefully inserted into the guide cannula, and drugs injected in a final volume of 500 nL over a 60-s period.
Baroreflex assessment
Twenty-four hours after femoral artery surgery, we connected the animals to the acquisition system to collect the pulsatile arterial pressure using an AECAD 04P preamplifier (AVS projects, Sao Paulo, Sao Paulo, Brazil) and an acquisition board (PowerLab 8/30, AD Instruments, New South Wales, Australia) connected to a computer. Mean arterial pressure (MAP) and HR values were derived from these pulsatile recordings and were online processed. We activated the baroreflex through the intravenous infusion of phenylephrine (70 μg/ml at 0.4 ml/min/kg), an alpha 1-adrenoreceptor agonist, or sodium nitroprusside (SNP, 100 μg/ml at 0.8 ml/min/kg), a nitric oxide donor, with the aid of an infusion pump (KD Scientific, Holliston, MA, USA). Phenylephrine and SNP were injected, in a random way, in each animal over 30 s and caused, respectively, an increase or a decrease in the BP [26]. All animals received phenylephrine and SNP at three different times: before, 10 min after, and 60 min after administration of drugs into DH.
Baroreflex function analysis
We constructed the baroreflex sigmoidal curves and the linear regressions by matching the BP-evoked increases and the reflex HR decreases as well as by matching the BP-evoked decrease and the reflex HR increases. These correlations were generated at three different times (before, 10 min after, and 60 min after the administration of drugs into the DH) and used to build the sigmoidal curves and the linear regressions for each rat [25, 26]. We used Boltzmann sigmoidal function [Y = Bottom + (Top − Bottom) / 1 + exp (BP50 – X / Slope)] to build the sigmoidal curves and determine sigmoidal baroreflex parameters: (i) lower heart rate plateau (P1, bpm), (ii) upper heart rate plateau (P2, bpm), (iii) difference between upper and lower plateau levels (range, bpm), and (iv) the average slope of the sigmoidal curve (G, bpm/mmHg) [37, 46]. According to Head and Mccarty, while P1 plateau is influenced by parasympathetic activity, the P2 plateau may be used as a measure of the baroreflex sympathetic tonus [37]. On the other hand, linear regressions were utilized to analyze the slopes of tachycardic and bradycardic baroreflex responses separately [25, 29]. The results of the sigmoid curves and linear regression curves were separately analyzed using one-way ANOVA followed by Dunnett’s post hoc test. Moreover, we collected the average 5 min of stable cardiovascular recordings to assess the baseline BP and HR. These baseline values before and after pharmacological treatment in the DH were compared using the Student’s t test. Results of statistical tests with P < 0.05 were considered significant.
Histological procedure
We euthanized the animals at the end of the experiments using an overdose of urethane. After, 500 nL of 1% Evan’s blue dye was bilaterally injected into the DH as a marker of injection sites. The chest was surgically opened, the descending aorta occluded, the right atrium cut off, and the brain perfused with saline and 10% formalin through the left ventricle. Brains were postfixed for 24 h at 4 °C, and 40-μm sections were cut with a cryostat (CM-1900, Leica, Wetzlar, Germany). We used the Rat Brain Atlas of Paxinos and Watson to localize the placement of the injection needles [62].
Experimental protocols
Seventeen groups of animals were used in this study (Fig. 1): three sets of animals that received bilateral microinjections of 500 nL of NMDA receptor antagonist (DL-AP7): 1 nmol (n = 8), 10 nmol (n = 8), or 50 nmol (n = 14) into the DH [27]; three sets of animals that received bilateral microinjections of 500 nL of AMPA/Kainate receptor antagonist (NBQX): 10 nmol (n = 7), 30 nmol (n = 5), or 100 nmol (n = 7) into the DH [66]; three sets of animals that received bilateral microinjections of 500 nL of GABAA receptor antagonist (Bicuculline): 0.01 nmol (n = 7), 0.1 nmol (n = 9), or 1 nmol (n = 10) into the DH [78]; three sets of animals that received bilateral microinjections of 500 nL of CB1 receptor antagonist (AM251): 1 pmol (n = 10), 10 pmol (n = 11), or 100 pmol (n = 9) into the DH [24]; one set of animals that received bilateral microinjections of 500 nL of NMDA receptor antagonist (1 nmol) plus CB1 receptor antagonist (100 pmol) (n = 12) into the DH; another two sets that received microinjections of 500 nL of DMSO 10% (n = 7) or NaOH 100 nM (n = 7) into the DH; and finally those ones that received bilateral microinjections of 500 nL of NMDA receptor antagonist (10 nmol) (n = 5) or CB1 receptor antagonist 100 pmol (n = 7) in the surrounding structures of the DH.
Results
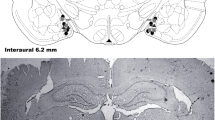
Figure 2 shows a photomicrograph of a coronal section of rat brain implanted with bilateral guide cannulae into the DH and diagrammatic representations of injection sites of drugs and vehicles administered into the DH or in the surrounding structures of the DH.
Photomicrograph of a coronal section of the hippocampus and the injection sites within the DH. Diagrammatic representations with the injection sites of the vehicle (gray circles), drugs into the DH (black circles), and in surrounding structures (white circles) of the DH. Coordinates according to the Rat Brain Atlas of Paxinos and Watson (1997)
Effects of NMDA receptor antagonist (DL-AP7 1, 10, or 50 nmol) injected into the DH
NMDA receptor antagonist, DL-AP7, injected into the DH did not alter the basal levels of MAP or HR (Table 1). Non-linear regression analysis showed that NMDA receptor antagonist 1 nmol (n = 8) did not change any parameters analyzed in sigmoid curve (Table 2 and Fig. 3). However, both doses 10 nmol (n = 8) and 50 nmol (n = 14) reduced the cardiac baroreflex activity (Table 2 and Fig. 3). Moreover, linear regression analysis shows that there were no differences in linear regression slopes of bradycardic (before = − 1.78 ± 0.15, 10 min after = − 1.69 ± 0.29, and 60 min after = − 1.68 ± 0.13 bpm/mmHg; F (2, 23) = 0.10; P > 0.05) and tachycardic (before = − 2.49 ± 0.33, 10 min after = − 2.41 ± 0.15, and 60 min after = − 2.37 ± 0.28 bpm/mmHg; F (2, 23) = 0.10; P > 0.05) responses after administration of DL-AP7 1 nmol (Fig. 3). However, DL-AP7 10 nmol reduced the bradycardic (before = − 1.95 ± 0.17 and 10 min after = − 1.33 ± 0.13 bpm/mmHg; F (2, 23) = 3.55; P < 0.05) and tachycardic (before = − 2.33 ± 0.18 and 10 min after = − 1.43 ± 0.15 bpm/mmHg; F (2, 23) = 5.40; P < 0.05) responses (Fig. 2). Likewise, DL-AP7 50 nmol reduced the bradycardic (before = − 2.23 ± 0.25 and 10 min after = − 1.47 ± 0.18 bpm/mmHg; F (2, 41) = 3.61; P < 0.05) and tachycardic (before = − 2.07 ± 0.22 and 10 min after = − 1.32 ± 0.19 bpm/mmHg; F (2, 41) = 4.15; P < 0.05) responses (Fig. 3). The effect of DL-AP7 on the baroreflex activity was reverted after 60 min (Fig. 3).
Left side: sigmoid curves generated before (r 2 = 0.86; 0.93; 0.84) and 10 min (r 2 = 0.86; 0.94; 0.78) and 60 min (r 2 = 0.88; 0.91; 0.84) after the administration into the DH of the NMDA receptor antagonist (DL-AP7) in doses of 1 nmol (n = 8), 10 nmol (n = 8), or 50 nmol (n = 14). Right side: bradycardia linear regression before (r 2 = 0.83; 0.78; 0.54) and 10 min (r 2 = 0.53; 0.75; 0.50) and 60 min (r 2 = 0.85; 0.73; 0.56) after the DL-AP7 injection into the DH. Tachycardia linear regression before (r 2 = 0.65; 0.85; 0.57) and 10 min (r 2 = 0.90; 0.75; 0.59) and 60 min (r 2 = 0.70; 0.69; 0.59) after the DL-AP7 injection into the DH
Effects of AMPA/Kainate receptor antagonist (NBQX 10, 30, or 100 nmol) injected into the DH
Bilateral administration of AMPA/Kainate receptor antagonist NBQX into the DH did not alter the basal levels of MAP or HR (Table 1). Non-linear regression analysis showed that AMPA/Kainate receptor antagonist NBQX 10 nmol (n = 7) and 30 nmol (n = 5) into the DH did not change any parameters analyzed in sigmoid curve (Table 2). However, the dose of 100 nmol (n = 7) decreased the cardiac baroreflex activity (Table 2 and Fig. 4). Linear regression analysis showed that there were no differences in linear regression slopes after administration of NBQX 10, 30, or 100 nmol. NBQX 10 nmol did not change bradycardic (before = − 1.80 ± 0.36, 10 min after = − 2.07 ± 0.23, and 60 min after = − 2.02 ± 0.27 bpm/mmHg; F (2, 20) = 0.22; P > 0.05) and tachycardic (before = − 1.83 ± 0.19, 10 min after = − 1.92 ± 0.36, and 60 min after = − 1.98 ± 0.26 bpm/mmHg; F (2, 20) = 0.10; P > 0.05) responses. NBQX 30 nmol did not change bradycardic (before = − 2.00 ± 0.31, 10 min after = − 1.77 ± 0.38, and 60 min after = − 1.80 ± 0.35 bpm/mmHg; F (2, 14) = 0.13; P > 0.05) and tachycardic (before = − 2.13 ± 0.69, 10 min after = − 1.54 ± 0.37, and 60 min after = − 2.04 ± 0.61 bpm/mmHg; F (2, 14) = 0.31; P > 0.05) responses. NBQX 100 nmol did not change bradycardic (before = − 1.52 ± 0.24, 10 min after = − 1.91 ± 0.28, and 60 min after = − 1.62 ± 0.15 bpm/mmHg; F (2, 20) = 0.73; P > 0.05) and tachycardic (before = − 2.72 ± 0.56, 10 min after = − 2.03 ± 0.35, and 60 min after = − 2.83 ± 0.31 bpm/mmHg; F (2, 20) = 1.06; P > 0.05) responses (Fig. 4).
Left side: sigmoid curves generated before (r 2 = 0.86; 0.75; 0.78) and 10 min (r 2 = 0.85; 0.80; 0.81) and 60 min (r 2 = 0.88; 0.77; 0.89) after the administration into the DH of the AMPA/Kainate receptor antagonist (NBQX) in doses of 10 nmol (n = 7), 30 nmol (n = 5), or 100 nmol (n = 7). Right side: bradycardia linear regression before (r 2 = 0.50) and 10 min (r 2 = 0.67) and 60 min (r 2 = 0.68) after the NBQX injection into the DH. Tachycardia linear regression before (r 2 = 0,78) and 10 min (r 2 = 0.53) and 60 min (r 2 = 0.69) after the NBQX injection into the DH
Effects of GABAA receptor antagonist (Bicuculline 0.01, 0.1, or 1 nmol) injected into the DH
GABAA receptor antagonist Bicuculline injected into the DH did not alter the basal levels of MAP or HR (Table 1). Non-linear regression analysis showed that GABAA receptor antagonist 0.01 nmol (n = 7) and 0.1 nmol (n = 9) did not change any parameters analyzed in sigmoid curve (Table 2 and Fig. 5). However, the dose of 1 nmol (n = 10) increased the cardiac baroreflex activity (Table 3 and Fig. 5). Moreover, linear regression analysis shows that there were no differences in linear regression slopes of bradycardic (before = − 2.26 ± 0.22, 10 min after = − 2.59 ± 0.22, and 60 min after = − 2.01 ± 0.21 bpm/mmHg; F (2, 20) = 1.35; P > 0.05) and tachycardic (before = − 2.36 ± 0.21, 10 min after = − 2.41 ± 0.27, and 60 min after = − 2.19 ± 0.28 bpm/mmHg; F (2, 20) = 0.20; P > 0.05) responses after administration of GABAA 0.01 nmol (Fig. 5) as well as there were no differences in linear regression slopes of bradycardic (before = − 2.25 ± 0.27, 10 min after = − 2.54 ± 0.39, and 60 min after = − 2.31 ± 0.23 bpm/mmHg; F (2, 26) = 0.26; P > 0.05) and tachycardic (before = − 2.03 ± 0.25, 10 min after = − 2.28 ± 0.34, and 60 min after = − 2.20 ± 0.36 bpm/mmHg; F (2, 26) = 0.15; P > 0.05) responses after administration of GABAA 0.1 nmol. However, GABAA 1 nmol increased the bradycardic (before = − 1.59 ± 0.19 and 10 min after = − 2.19 ± 0.13 bpm/mmHg; F (2, 29) = 4.59; P < 0.05) response, but this dose did not change the tachycardic (before = − 2.16 ± 0.27, 10 min after = − 2.75 ± 0.40, and 60 min after = − 2.17 ± 0.30 bpm/mmHg; F (2, 29) = 1.06; P > 0.05) response (Fig. 5). The effect of GABAA 1 nmol on the baroreflex activity was reverted after 60 min (Fig. 5).
Left side: sigmoid curves generated before (r 2 = 0.90; 0.86; 0.85) and 10 min (r 2 = 0.91; 0.82; 0.89) and 60 min (r 2 = 0.88; 0.84; 0.81) after the administration into the DH of the GABAA receptor antagonist (Bicuculline) in doses of 0.01 nmol (n = 7), 0.1 nmol (n = 9) or 1 nmol (n = 10). Right side: bradycardia linear regression before (r 2 = 0.60; 0.72; 0.71) and 10 min (r 2 = 0.77; 0.78; 0.82) and 60 min (r 2 = 0.72; 0.76; 0.70) after the AM251 injection into the DH. Tachycardia linear regression before (r 2 = 0,73; 0,64; 0,55) and 10 min (r 2 = 0.70; 0.87; 0.66) and 60 min (r 2 = 0.59; 0.63; 0.57) after the AM251 injection into the DH
Effects of CB1 receptor antagonist (AM251 1, 10, or 100 pmol) injected into the DH
CB1 receptor antagonist injection into the DH did not alter the basal levels of MAP or HR (Table 1). Non-linear regression analysis showed that CB1 receptor antagonist AM251 1 pmol (n = 10) did not change any parameters analyzed in sigmoid curve (Table 3 and Fig. 6). However, both doses of 10 pmol (n = 11) and 100 pmol (n = 9) increased the cardiac baroreflex activity (Table 3 and Fig. 6) analyzed in the sigmoid curve. Moreover, linear regression analysis shows no differences in linear regression slopes of bradycardic (before = − 1.51 ± 0.28, 10 min after = − 1.75 ± 0.16, and 60 min after = − 1.57 ± 0.16 bpm/mmHg; F (2, 29) = 0.37; P > 0.05) and tachycardic (before = − 1.73 ± 0.17, 10 min after = − 2.23 ± 0.24, and 60 min after = − 1.80 ± 0.25 bpm/mmHg; F (2, 29) = 1.55; P > 0.05) responses after the administration of AM251 1 pmol (Fig. 6). However, AM251 10 pmol increased the bradycardic (before = − 1.45 ± 0.14 and 10 min after = − 1.94 ± 0.13 bpm/mmHg; F (2, 32) = 3.84; P < 0.05) and tachycardic (before = − 1.92 ± 0.12 and 10 min after = − 2.59 ± 0.15 bpm/mmHg; F (2, 32) = 3.71; P < 0.05) responses (Fig. 6). Likewise, AM251 100 pmol increased bradycardic (before = − 1.55 ± 0.17 and 10 min after = − 2.28 ± 0.18 bpm/mmHg; F (2, 26) = 4.28; P < 0.05) and tachycardic (before = − 1.62 ± 0.20 and 10 min after = − 2.39 ± 0.20 bpm/mmHg; F (2, 26) = 3.46; P < 0.05) responses (Fig. 6). The effect of AM251 on the baroreflex activity was reverted after 60 min (Fig. 6).
Left side: sigmoid curves generated before (r 2 = 0.85; 0.81; 0.87) and 10 min (r 2 = 0.89; 0.91; 0.89) and 60 min (r 2 = 0.85; 0.84; 0.86) after the administration into the DH of the CB1 receptor antagonist (AM251) in doses of 1 pmol (n = 10), 10 pmol (n = 11), or 100 pmol (n = 9). Right side: bradycardia linear regression before (r 2 = 0.60; 0.72; 0.71) and 10 min (r 2 = 0.77; 0.78; 0.82) and 60 min (r 2 = 0.72; 0.76; 0.70) after the AM251 injection into the DH. Tachycardia linear regression before (r 2 = 0.73; 0.64; 0.55) and 10 min (r 2 = 0.70; 0.87; 0.66) and 60 min (r 2 = 0.59; 0.63; 0.57) after the AM251 injection into the DH
Effects of CB1 receptor antagonist (AM251 100 pmol) + NMDA receptor antagonist (DL-AP7 1 nmol) injected into the DH
Bilateral administration of AM251 100 pmol and DL-AP7 1 nmol into the DH (n = 12) did not alter the basal levels of MAP (before = 101 ± 3 and after = 104 ± 4 mmHg; t = 1.2; P > 0.05) or HR (before = 358 ± 9 and after = 353 ± 8 bpm; t = 1.3; P > 0.05). Non-linear regression analysis showed that DL-AP7 1 nmol into DH was able to inhibit the effect of AM251 100 pmol on baroreflex activity (Table 3 and Fig. 7). Corroborating these results, linear regression analysis showed that DL-AP7 1 nmol into DH was able to inhibit the effect of AM251 100 pmol on linear regression slopes of bradycardic (before = − 1.72 ± 0.26, 10 min after = − 2.11 ± 0.27, and 60 min after = − 1.91 ± 0.24 bpm/mmHg; F (2, 35) = 0.58; P > 0.05) and tachycardic (before = − 2.39 ± 0.26, 10 min after = − 2.68 ± 0.32, and 60 min after = − 2.52 ± 0.26 bpm/mmHg; F (2, 35) = 0.26; P > 0.05) responses (Fig. 7).
Left side: sigmoid curves before (r 2 = 0.86) and 10 min (r 2 = 0.85) and 60 min (r 2 = 0.87) after the co-administration of the drugs AM251 100 pmol (CB1 receptor antagonist) and DL-AP7 1 nmol (NMDA receptor antagonist) into the DH (n = 12). Right side: bradycardia linear regression before (r 2 = 0.51) and 10 min (r 2 = 0.59) and 60 min (r 2 = 0.58) after the co-administration of the drugs AM251 100 pmol and DL-AP7 1 nmol into the DH. Tachycardia linear regression before (r 2 = 0.70) and 10 min (r 2 = 0.61) and 60 min (r 2 = 0.67) after the co-administration of the drugs AM251 100 pmol and DL-AP7 1 nmol into the DH
Effect on baroreflex activity of vehicles (DMSO 10% or NaOH 100 nM) administered into the DH and drugs (DL-AP7 10 nmol or AM251 100 pmol) injected in the surrounding structures of the DH
DMSO 10% bilaterally administered into the DH (n = 7) did not alter the basal levels of MAP (before = 92 ± 2 and after = 88 ± 1 mmHg; t = 1.1; P > 0.05) or HR (before = 362 ± 11 and after = 358 ± 9 bpm; t = 0.9; P > 0.05). Moreover, linear regression analysis showed that there were no differences in linear regression slopes of bradycardic (before = − 1.90 ± 0.16 and after = − 2.11 ± 0.28 bpm/mmHg; t = 0.6; P > 0.05) and tachycardic (before = − 2.42 ± 0.23 and after = − 2.41 ± 0.19 bpm/mmHg; t = 0.1; P > 0.05) responses (Fig. 8). Likewise, bilateral administration of NaOH 100 nM into DH (n = 7) did not alter the basal levels of MAP (before = 99 ± 3 and after = 96 ± 3 mmHg; t = 1.3; P > 0.05) or HR (before = 361 ± 9 and after = 357 ± 8 bpm; t = 1.1; P > 0.05). Linear regression analysis showed that there were no differences in linear regression slopes of bradycardic (before = − 1.71 ± 0.17 and after = − 1.62 ± 0.18 bpm/mmHg; t = 0.3; P > 0.05) and tachycardic (before = − 1.73 ± 0.14 and after = − 1.91 ± 0.15 bpm/mmHg; t = 0.74; P > 0.05) responses (Fig. 8).
NaOH 100 nM: linear regression generated before (r 2 = 0.78) and 10 min (r 2 = 0.85) after the NaOH 100 nM injection into the DH (n = 7). DMSO 10%: linear regression generated before (r 2 = 0.85) and 10 min (r 2 = 0.82) after the DMSO 10% injection into the DH (n = 7). DL-AP7 10 nmol: linear regression generated before (r 2 = 0.86) and 10 min (r 2 = 0.85) after the DL-AP7 10 nmol injection in the surrounding structures of the DH (n = 5). AM251 100 pmol: linear regression generated before (r 2 = 0.86) and 10 min (r 2 = 0.82) after the AM251 100 pmol injection in the surrounding structures of the DH (n = 7)
In the same way, bilateral administration of DL-AP7 10 nmol in the surrounding structures of the DH (n = 5) did not alter the basal levels of MAP (before = 103 ± 5 and after = 104 ± 4 mmHg; t = 1.1; P > 0.05) or HR (before = 352 ± 7 and after = 355 ± 9 bpm; t = 1.0; P > 0.05). Linear regression analysis showed that there were no differences in linear regression slopes of bradycardic (before = − 2.21 ± 0.15 and after = − 2.45 ± 0.16 bpm/mmHg; t = 1.1; P > 0.05) and tachycardic (before = − 2.27 ± 0.28 and after = − 2.42 ± 0.15 bpm/mmHg; t = 0.4; P > 0.05) responses (Fig. 8). Moreover, bilateral administration of AM251 100 pmol in the surrounding structures of the DH (n = 7) did not alter the basal levels of MAP (before = 99 ± 7 and after = 103 ± 6 mmHg; t = 0.8; P > 0.05) or HR (before = 348 ± 9 and after = 352 ± 9 bpm; t = 0.9; P > 0.05). Linear regression analysis showed that there were no differences in linear regression slopes of bradycardic (before = − 1.79 ± 0.22 and after = − 1.68 ± 0.18 bpm/mmHg; t = 0.5; P > 0.05) and tachycardic (before = − 1.80 ± 0.12 and after = − 1.82 ± 0.18 bpm/mmHg; t = 0.6; P > 0.05) responses (Fig. 8).
Discussion
The present work is the first to demonstrate that the glutamatergic, GABAergic, and endocannabinoid neurotransmissions within the DH are involved in the modulation of the cardiac baroreflex function. Our results show that glutamate within the hippocampus facilitates the cardiac baroreflex function through acting at NMDA and AMPA/Kainate receptors while both neurotransmitters GABA, acting at GABAA receptors, and endocannabinoids, acting at CB1 receptors, reduce cardiac baroreflex responses. Moreover, we found an interaction between glutamatergic and endocannabinoid neurotransmission in the DH in modulating the cardiac baroreflex function once NMDA receptor antagonist in the dose of 1 nmol inhibited the enhancement of the cardiac baroreflex response promoted by the dose of 100 pmol of CB1 receptor antagonist. Furthermore, the vehicles of the drugs administered into the DH did not alter cardiac baroreflex responses, as well as doses of the antagonists administered in the DH surrounding areas, did not change the cardiac baroreflex function. Thus, these results reinforce a site-dependent effect of the drugs injected into the DH.
The baroreflex provides an essential feedback to the CNS for moment-to-moment control of the cardiovascular function to maintain BP within a narrow functional range [33, 63]. Baroreceptors are peripheral afferent neurons located in the aortic arch and carotid sinus [22]. They convert mechanical stimuli derived from oscillations in BP into action potentials driven to the nucleus of the solitary tract (NTS) [22, 33]. The NTS is a medullary area that integrates the BP information from the baroreceptors and sends projections to other brainstem areas influencing the sympathetic and parasympathetic drive to heart and vessels [33]. Several studies have shown that forebrain areas can also modulate the baroreflex function: medial prefrontal cortex (MPFC) [26], bed nucleus of stria terminalis (BNST) [15], hypothalamus [14], amygdala [29], as well as the hippocampus. We have shown that the DH blockade increases the heart rate reflex responses, suggesting that the DH has an inhibitory influence on cardiac baroreflex function [28].
In spite of DH does not project directly to brainstem areas involved in cardiovascular control [11, 33, 57], it has extensive connections with the BNST [17, 19, 61]. BNST plays a role in the baroreflex modulation [1, 2, 15] and sends projections to brainstem areas related to the neurocircuitry of the baroreflex [31, 32, 40]. Thus, BNST may be a “relay area” to the modulatory action of the DH in the cardiac baroreflex function. Further studies are required to verify the pathways involved in the baroreflex modulation promoted by the DH.
Although the DH has been classically implicated in memory and spatial navigation [21, 65], studies show the involvement of the DH in autonomic responses during aversive conditions. The rats’ DH blockade before the re-exposure to an aversive context did not change the behavioral consequences (“freezing”) of animals, but it reduced the increase in both BP and HR [67]. Indeed, DH inhibition may also attenuate both BP and HR increase elicited by acute restraint stress [73]. Thus, these results suggest that DH plays a major role in cardiovascular modulation in threat situations.
In the present study, glut facilitates baroreflex function via NMDA and non-NMDA receptor activation. In the same way, a previous work showed that the administration of a NMDA receptor antagonist in the ventral hippocampus (VH) was able to inhibit the increase in the HR and BP promoted by the local infusion of glut in the VH [70]. Moreover, the intravenous pretreatment with either a ganglionic blocker or a β1-adrenergic receptor antagonist abolished those cardiovascular responses glut-evoked in the VH [70]. Thus, these results suggest that glut elicits cardiovascular responses through NMDA receptor in the VH and these cardiovascular responses glut-evoked are mediated by the sympathetic nervous system.
The injection of a NMDA receptor antagonist into the DH attenuates the increase in the BP and HR as well as the reduction in tail skin temperature of rats submitted to the restraint stress model [56]. Moreover, administration of a NMDA receptor antagonist into the DH reduces the “freezing behavior” and cardiovascular responses of animals submitted to the contextual fear conditioning model [23]. Taken together, these results suggest that NMDA receptors within the DH have a facilitative influence in stress-evoked behavior and autonomic responses.
On the other hand, administration of a CB1 receptor antagonist into the DH reduces the “freezing behavior” and cardiovascular responses of animals submitted to the contextual fear conditioning model [78]. Furthermore, the NMDA receptor antagonist was able to inhibit the increase in both “freezing behavior” and cardiovascular responses promoted by the CB1 receptor antagonist [78], suggesting that the changes evoked by CB1 receptors involve the glutamate and its action on NMDA receptors. These results are in accordance with the present study showing that endocannabinoid system modulates hippocampal glutamate-related responses.
In the hippocampus, CB1 receptors are found in glutamatergic neurons [45]. Excessive activation of glutamatergic transmission in the hippocampus is considered as a key pathogenic event leading to epileptiform seizures [6]. Endocannabinoid system is important in the control of neuronal activity through CB1 receptors [30, 52]. Balanced control of neuronal activity is central in maintaining function and viability of neuronal circuits [6]. The endocannabinoid system tightly controls neuronal excitability, providing substantial endogenous protection against kainic acid (KA)-induced seizures [55]. Cardiovascular autonomic dysfunction in seizures is a major cause of sudden unexpected death in epilepsy [53]. Seizures are associated with altered autonomic activity [54] and decrease heart rate variability [80]. Moreover, a study in rats showed that sustained hippocampal seizure activity was accompanied by progressive baroreflex impairment [81]. Since our results showed that endocannabinoids and glutamate in the hippocampus are important to control of baroreflex function, we suggest that the imbalance in endocannabinoid and glutamatergic activity found in seizure patients may contribute to autonomic and baroreflex failure in the epileptic crisis.
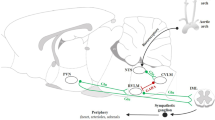
Glutamatergic pyramidal neurons are the main postsynaptic target of GABAergic interneurons [12]. These interneurons control different domains of glutamatergic principal neurons at precise moments during hippocampal activity [12, 75], and the activation of these interneurons results in inhibitory postsynaptic currents (IPSCs) in pyramidal cells [82]. Therefore, since the GABA interneurons in the hippocampus are arranged in a configuration that permits to control the activity of glutamatergic pyramidal neurons, we speculate that GABAergic and endocannabinoid neurotransmissions modulate the glutamatergic neurotransmission activity within the DH controlling the baroreflex function (Fig. 9).
A putative mechanism involving glutamate, GABA, and endocannabinoids within the dorsal hippocampus in the control of baroreflex function. We have suggested that presynaptic glutamate release facilitates the baroreflex function through NMDA and AMPA/Kainate receptors activation. However, the NMDA receptor activation may elicit the endocannabinoid synthesis in the postsynaptic neuron that in turn activate cannabinoid receptor subtype 1 (CB1) in the presynaptic neuron decreasing the baroreflex function through the inhibition of glutamate exocytose. On the other hand, GABA interneurons also modulate the activity of glutamatergic principal neurons. The inhibitory actions of GABA on GABAA receptors hyperpolarize the glutamatergic neurons, decreasing the baroreflex function
Conclusion
During defensive reactions, there is a “delay” in the baroreflex response that allows an increase in HR along with a rise in BP [16, 58, 72]. In the present study, we have shown that endocannabinoid neurotransmission through the CB1 receptor or GABAergic neurotransmission through the GABAA receptor in the DH may blunt heart rate baroreflex responses. Therefore, we speculate that the activation of these pathways within the DH may modulate the baroreflex function in threat situations to adjust the blood perfusion to organs and tissues needed for flight or fight since the baroreflex function is reduced during both an aversive condition and activating CB1 or GABAA receptors.
However, our results are limited in demonstrating the involvement of the glut, GABA, and endocannabinoids within the DH in the cardiac baroreflex modulation. The neural pathways upon which the DH modulates the baroreflex function as well as the conditions in which the DH is necessary for the baroreflex function will be the target of future studies. Therefore, our findings support the possibility that the endocannabinoid, glutamatergic, and GABAergic neurotransmissions interact with each other within the DH to modulate the cardiac baroreflex function (Fig. 9).
References
Alves FH, Crestani CC, Gomes FV, Guimaraes FS, Correa FM, Resstel LB (2010) Cannabidiol injected into the bed nucleus of the stria terminalis modulates baroreflex activity through 5-HT1A receptors. Pharmacol Res 62:228–236
Alves FH, Crestani CC, Resstel LB, Correa FM (2009) Bed nucleus of the stria terminalis N-methyl-D-aspartate receptors and nitric oxide modulate the baroreflex cardiac component in unanesthetized rats. J Neurosci Res 87:1703–1711
Anand BK, Dua S (1956) Circulatory and respiratory changes induced by electrical stimulation of limbic system (visceral brain). J Neurophysiol 19:393–400
Andy O, Akert M (1953) Electrically induced seizure discharges from Ammon's formation, fornix, thalamus and cingulate gyrus in the cat and monkey. Electroencephalogr Clin Neurophysiol 5:320
Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J (2004) Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev 28:273–283
Ben-Ari Y, Cossart R (2000) Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci 23:580–587
Busnardo C, Ferreira-Junior NC, Cruz JC, Machado BH, Correa FM, Resstel LB (2013) Cardiovascular responses to ATP microinjected into the paraventricular nucleus are mediated by nitric oxide and NMDA glutamate receptors in awake rats. Exp Physiol 98:1411–1421
Busnardo C, Tavares RF, Antunes-Rodrigues J, Correa FM (2007) Cardiovascular effects of L-glutamate microinjection in the supraoptic nucleus of unanaesthetized rats. Neuropharmacology 52:1378–1384
Carlson HB, Gellhorn E, Darrow CW (1941) Representation of the sympathetic and parasympathetic nervous systems in the forebrain of the cat. Arch Neurol Psychol 45:105–116
Carta M, Fievre S, Gorlewicz A, Mulle C Kainate receptors in the hippocampus. Eur J Neurosci 39:1835–1844
Castle M, Comoli E, Loewy AD (2005) Autonomic brainstem nuclei are linked to the hippocampus. Neuroscience 134:657–669
Chamberland S, Topolnik L Inhibitory control of hippocampal inhibitory neurons. Front Neurosci 6:165
Chianca DA Jr, Lin LH, Dragon DN, Talman WTNMDA (2004) Receptors in nucleus tractus solitarii are linked to soluble guanylate cyclase. Am J Physiol Heart Circ Physiol 286:H1521–H1527
Crestani CC, Alves FH, Busnardo C, Resstel LB, Correa FM (2010) N-methyl-D-aspartate glutamate receptors in the hypothalamic paraventricular nucleus modulate cardiac component of the baroreflex in unanesthetized rats. Neurosci Res 67:317–326
Crestani CC, Alves FH, Resstel LB, Correa FM (2008) Bed nucleus of the stria terminalis alpha(1)-adrenoceptor modulates baroreflex cardiac component in unanesthetized rats. Brain Res 1245:108–115
Crestani CC, Tavares RF, Alves FH, Resstel LB, Correa FM (2010) Effect of acute restraint stress on the tachycardiac and bradycardiac responses of the baroreflex in rats. Stress 13:61–72
Cullinan WE, Herman JP, Watson SJ (1993) Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol 332:1–20
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
Dillingham CM, Erichsen JT, O'Mara SM (2015) Aggleton JP, and Vann SD. Fornical and non-fornical projections from the rat hippocampal formation to the anterior thalamic nuclei, Hippocampus
Ding R, Asada H, Obata K (1998) Changes in extracellular glutamate and GABA levels in the hippocampal CA3 and CA1 areas and the induction of glutamic acid decarboxylase-67 in dentate granule cells of rats treated with kainic acid. Brain Res 800:105–113
Dong HW, Petrovich GD, Watts AG, Swanson LW (2001) Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol 436:430–455
Drummond HA, Welsh MJ, Abboud FM (2001) ENaC subunits are molecular components of the arterial baroreceptor complex. Ann N Y Acad Sci 940:42–47
Fabri DR, Hott SC, Reis DG, Biojone C, Correa FM, Resstel LB The expression of contextual fear conditioning involves activation of a NMDA receptor-nitric oxide-cGMP pathway in the dorsal hippocampus of rats. Eur Neuropsychopharmacol 24:1676–1686
Ferreira-Junior NC, Fedoce AG, Alves FH, Correa FM, Resstel LB Medial prefrontal cortex endocannabinoid system modulates baroreflex activity through CB(1) receptors. Am J Physiol Regul Integr Comp Physiol 302:R876–R885
Ferreira-Junior NC, Fedoce AG, Alves FH, Correa FM, Resstel LB (2012) Medial prefrontal cortex endocannabinoid system modulates baroreflex activity through CB(1) receptors. Am J Physiol Regul Integr Comp Physiol 302:R876–R885
Ferreira-Junior NC, Fedoce AG, Alves FH, Resstel LB (2013) Medial prefrontal cortex N-methyl-D-aspartate receptor/nitric oxide/cyclic guanosine monophosphate pathway modulates both tachycardic and bradycardic baroreflex responses. J Neurosci Res 91:1338–1348
Ferreira-Junior NC, Fedoce AG, Alves FH, Resstel LB Medial prefrontal cortex N-methyl-D-aspartate receptor/nitric oxide/cyclic guanosine monophosphate pathway modulates both tachycardic and bradycardic baroreflex responses. J Neurosci Res 91:1338–1348
Ferreira-Junior NC, Lagatta DC, Fabri DR, Alves FH, Correa FM, Resstel LB (2016) Hippocampal subareas arranged in the dorsal-ventral axis modulate cardiac baroreflex function in a site-dependent manner in rats. Exp Physiol
Fortaleza EA, Ferreira-Junior NC, Lagatta DC, Resstel LB, Correa FM (2015) The medial amygdaloid nucleus modulates the baroreflex activity in conscious rats. Auton Neurosci 193:44–50
Freund TF, Katona I, Piomelli D (2003) Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83:1017–1066
Giancola SB, Roder S, Ciriello J (1993) Contribution of caudal ventrolateral medulla to the cardiovascular responses elicited by activation of bed nucleus of the stria terminalis. Brain Res 606:162–166
Gray TS, Magnuson DJ (1987) Neuropeptide neuronal efferents from the bed nucleus of the stria terminalis and central amygdaloid nucleus to the dorsal vagal complex in the rat. J Comp Neurol 262:365–374
Guyenet PG (2006) The sympathetic control of blood pressure. Nat Rev Neurosci 7:335–346
Hagihara H, Ohira K, Toyama K, Miyakawa T Expression of the AMPA receptor subunits GluR1 and GluR2 is associated with granule cell maturation in the dentate gyrus. Front Neurosci 5:100
Haring M, Guggenhuber S, Lutz B Neuronal populations mediating the effects of endocannabinoids on stress and emotionality. Neuroscience 204:145–158
Hashimoto T, Kimori M, Nakamura Y, Kuriyama K (1989) Effect of NC-1100 [1-(3,4-dimethoxyphenyl)-2-(4-diphenylmethylpiperazinyl) ethanol dihydrochloride] on gamma-aminobutyric acid (GABA) metabolism in rat brain: analysis using stroke-prone spontaneously hypertensive rat. Jpn J Pharmacol 50:131–139
Head GA, McCarty R (1987) Vagal and sympathetic components of the heart rate range and gain of the baroreceptor-heart rate reflex in conscious rats. J Auton Nerv Syst 21:203–213
Herron CE, Lester RA, Coan EJ, Collingridge GL (1985) Intracellular demonstration of an N-methyl-D-aspartate receptor mediated component of synaptic transmission in the rat hippocampus. Neurosci Lett 60:19–23
Higuera-Matas A, Miguens M, Coria SM, Assis MA, Borcel E, del Olmo N, Ambrosio E (2012) Sex-specific disturbances of the glutamate/GABA balance in the hippocampus of adult rats subjected to adolescent cannabinoid exposure. Neuropharmacology 62:1975–1984
Holstege G, Meiners L, Tan K (1985) Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res 58:379–391
Ichida T, Takeda K, Sasaki S, Nakagawa M, Hashimoto T, Kuriyama K (1996) Age-related decrease of gamma-aminobutyric acid (GABA) release in brain of spontaneously hypertensive rats. Life Sci 58:209–215
Kaada BR (1951) Somatomotor, autonomic and electrocorticographic responses to electrical stimulation of rhinencephalic and other structures in primates, cat and dog. Acta Physiol Scand 83:241–285
Kaada BR, Jansen J Jr, Andersen P (1953) Stimulation of the hippocampus and medial cortical areas in unanesthetized cats. Neurology 3:844–857
Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF (1999) Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19:4544–4558
Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF (2006) Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci 26:5628–5637
Korner PI, Shaw J, West MJ, Oliver JR (1972) Central nervous system control of baroreceptor reflexes in the rabbit. Circ Res 31:637–652
Lagatta DC, Ferreira-Junior NC, Resstel LB (2015) Medial prefrontal cortex TRPV1 channels modulate the baroreflex cardiac activity in rats. Br J Pharmacol 172:5377–5389
Lupica CR, Hu Y, Devinsky O, Hoffman AF Cannabinoids as hippocampal network administrators. Neuropharmacology 124:25–37
Mac LP (1949) Psychosomatic disease and the visceral brain; recent developments bearing on the Papez theory of emotion. Psychosom Med 11:338–353
Maclean PD (1952) Some psychiatric implications of physiological studies on frontotemporal portion of limbic system (visceral brain). Electroencephalogr Clin Neurophysiol 4:407–418
Maclean PD, Delgado JM (1953) Electrical and chemical stimulation of frontotemporal portion of limbic system in the waking animal. Electroencephalogr Clin Neurophysiol 5:91–100
Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B (2003) CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302:84–88
Massey CA, Sowers LP, Dlouhy BJ, Richerson GB Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol 10:271–282
Meyer S, Strittmatter M Autonomic changes with seizures correlate with postictal EEG suppression. Neurology 80:1538–1539
Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R, Kelsch W, Jacob W, Marsch R, Ekker M, Long J, Rubenstein JL, Goebbels S, Nave KA, During M, Klugmann M, Wolfel B, Dodt HU, Zieglgansberger W, Wotjak CT, Mackie K, Elphick MR, Marsicano G, Lutz B (2006) The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 51:455–466
Moraes-Neto TB, Scopinho AA, Biojone C, Correa FM, Resstel LB Involvement of dorsal hippocampus glutamatergic and nitrergic neurotransmission in autonomic responses evoked by acute restraint stress in rats. Neuroscience 258:364–373
Moser MB, Moser EI (1998) Distributed encoding and retrieval of spatial memory in the hippocampus. J Neurosci 18:7535–7542
Nosaka S (1996) Modifications of arterial baroreflexes: obligatory roles in cardiovascular regulation in stress and poststress recovery. Jpn J Physiol 46:271–288
Notman R, Noro M, O'Malley B, Anwar J (2006) Molecular basis for dimethylsulfoxide (DMSO) action on lipid membranes. J Am Chem Soc 128:13982–13983
O'Mara SM, Commins S, Anderson M, Gigg J (2001) The subiculum: a review of form, physiology and function. Prog Neurobiol 64:129–155
Pacak K, Palkovits M, Kopin IJ, Goldstein DS (1995) Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: in vivo microdialysis studies. Front Neuroendocrinol 16:89–150
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. Academic Press, Inc., San Diego, CA
Pilowsky PM, Goodchild AK (2002) Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens 20:1675–1688
Piomelli D (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4:873–884
Rademacher DJ, Patel S, Hopp FA, Dean C, Hillard CJ, Seagard JL (2003) Microinjection of a cannabinoid receptor antagonist into the NTS increases baroreflex duration in dogs. Am J Physiol Heart Circ Physiol 284:H1570–H1576
Resstel LB, Correa FM (2006) Injection of l-glutamate into medial prefrontal cortex induces cardiovascular responses through NMDA receptor—nitric oxide in rat. Neuropharmacology 51:160–167
Resstel LB, Joca SR, Correa FM, Guimaraes FS (2008) Effects of reversible inactivation of the dorsal hippocampus on the behavioral and cardiovascular responses to an aversive conditioned context. Behav Pharmacol 19:137–144
Ruit KG, Neafsey EJ (1988) Cardiovascular and respiratory responses to electrical and chemical stimulation of the hippocampus in anesthetized and awake rats. Brain Res 457:310–321
Saleh TM, Connell BJ (2003) Estrogen-induced autonomic effects are mediated by NMDA and GABAA receptors in the parabrachial nucleus. Brain Res 973:161–170
Santini CO, Fassini A, Scopinho AA, Busnardo C, Correa FM, Resstel LB The ventral hippocampus NMDA receptor/nitric oxide/guanylate cyclase pathway modulates cardiovascular responses in rats. Auton Neurosci 177:244–252
Sasaki S, Lee LC, Nakamura Y, Iyota I, Fukuyama M, Inoue A, Takeda K, Yoshimura M, Nakagawa M, Ijichi H (1986) Hypotension and hypothalamic depression produced by intracerebroventricular injections of GABA in spontaneously hypertensive rats. Jpn Circ J 50:1140–1148
Schlor KH, Stumpf H, Stock G (1984) Baroreceptor reflex during arousal induced by electrical stimulation of the amygdala or by natural stimuli. J Auton Nerv Syst 10:157–165
Scopinho AA, Lisboa SF, Guimaraes FS, Correa FM, Resstel LB, Joca SR (2013) Dorsal and ventral hippocampus modulate autonomic responses but not behavioral consequences associated to acute restraint stress in rats. PLoS One 8:e77750
Siegel A, Flynn JP (1968) Differential effects of electrical stimulation and lesions of the hippocampus and adjacent regions upon attack behavior in cats. Brain Res 7:252–267
Sik A, Penttonen M, Ylinen A, Buzsaki G (1995) Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci 15:6651–6665
Smith WK (1944) The results of stimulation of the uncus and adjacent portions of the hippocampal gyrus. Fed Proc 3:43
Spencer SE, Sawyer WB, Loewy AD (1988) L-glutamate stimulation of the zona incerta in the rat decreases heart rate and blood pressure. Brain Res 458:72–81
Spiacci GB, Antero LS, Reis DG, Lisboa SF, Resstel LB Dorsal hippocampus cannabinoid type 1 receptors modulate the expression of contextual fear conditioning in rats: involvement of local glutamatergic/nitrergic and GABAergic neurotransmissions. Eur Neuropsychopharmacol 26:1579–1589
Strange BA, Witter MP, Lein ES, Moser EI (2014) Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 15:655–669
Surges R, Henneberger C, Adjei P, Scott CA, Sander JW, Walker MC (2009) Do alterations in inter-ictal heart rate variability predict sudden unexpected death in epilepsy? Epilepsy Res 87:277–280
Tsai CY, Chan JY, Hsu KS, Chang AY, Chan SH Brain-derived neurotrophic factor ameliorates brain stem cardiovascular dysregulation during experimental temporal lobe status epilepticus. PLoS One 7:e33527
Vida I, Halasy K, Szinyei C, Somogyi P, Buhl EH, Unitary IPSP (1998) Evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J Physiol 506(Pt 3):755–773
Werling LL, Nadler JV (1982) Complex binding of L-[3H]glutamate to hippocampal synaptic membranes in the absence of sodium. J Neurochem 38:1050–1062
Willette RN, Barcas PP, Krieger AJ, Sapru HN (1983) Vasopressor and depressor areas in the rat medulla. Identification by microinjection of L-glutamate. Neuropharmacology 22:1071–1079
Xu JY, Chen C Endocannabinoids in synaptic plasticity and neuroprotection. Neuroscientist 21:152–168
Acknowledgments
The authors thank Camargo, L.H. for their technical help.
Funding
Ferreira-Junior has a FAPESP doctoral fellowship (2011/19494-8). The grants that supported the present research were from the CNPq (305996/2008-8 and 470042/2009-5), FAPESP (2011/07332-3), and FAEPA.
Author information
Authors and Affiliations
Contributions
N.C.F.J. and L.B.M.R. conceived and designed the research; N.C.F.J. and D.C.L. performed experiments; N.C.F.J. and D.C.L. analyzed the data; N.C.F.J. and L.B.M.R. interpreted the results of experiments; N.C.F.J. prepared the figures; N.C.F.J. drafted the manuscript; N.C.F.J., D.C.L. and L.B.M.R. edited and revised the manuscript; and all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ferreira-Junior, N.C., Lagatta, D.C. & Resstel, L.B.M. Glutamatergic, GABAergic, and endocannabinoid neurotransmissions within the dorsal hippocampus modulate the cardiac baroreflex function in rats. Pflugers Arch - Eur J Physiol 470, 395–411 (2018). https://doi.org/10.1007/s00424-017-2083-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-017-2083-y