Abstract
Ventral medial prefrontal cortex (vMPFC) glutamatergic neurotransmission has a facilitatory role on cardiac baroreflex activity which is mediated by NMDA receptors activation. Corticotrophin releasing factor receptors type1 and 2 (CRF1 and CRF2), present in the vMPFC, are colocalized in neurons containing glutamate vesicles, suggesting that such receptors may be involved in glutamate release in this cortical area. Therefore, our hypothesis is that the CRF1 and CRF2 receptors can modulate the baroreflex bradycardic and tachycardic responses. In order to prove this assumption, male Wistar rats had bilateral stainless steel guide cannula implanted into the vMPFC, and baroreflex was activated by intravenous infusion of phenylephrine or sodium nitroprusside through a vein catheter. A second catheter was implanted into the femoral artery for cardiovascular measurements. The CRF1 receptor antagonist administration in either infralimbic cortex (IL) or prelimbic cortex (PL), vMPFC regions, was unable to change the bradycardic responses but increased the slope of the baroreflex tachycardic activity. Microinjection of the CRF2 receptor antagonist into the IL and PL did not alter ether bradycardic nor tachycardic baroreflex responses. The administration of the non-selective CRF receptors agonist, urocortin in these areas, did not modify bradycardic responses but decreased tachycardia slope of the baroreflex. CRF1 receptor antagonist administration prior to non-selective CRF agonist in vMPFC prevented the tachycardic responses reduction. However, CRF2 receptor antagonism could not prevent the effect of CRF receptors agonist. These results suggest that IL and PL CRF1 but not CRF2 receptors have an inhibitory role on the baroreflex tachycardic activity. Furthermore, they have no influence on baroreflex bradycardic activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blood pressure (BP) needs to be adequately maintained and reasonably constant throughout life since excessive variability represents a major risk factor for harmful events to health [24]. The cardiovascular parameters can be influenced by functional factors such as cardiac output, peripheral vascular resistance (PVR), and heart rate (HR) [11]. Arterial baroreflex is a neural mechanism that regulates BP in the short and medium term, which is essential to prevent BP and HR oscillations. This modulation is mediated by mechanoreceptors sensitive to arterial stretching present in the carotid sinus and aortic arch, the so-called baroreceptors [32].
The baroreceptors are connected to brainstem structures, such as the solitary tract nucleus, ambigus nucleus, the caudal ventrolateral medulla, and the ventrolateral-lateral medulla. Interestingly, structures of the limbic system and forebrain send projections to brainstem regions [6, 13, 36]. In this context, the prefrontal cortex (PFC) is a limbic structure with a fundamental role in the integration of emotional and cardiovascular responses through the autonomic nervous system. This structure can be subdivided into the lateral prefrontal cortex (LPFC) and medial (MPFC) cortex. The ventral portion of the MPFC (vMPFC) comprises the infralimbic (IL), prelimbic (PL), and dorso penducular (DP) cortices [2]. Some studies show that glutamatergic neurotransmission of IL and PL has a facilitatory role on the tachycardic and bradycardic responses of baroreflex, through NMDA receptors activation and production of nitric oxide [14, 35, 21].
Glutamatergic neurotransmission has been proposed to be modulated by the corticotropin-releasing factor (CRF) receptors since its receptors are colocalized with glutamate vesicles in vMPFC neurons [34]. CRF is produced mainly by cells of the parvocellular division of paraventricular nucleus, which is located in hypothalamus. It plays an important role in the regulation of the hypothalamic-pituitary-adrenal (HPA) axis [41]. CRF receptors are coupled to the Gs protein [10, 26, 31] and are divided into CRF type 1 (CRF1) and CRF type 2 (CRF2). Both receptors are highly expressed in the central nervous system [3]. Some studies demonstrate that the CRF1 and CRF2 receptors present limbic areas, such as the bed nucleus of the stria terminalis (BNST), play a role in modulating the cardiovascular component of the conditioned emotional response [4, 29], demonstrating the role of CRF present in the limbic system in the modulation of cardiovascular response. However, despite the activity modulation in the prefrontal cortex by the CRF has already been observed in the study by Meng et al, [25], its role in the modulation of particular cardiovascular responses, such as baroreflex, is still uncertain.
Considering that vMPFC NMDA receptors can modulate the baroreflex activity [14], that CRF receptors are expressed in glutamatergic neurons in vMPFC [34], and that CRF1 and CRF2 receptors of other brain structures have been associated with cardiovascular regulation [4, 29], the hypothesis of the present study is that vMPFC CRF1 and CRF2 receptor are involved in the modulation of baroreceptor reflex arc.
Methods
Animals
Fifty-two male Wistar rats weighing 230–270 g were used in the present experiments from the colony of pathogen-free rats maintained by the Pharmacy School of Ribeirão Preto, University of São Paulo (USP). All animals were kept in the Animal Care Unit. The animals were housed in groups of five per cage (41 × 33 × 17 cm) in a temperature-controlled room (24 ± 1 °C) under a 12 h light/dark cycle (lights on at 06:00 A.M.) and were given water and food ad libitum. All experiments were performed in accordance with Ethical Principles for Animal Experimentation followed by the Brazilian Committee for Animal Experimentation (COBEA) and approved by the Committee of Ethics in Animal Research of the School of Medicine of Ribeirão Preto, University of São Paulo (number 101/09/2015).
Drugs
The following drugs were used: a CRF1 receptor antagonist (CP376395; Tocris, Westwoods Business Park Ellisville, MO, USA) and a CRF2 receptor antagonist (K41498; Tocris, Westwoods Business Park Ellisville, MO, USA). They were both dissolved in saline (0.9% NaCl). In addition, a CRF receptor agonist (Urocortin-K41498; Tocris, Westwoods Business Park, Ellisville, MO, USA) was used. Urocortin was dissolved in DMSO 10% in saline (0.9% NaCl). The solutions were prepared immediately before use and were kept on ice and protected from light during the experimental sessions.
Surgical procedure
Four days before the experiment, rats were anaesthetized using tribromoethanol (250 mg kg−1, i.p., Sigma, St. Louis, MO, USA). After local anesthesia with 2% lidocaine, the skull was surgically exposed, and stainless steel guide cannulae (26G) were bilaterally implanted into the vMPFC using a stereotaxic apparatus (Stoelting, Wood Dale, IL, USA). Stereotaxic coordinates for cannulae implantation into the prelimbic (PL) and infralimbic (IL) regions of vMPFC were based from the Rat Brain Atlas of Paxinos and Watson (1997). It was used antero-posterior = + 3.4 mm, lateral = 2.5 mm from the medial suture, and lateral inclination of 24°. Vertical coordinates from the skull were 3.2 mm and 3.3 mm for the PL and IL, respectively. Cannulae were fixed to the skull with dental cement and one metal screw fixed to the skull. After surgery, animals were treated with a polyantibiotic preparation of streptomycins/penicillins (i.m., Pentabiotico®, Fort Dodge, Campinas, São Paulo, Brazil) to prevent infection and with the non-steroidal anti-inflammatory flunixine meglumine (s.c., Banamine®, Schering Plough, Cotia, São Paulo, Brazil) for analgesia. One day before the experiment, rats were anaesthetized with tribromoethanol (250 mg kg-1, i.p.), and a catheter (a 4 cm segment of PE-10 that was heat-bound to a 13 cm segment of PE-50, Clay Adams, Parsippany, NJ, USA) was inserted into the femoral artery, in order to record BP. A second catheter was implanted into the femoral vein for the infusion of vasoactive substances. Both catheters were inserted under the skin and exteriorized on the animal’s dorsum. After surgery, treatment with anti-inflammatory drugs was repeated.
Measurement of cardiovascular responses
The pulsatile arterial pressure of freely moving animals was recorded using an ML870 preamplifier (LabChart, USA) and an acquisition board (PowerLab, AD Instruments, USA) connected to a computer. Mean arterial pressure (MAP) and heart rate (HR) values were derived from pulsatile recordings and processed online. The needles (33G, Small Parts, Miami Lakes, FL, USA) used for microinjection into the vMPFC were 1 mm longer than the guide cannulas and were connected to a 1-μL syringe (7002-H, Hamilton Co., Reno, NV, USA) through PE-10 tubing. The needle was carefully inserted into the guide cannula, and drugs were injected with an infusion pump (KD Scientific, Holliston, MA, USA). in vMPFC (200 nL) over a 5-s period. After a 30-s period, the needle was removed and inserted into the second guide cannula for microinjection into the contralateral vMPFC.
Baroreflex assessment
The baroreflex was activated by phenylephrine (α1 adrenoceptor agonist; 50 μg kg-1; 0.34 mL min-1, Sigma, St. Louis, MO,USA) or sodium nitroprusside (SNP; NO donor; 50 μg kg-1; 0.8 mL min-1, Sigma, St. Louis, MO,USA) infusion using an infusion pump (KD Scientific, Holliston, MA, USA). The phenylephrine or SNP infusion lasted 30–40 s and induced an increase and decrease in BP in order to evoke tachycardic or bradycardic responses, respectively [1].
Method used to evaluate baroreflex activity
Baroreflex curves were constructed, matching MAP variations with HR responses. Paired values for variations in MAP (ΔMAP) and HR (ΔHR) were plotted to create sigmoid curves for each rat, which were used to determine baroreflex activity to analyze bradycardic and tachycardic responses separately; HR values matching 10, 20, 30, and 40 mmHg of MAP changes were calculated [1, 36]. Values were plotted to create linear regression curves for each rat, and their slopes were compared to determine changes in baroreflex gain.
Experimental protocols
All groups of animals used in our study received three sets of phenylephrine or SNP infusion to determine control values of baroreflex activity. Posteriorly, the first group received microinjections of CRF1 antagonist (0.45 or 4.5 nmol) in the IL region or 4.5 nmol in PL region. The second group received microinjections of CRF2 antagonist 4.5 nmol in IL or in PL region. The third group received microinjections of non-selective CRF agonist (0.02 or 0.2 nmol) in only the IL region to describe an effective dose. The fourth group received microinjections of CRF2 antagonist (4.5 nmol) prior to non-selective CRF agonist (0.2 nmol) in the vMPFC. The last group was injected with CRF1 antagonist (0.45 nmol) prior to non-selective CRF agonist (0.2 nmol) in vMPFC. In both groups, the second compound was always injected 5 min after the first one. In all experimental groups, phenylephrine and SNP infusion were repeated 10 and 60 min after the bilateral vMPFC microinjections. The effective doses of these drugs were obtained based on previous experiments by our group that have not yet been published. Regarding this matter, such concentrations of these compounds were able to change cardiovascular responses in rats submitted to the contextual fear conditioning, when injected in the bed nucleus of the stria terminalis (data not published).
Histological procedure
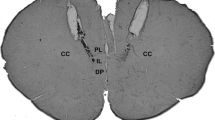
At the end of the experiments, the rats were anaesthetized with urethane (1.25 g kg-1, i.p.), and 200 nL of 1% Evan’s blue dye was bilaterally injected into the IL and PL regions of vMPFC as a marker of injection sites. The chest was surgically opened, the descending aorta occluded, the right atrium severed, and the brain perfused with saline and 10% formalin through the left ventricle. Brains were post-fixed for 24 h at 4 °C, and 40-μm sections were cut with a cryostat (CM-1900, Leica, Wetzlar, Germany). The actual placement of the injection needles was verified in serial sections, using as reference the Rat Brain Atlas of Paxinos and Watson, 1997( Fig. 1).
Representative photomicrography of an injection site. (a) Diagrammatic representations showing the microinjection correct sites of the drugs (dark circles) in the IL and PL regions and of areas outside the IL and PL regions (gray circles) (left and right), based on the Rat Brain Atlas of Paxinos and Watson. (b) Representative photomicrography of an injection site in the IL and PL regions of vMPFC (bar = 1 mm)
Data analysis
Baseline cardiovascular values before and after pharmacological treatment into the IL and PL portions of vMPFC were compared using Student’s t test. Baroreflex activity was analyzed using sigmoid curves which were characterized as five parameters: (i) P1 (beats min-1) lower heart rate plateau and P2 (beats min-1) upper heart rate plateau; (ii) heart rate range (beats min-1), difference between upper and lower plateau levels (ΔP); and (iii) average gain (G, beats min-1 mmHg-1 ), which is the average slopes of the non-linear curves. Significant differences among sigmoid curves or linear regression parameters were analyzed using one-way ANOVA followed by the Dunnett’s post hoc test. The slope of linear regression curves (Δ HR vs. Δ MAP) before and 10 and 60 min after microinjection of each treatment was determined, and results were analyzed to detect alterations in cardiac baroreflex gain using one-way ANOVA followed by Dunnett’s post hoc test. Results of statistical tests with p < 0.05 value were considered significant.
Results
Figure 1 shows a representative photomicrograph of IL and PL regions of a vMPFC coronal section of an animal used in the present study. Also, we show a diagrammatic representations of vMPFC microinjection sites of all experimental groups used in this study.
Effects of bilateral microinjections of combined vehicles (10%DMSO and NaCl 0.9%) into the vMPFC and also CRF1 antagonist in vMPFC surrounding structures on cardiac baroreflex activity
In order to exclude the possible effects of the vehicles used, we performed the injection of the combination of vehicles into the vMPFC, as a control group. Microinjection of 10% DMSO preceded by NaCl 0.9% into the vMPFC (n = 5) did not change basal levels of HR (before = 387 ± 11; after = 372 ± 9 bpm; t = 0.09; p > 0.05) and MAP (before = 99 ± 1.03; after = 100 ± 1.12 mmHg; t = 0.05; p > 0.05). Moreover, there was no alteration in the slope of the regression line curve of both the bradycardic (before = − 1.52 ± 0.02; 10 min = − 1.59 ± 0.04; 60 min = − 1.55 ± 0.09; F (2,14) = 0.51; p > 0.05) and tachycardic (before = − 1.87 ± 0.05; 10 min = − 1.86 ± 0.04; 60 min = − 1.88 ± 0.02; F (2,14) = 0.83; p > 0.05) baroreflex responses. Sigmoid curve parameters (G, P1, P2, ∆P) were also not affected (data not shown).
In another group of animals, CRF1 antagonist (CP376395, 4.5 nmol) was injected into vMPFC surrounding structures in order to confirm a site-specific effect. Microinjection of the compound into the vMPFC (n = 5) did not change basal levels of HR (before = 323 ± 9; after = 333 ± 14 bpm; t = 0.10; p > 0.05) and MAP (before = 107 ± 1.03; after = 104 ± 0.12 mmHg; t = 0.07; p > 0.05). Additionally, there was no alteration in the slope of the regression line curve of both the bradycardic (before = − 1.44 ± 0.15; 10 min = − 1.49 ± 0.09; 60 min = − 1.52 ± 0.11; F (2,14) = 0.91; p > 0.05) and tachycardic (before = − 1.62 ± 0.08; 10 min = − 1.54 ± 0.06; 60 min = − 1.58 ± 0.09; F (2,14) = 0.31; p > 0.05) baroreflex responses. Sigmoid curve parameters (G, P1, P2, ∆P) were also not affected (data not shown).
Effects of CRF-1 receptor antagonism in the vMPFC on cardiac baroreflex activity
Bilateral microinjections of CRF1 antagonist CP376395 (0.45 nmol/ 200 nL; n = 6) in IL region in the vMPFC did not modify the baseline MAP (before = 99.4 ± 3.46, after = 99.1 ± 3. 35 mmHg, t = 0.27, p > 0.05) and HR (before = 382 ± 24, after = 382 ± 24 bpm: t = 0.07, p > 0.05) values. Linear regression indicates that the microinjections in the vMPFC did not cause alterations in the baroreflex response, in neither the bradycardic (before = − 2.59 ± 0.87, 10 min later = − 2.80 ± 0.23, 60 min later = − 2.61 ± F (2,17) = 0.02; p > 0.05) nor tachycardic components (before = − 2.02 ± 0.41, 10 min later = − 2.66 ± 0.34, 60 min later = − 2.55 ± 0.27; F (2,17) = 0.39; p > 0.05; Fig. 2a). In addition, non-linear regression values (G, P1, P2, and ΔP) were not altered (Table 1).
Graphs representative of the variation in mean arterial pressure over the variation in heart rate: (Top) Linear regression curves correlating the Δ MAP and Δ HR responses before, 10 min and 60 min after bilateral microinjection of CRF1 antagonist CP376395 0.45 nmol in vMPFC (a), 4.5 nmol in IL (b), and 4.5 nmol in PL (c). Correlation values r2 for the bradycardic regression curves were 0.23, 0.84, and 0.49 for the data generated before; 0.83, 0.65, and 0.38 for the data generated 10 min after; and 0.32, 0.70, and 0.86 for the data generated after 60 min of the bilateral microinjection of CP376395 in the respective doses. The correlation values r2 for the tachycardic regression curves were 0.52, 0.76, and 0.89 for the data generated before; 0.58, 0.76, and 0.83 for the data generated 10 min after; and 0.55, 0.79, and 0.88 for the data generated 60 min after microinjection of CP376395 in the respective doses. (Bottom) Non-linear regression coefficients correlating mean arterial pressure (Δ MAP) and heart rate (ΔHR) before (R2 = 0.67, 0.92, 0.90), 10 min (R2 = 0.88, 0.90, 0.93), and 60 min (R2 = 0.76, 0.93, 0.91) after bilateral microinjection of CP376395 in the vMPFC (Table 1)
In another group of animals, bilateral microinjections of CRF1 antagonist CP376395 (4.5 nmol/200 nL; n = 7) in IL region did not change the basal levels of MAP (before = 103.6 ± 4.96, after = 103.0 ± 4.74 mmHg, p > 0.05) and HR (before = 383.9 ± 18, after = 384.4 ± 20 bpm, t = 0.27, p > 0.05). In addition, there were no difference in the slope of linear regression curve of the bradycardic component (before = 1.49 ± 0.48, 10 min = − 1.93 ± 0.67, 60 min later = − 0.85 ± 1.22; F (2,17) = 2.89; p > 0.05). However, there was an increase in the tachycardic slope of linear regression (before = − 1.72 ± 0.37, 10 min later = − 2.84 ± 0.24, 60 min later = − 1.84 ± 0.55 bpm; F (2,17) = 15.47; p < 0.05; Fig. 2b). Furthermore, some non-linear regression parameters (G, P2, and ΔP) were also enhanced (Table 1).
In another group of animals, the bilateral microinjections of CRF1 antagonist CP376395 (4.5 nmol/ 200 nL; n = 5) in PL region in the vMPFC was unable to change the baseline values of MAP (before = 97 ± 23, after = 97 ± 23 mmHg; t = 1.50; p > 0.05) and HR (before = 304 ± 43, after = 305 ± 24 bpm; t = 0.54; p > 0.05) as in the IL region. Microinjections in PL region did not alter the baroreflex bradycardic response (before = − 1.78 ± 0.12, 10 min later = − 1.94 ± 0.08, 60 min later = − 1.78 ± 0.55; F (2,17) = 1.08; p > 0.05), but increased the slope of the tachycardic component (before = − 2.31 ± 0.04, 10 min later = − 2.98 ± 0.43, 60 min later = − 2.58 ± 0.35; F (2,17) = 3.91; p < 0.05) (Fig. 2c). In addition, the non-linear regression values (P1 and ΔP) were not altered, while G and P2 were enhanced (Table 1).
Effects of CRF-2 receptor antagonism in the vMPFC on cardiac baroreflex activity
Bilateral microinjections of CRF-2 antagonist K41498 (4.5 nmol) in the IL region of vMPFC (n = 6) did not change the baseline values of MAP (before = 157 ± 12, after = 157 ± 19 mmHg, t = 0.23, p > 0.05) and HR (before = 333 ± 24, after = 332 ± 32 bpm, t = 1.54, p > 0.05). Linear regression analysis of baroreflex indicates that microinjections in IL vMPFC did not alter either the bradycardic component (before = − 2.00 ± 0.41, 10 min later = − 2.19 ± 0.46, 60 min later = − 1.98 ± 0.41, F (2,17) = 0.30, p > 0.05) or tachycardic component (before = − 2.90 ± 0.30, 10 min later = − 2.45 ± 0.17, 60 min after = − 2.70 ± 0.33, F (2,17) = 0.25, p > 0.05; Fig. 3a). In addition, the non-linear regression values (G, P1, P2, and ΔP) were not altered (Table 2).
Graphs representative of the variation in mean arterial pressure over the variation in heart rate: (Top) Linear regression curves correlating the Δ MAP and Δ HR responses before and 10 min and 60 min after bilateral microinjection of CRF2 antagonist K41498 of 4.5 nmol in IL (a) and 4.5 nmol in PL (b). Correlation values r2 for the bradycardic regression curves were 0.60 and 0.63 for the data generated before, 0.67 and 0.58 for the data generated 10 min after, and 0.63, and 0.75 for the data generated after 60 min of the bilateral microinjection of K41498 4.5 nmol in the respective doses. The correlation values r2 for the tachycardic regression curves were 0.57 and 0.73 for the data generated before, 0.75 and 0.43 for the data generated 10 min after, and 0.55 and 0.85 for the data generated after 60 min after microinjection of K41498 in the respective doses. (Bottom) Non-linear regression coefficients correlating mean arterial pressure (Δ MAP) and heart rate (ΔHR) before (R2 = 0.84, 0.86), 10 min (R2 = 0.90, 0.79), and 60 min (R2 = 0.86, 0.91) after bilateral microinjection of K41498 in the vMPFC (Table 2)
Additionally, in another group of animals, the bilateral microinjections of CRF2 antagonist K41498 (4.5 nmol) in the PL region of vMPFC (n = 5) did not modify the baseline values of MAP (before = 133 ± 27, after = 134 ± 31 mmHg, t = 0.43, p > 0.05) and HR (before = 308 ± 17, after = 312 ± 12 bpm, t = 1.99, p > 0.05). Linear regression analysis of the baroreflex indicates that microinjections in vMPFC did not change in either the bradycardic (before = − 2.13 ± 0.38, 10 min later = − 2.10 ± 0.41, 60 min later = − 2.24 ± 0.30, F (2,17) = 0.37, p > 0.05) or tachycardic components (before = − 2.23 ± 0.31, 10 min later = − 1.73 ± 0.47, 60 min after = − 2.07 ± 0.19, F (2,17) = 0.73, p > 0.05; Fig. 3b). In addition, the non-linear regression values (G, P1, P2, and ΔP) were not altered (Table 2).
Effects of bilateral microinjection in the vMPFC of a non-selective CRF receptor agonist on cardiac baroreflex activity
This experiment was built to analyze the sum of the agonist’s activity when microinjected in the two regions of the vMPFC (IL and PL), in order to be able to proceed with our next experiment. Bilateral microinjections the non-selective receptors agonist, urocortin 0.02 nmol (n = 5) in the vMPFC (PL and IL) did not modify baseline MAP (before = 91 ± 6, after = 91 ± 5 mmHg, t = 0.25, p > 0. 05) and HR values (previously = 386 ± 15, after = 396 ± 19 bpm, t = 2.15, p > 0.05). Baroreflex linear regression analysis indicates that microinjection of urocortin in the vMPFC did not switch baroreflex responses, both bradycardic (before = − 2.39 ± 0.89, 10 min later = − 1.85 ± 0.27, 60 min later = − 2.43 ± 0.76, F (2,17) = 0.16, p > 0.05) and tachycardic component (before = − 2.18 ± 0.27, 10 min later = 2.40 ± 0.17, 60 min later = − 2.20 ± 0.25; F (2,17) = 0.70, p > 0.05; Fig. 4a). In addition, non-linear regression values (G, P1, and P2) were not altered either (Table 3).
Graphs representative of the variation in mean arterial pressure over the variation in heart rate: (Top) Linear regression curves correlating the Δ MAP and Δ HR responses before and 10 min and 60 min after bilateral microinjection CRF agonist urocortin of 0.02 nmol (a) and 0.2 nmol (B), both in the IL region into the vMPFC. Correlation values r2 for the bradycardic regression curves were 0.29 and 0.79 for the data generated before, 0.25 and 0.91 for the data generated 10 min after, and 0.27 and 0.65 for the data generated after 60 min of the bilateral microinjection of urocortin in the respective doses. The correlation values r2 for the tachycardic regression curves were 0.60 and 0.62 for the data generated before, 0.73 and 0.59 for the data generated 10 min after, and 0.65 and 0.61 for the data generated 60 min after microinjection of urocortin in the respective doses. (Bottom) Non-linear regression coefficients correlating mean arterial pressure (Δ MAP) and heart rate (ΔHR) before (R2 = 0.73, 0.83), 10 min (R2 = 0.77, 0.91), and 60 min (R2 = 0.76, 0.83) after bilateral microinjection of urocortin in the vMPFC (Table 3)
In another group of animals, bilateral microinjections of urocortin 0.2 nmol (n = 6) in the vMPFC (PL and IL) did not change baseline MAP values (before = 104 ± 0.87, after = 103 ± 0.35 mmHg; t = 0.47, p > 0.05) and HR (before = 308 ± 32, after = 308 ± 46 bpm, t = 0.13, p > 0.05). Baroreflex linear regression curves indicate that urocortin microinjection in the vMPFC did not modify the bradycardic response (before = − 1.78 ± 0.26; 10 min later = − 2.01 ± 0.13; 60 min later = − 1.61 ± 0.23; F (2,17) = 0.85; p > 0.05 ), while it decreased tachycardic component slope ( before = − 2.52 ± 0.32, 10 min later = − 1.53 ± 0.16, 60 min later = − 2.30 ± 0.33; F (2,17) = 4.58, p < 0.05; Fig 4b). In addition, the non-linear regression values (G, P2 ) were also altered (Table 3).
Effects of bilateral CRF-1 or CRF-2 antagonism prior to microinjection of urocortin in the IL region in the vMPFC on cardiac baroreflex activity
The microinjections of CRF-1 antagonist CP376395 (0.45 nmol n = 6) prior to effective dose of CRF agonist urocortin (0.2 nmol) in the IL region in the vMPFC did not change the baseline values of MAP (before = 157 ± 12, after = 160 ± 10 bpm, t = 1.02, p > 0.05) and HR (before = 333 ± 24, after = 332 ± 32 bpm, t = 1.54, p > 0.05). Linear regression of the baroreflex indicates that microinjections in the IL region in the vMPFC did not alter bradycardic component (before = − 2.00 ± 0.41, 10 min later = − 2.19 ± 0.46, 60 min later = − 1.98 ± 0.41; F (2,17) = 0.13; p > 0.05) and also did not prevent tachycardic response increase (before = − 2.90 ± 0.30, 10 min later = − 2.45 ± 0.17, 60 min later = 2.70 ± 0.33, F (2,17) = 0.49, p > 0.05; Fig. 5a). In addition, non-linear regression values (P1, P2, and ΔP) were not altered (Table 4).
Graphs representative of the variation in mean arterial pressure over the variation in heart rate: (Top) Linear regression curves correlating the Δ MAP and Δ HR responses before and 10 min and 60 min after bilateral microinjection of CRF1 0.45 nmol antagonist CP376395 (a) or CRF2 0.45 nmol antagonist K41498 (b) prior to urocortin 0.02 CRF non-selective agonist nmol both in IL region of vMPFC. Correlation values r2 for the bradycardic regression curves were 0.35 and 0.63 for the data generated before, 0.50 and 0.68 for the data generated 10 min after, and 0.63 and 0.59 for the data generated 60 min after the bilateral microinjection of CP376395 0.45 nmol or K41498 0.45 nmol prior to urocortin 0.02 nmol in the respective doses. The correlation values r2 for the tachycardic regression curves were 0.83 and 0.41 for the data generated before, 0.55 and 0.23 for the data generated after 10 min, and 0.79 and 0.36 for the data generated after 60 min after microinjection of CP376395 0.45 nmol or K41498 0.45 nmol prior to urocortin 0.2 nmol both in IL region of vMPFC. (Bottom) Non-linear regression coefficients correlating mean arterial pressure (Δ MAP) and heart rate (ΔHR) before (R2 = 0.86, 0, 78), 10 min (R2 = 0.80, 0.82), and 60 min (R2 = 0.89, 0.80) after bilateral microinjection of CP376395 or K41498 prior to urocortin both in the IL region of vMPFC (Table 4)
In another group of animals, the microinjections of CRF2 antagonist K41498 (0.45 nmol, n = 6) prior to urocortin (0.2 nmol) in the IL region in the vMPFC did not change the baseline values of MAP (before = 121 ± 33, after = p > 0.05 ) and HR (before = 324 ± 11, after = 322 ± 14 bpm, t = 1.94, p > 0.05). Baroreflex linear regression indicates that the microinjections in vMPFC did not change the bradycardic component (before = − 1.23 ± 0.22; 10 min later = − 1.51 ± 0.24; 60 min later = − 1.24 ± 0.24; F (2,17) = 2.47; p > 0.05). However, CRF2 antagonist associated with the urocortin was able to enable the reduction in tachycardic component slope induced by urocortin (before = − 2.06 ± 0.35, 10 min later = − 0.89 ± 0.22, 60 min later = − 1.99 ± 0.40; F (2,17) = 9.99, p < 0.05; Fig. 5b). Moreover, non-linear regression values (P1 and P2) were also altered (Table 4).
Discussion
Our results demonstrate for the first time that the CRF1 receptor located in IL and PL regions of the vMPFC negatively modulates tachycardic reflex. Differently, the bradycardic response did not show significant alterations due to vMPFC CRF1 antagonism, suggesting no involvement of these receptors in the parasympathetic activity of baroreflex (Figs. 2b and c and 5b). In addition, we demonstrated that the non-selective vMPFC CRF receptor agonism decreased the tachycardic reflex, which was inhibited by CRF1 antagonism, but not by CRF2 blockade in the area (Figs. 4b, 5a and b).
Despite some evidence showing that CRF2 receptors could modulate autonomic responses in vMPFC [3, 29, 30], CRF2 antagonism was unable to modify cardiac activity in our study (Figs. 3a, b and 5a). This result corroborates with the previous data from the literature that demonstrated CRF1 and CRF2 receptors could play different roles in baroreflex modulation, such as in BNST [30]. This lack of effect can be related to the dose used in the study, and further studies are necessary to better understand the function of CRF2 in the vMPFC in cardiovascular modulation. However, it is important to mention that urocortin used in the present study has a greater affinity to CRF2 receptor (Ki = 1.5) then to CRF1 (Ki = 13) (K41498; Tocris, Westwoods Business Park, Ellisville, MO, USA). This information reinforces that the effect of urocortin on baroreflex activity was due to CRF1 activation, once that even with a greater affinity to CRF2, the effect of urocortin was not blocked by CRF2 antagonist. Based on this, we can suggest that CRF2 receptors present in vMPFC do not participate in the baroreflex modulation.
Evidence from the literature have also shown that the PL and IL subregions of vMPFC have opposite roles in the cardiac modulation since PL or IL ablation with cobalt facilitated or reduced, respectively, the tachycardic responses in animals submitted to restraint stress [40]. These different responses could be accounted for their different neural projection patterns. For example, IL densely innervates the NTS from the brainstem, central, medial, basomedial, and cortical amygdala nuclei, while the PL sends projections to the ventral tegmental area and the basolateral nucleus of the amygdala [15, 43]. In other words, IL mainly distributes to autonomic/visceral-related sites, supporting its role in visceromotor activity [42], whereas PL primarily projects to limbic sites that reportedly affect emotion and cognition [19]. Another divergent role of IL and PL regions was highlighted by Frysztak and Neafsey (1994). Such authors observed that the increase in HR during fear conditioning response had a different influence of the IL and PL [17]. In our study, however, we found no difference on the PL or IL CRF1 modulation on tachycardic reflex (Fig. 2b and c). These discrepancies may be explained by the fact that the animals were not subjected to stressful stimuli during the protocols. However, our results are in agreement with other studies which show that PL and IL CB1 and TRPV1 receptors equally modulate the baroreflex function in [22].
On the other hand, the similar pattern of baroreflex modulation exerted by PL and IL CRF1 receptors may be explained by their common neuronal connections to the periaqueductal gray matter (PAG) [16]. This assumption is reinforced by the study of Pelosi and colleagues (2007), which demonstrated that PAG has an inhibitory role on tachycardic reflex, with no influence on the bradycardic response [33], which is very similar to the results showed in the present study (Figs. 2b, c and 4b).
Taken this information together, we suggest that CRF1 receptors in both PL and IL regions equally modulate the baroreflex tachycardic response, possibly because of their location in neurons directed to similar neuroanatomical relays, such as PAG [15].
It has been demonstrated that NMDA glutamate receptors of vMPFC have a facilitating role in the bradycardic and tachycardic activity of baroreflex [14, 35]. Since CRF1 receptors are colocalized with glutamate vesicles in vMPFC [34], we expected that CRF1 receptors would also exert a facilitatory role on both responses, possibly by stimulating glutamate release. However, our study pointed to another direction. We have seen that CRF1 receptors in vMPFC inhibit the tachycardic activity of the baroreflex, with no influence on the bradycardic response. Consequently, we suggest that these receptors may modulate the activity of another cortical neurotransmission.
Regarding this matter, it was shown that intracerebroventricular administration of CRF in rats increases adrenergic activity in PFC [44]. In addition, stressful stimuli led to increased levels of noradrenaline (NE) in the PFC, which could be a result of CRF activity into the area [23]. Indeed, a CRF1 receptor antagonist injected in PFC abolished the effect on NE levels [23], indicating that NE release may be enhanced by CRF1 activation. Based on this, our group hypothesized that CRF1 receptor could modulate the baroreflex tachycardic activity by modulating noradrenergic neurotransmission inside vMPFC. Previous data from our group observed that microinjection of the alpha-1 adrenergic receptor antagonist in the MPFC was unable to alter baroreflex parasympathetic activity, which is in line with our results [37]. However, in the mentioned study, it was not investigated the influence of such receptors on tachycardic activity. It is still not clear if vMPFC noradrenergic neurotransmission is indeed involved with baroreflex modulation. Nevertheless, through our study, we can propose that NE neurotransmission would modulate the outputs from vMPFC to brain stem nuclei that ultimately leads to tachycardic activity reduction of the baroreflex.
It is known that patients with major depressive disorder present greater general sympathetic activity and reduced parasympathetic discharge. Moreover, post-traumatic stress disorder (PTSD) patients seem to have persistent hyperarousal symptoms possibly due to high sympathetic outflow coupled with lower parasympathetic cardiac control [8]. In both cases, the diseases are linked with CRF alterations [18, 20, 27]. In fact, some patients with PTSD and depression have elevated levels of CRF in their cerebrospinal fluid, which positively correlates with symptom severity [5, 9, 12, 28, 38]. Importantly, the microinjection of CRF in the vMPFC of mice increased generalized anxiety-like behavior, which is usually followed by baroreflex adjustments [7]. Moreover, PTSD and depression are correlated to dysfunction in MPFC [18, 20, 27]. Interestingly, depression and PTSD are intimately linked to dysregulated and maladaptive response to stress, in which the CRF receptor could play a role [38, 39]. Based on this, we propose that changes in the activity of vMPFC CRFergic neurons through adrenergic system may have an association with cardiovascular alterations in those psychiatric disorders. In addition, we can postulate the hypothesis that vMPFC CRF1 receptors may counteract an exaggerated elevation of the cardiac activity during stressful stimuli and in stress-related disorders as well.
In conclusion, the present study demonstrates that CRF1, but not CRF2 receptors in the IL and PL regions of MPFC negatively, modulates the tachycardic response of baroreflex.
Data availability
N/A
References
Alves FH, Crestani CC, Resstel LB, Correa FM (2009) N-methyl-D-aspartate receptors in the insular cortex modulate baroreflex in unanesthetized rats. Auton Neurosci 147:56–63. https://doi.org/10.1016/j.autneu.2008.12.015
Angelo Machado LMH (2003) Neuroanatomia Funcional, Machado, 3th edição.249-268
Bale TL, Vale WW (2004) CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44:525–557. https://doi.org/10.1146/annurev.pharmtox.44.101802.121410
Bangasser DA, Kawasumi Y (2015) Cognitive disruptions in stress-related psychiatric disorders: a role for corticotropin releasing factor (CRF). Horm Behav 76:125–135. https://doi.org/10.1016/j.yhbeh.2015.04.003
Banki CM, Karmacsi L, Bissette G, Nemeroff CB (1992) CSF corticotropin-releasing hormone and somatostatin in major depression: response to antidepressant treatment and relapse. Eur Neuropsychopharmacol 2:107-113. https://doi.org/10.1016/0924-977x(92)90019-5
Berntson GGC, J.T. (2007) Heart rate variability: stress and psychiatric conditions. Dynamic Electrocardiography. https://doi.org/10.1002/9780470987483.ch7
Bijlsma EY, van Leeuwen ML, Westphal KG, Olivier B, Groenink L (2011) Local repeated corticotropin-releasing factor infusion exacerbates anxiety- and fear-related behavior: differential involvement of the basolateral amygdala and medial prefrontal cortex. Neuroscience 173:82-92. https://doi.org/10.1016/j.neuroscience.2010.11.026
Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH (2007) Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosom Med 69:935-943. https://doi.org/10.1097/PSY.0b013e31815a8f6b
Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS (1997) Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry 154:624–629. https://doi.org/10.1176/ajp.154.5.624
Chae JI, Ju SK, Lee MK, Park JH, Yoon JH, Shim JH, Lee DS (2009) cDNA cloning and analysis of tissue-specific gene expression of rat urocortin II. Mol Biol (Mosk) 43:91–96
Constanzo LS (2009) Fisiologia. In: 3th edição
Elbedour S, Baker A, Shalhoub-Kevorkian N, Irwin M, Belmaker RH (1999) Psychological responses in family members after the Hebron massacre. Depress Anxiety 9:27-31.https://doi.org/10.1002/(SICI)1520-6394(1999)9:1<27::AID-DA4>3.0.CO;2-W
Ferreira-Junior NC, Fedoce AG, Alves FH, Correa FM, Resstel LB (2012) Medial prefrontal cortex endocannabinoid system modulates baroreflex activity through CB(1) receptors. Am J Physiol Regul Integr Comp Physiol 302:R876–R885. https://doi.org/10.1152/ajpregu.00330.2011
Ferreira-Junior NC, Fedoce AG, Alves FH, Resstel LB (2013) Medial prefrontal cortex N-methyl-D-aspartate receptor/nitric oxide/cyclic guanosine monophosphate pathway modulates both tachycardic and bradycardic baroreflex responses. J Neurosci Res 91:1338–1348. https://doi.org/10.1002/jnr.23248
Fisk GD, Wyss JM (2000) Descending projections of infralimbic cortex that mediate stimulation-evoked changes in arterial pressure. Brain research 859:83-95. https://doi.org/10.1016/s0006-8993(00)01935-1
Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R (2000) Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J Comp Neurol 422:556-578. https://doi.org/10.1002/1096-9861(20000710)422:4<556::AID-CNE6>3.0.CO;2-U
Frysztak RJ, Neafsey EJ (1994) The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain research 643:181-193. https://doi.org/10.1016/0006-8993(94)90024-8
Gold PW, Chrousos GP (2002) Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry 7:254–275. https://doi.org/10.1038/sj.mp.4001032
Kalivas PW, Jackson D, Romanidies A, Wyndham L, Duffy P (2001) Involvement of pallidothalamic circuitry in working memory. Neuroscience 104:129-136. https://doi.org/10.1016/s0306-4522(01)00054-9
Kasckow JW, Baker D, Geracioti TD, Jr. (2001) Corticotropin-releasing hormone in depression and post-traumatic stress disorder. Peptides 22:845-851. https://doi.org/10.1016/s0196-9781(01)00399-0
Kuroda M, Yokofujita J, Murakami K (1998) An ultrastructural study of the neural circuit between the prefrontal cortex and the mediodorsal nucleus of the thalamus. Prog Neurobiol 54:417–458. https://doi.org/10.1016/s0301-0082(97)00070-1
Lagatta DC, Ferreira-Junior NC, Resstel LB (2015) Medial prefrontal cortex TRPV1 channels modulate the baroreflex cardiac activity in rats. Br J Pharmacol 172:5377–5389. https://doi.org/10.1111/bph.13327
Lorrain DS, Baccei CS, Correa LD, Bristow LJ (2005) Comparison of the effects of diazepam, the CRF1 antagonist CP-154,526 and the group II mGlu receptor agonist LY379268 on stress-evoked extracellular norepinephrine levels. Neuropharmacology 48:927-935. https://doi.org/10.1016/j.neuropharm.2004.12.022
Mancia G, Parati G (2003) The role of blood pressure variability in end-organ damage. J Hypertens Suppl 21:S17–S23. https://doi.org/10.1097/00004872-200307006-00004
Meng QY, Chen XN, Tong DL, Zhou JN (2011) Stress and glucocorticoids regulated corticotropin releasing factor in rat prefrontal cortex. Mol Cell Endocrinol 342:54-63. https://doi.org/10.1016/j.mce.2011.05.035
Myers DA, Trinh JV, Myers TR (1998) Structure and function of the ovine type 1 corticotropin releasing factor receptor (CRF1) and a carboxyl-terminal variant. Mol Cell Endocrinol 144:21-35. https://doi.org/10.1016/s0303-7207(98)00157-9
Nemeroff CB, Vale WW (2005) The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry 66(Suppl 7):5–13
Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W (1984) Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 226:1342–1344. https://doi.org/10.1126/science.6334362
Oliveira LA, Almeida J, Benini R, Crestani CC (2015) CRF1 and CRF2 receptors in the bed nucleus of the stria terminalis modulate the cardiovascular responses to acute restraint stress in rats. Pharmacol Res 95-96:53-62. https://doi.org/10.1016/j.phrs.2015.03.012
Oliveira LA, Almeida J, Gomes-de-Souza L, Benini R, Crestani CC (2017) CRF1 and CRF2 receptors in the bed nucleus of stria terminalis differently modulate the baroreflex function in unanesthetized rats. Eur J Neurosci 46:1805–1812. https://doi.org/10.1111/ejn.13622
Oshida Y, Ikeda Y, Chaki S, Okuyama S (2004) Monkey corticotropin-releasing factor1 receptor: Complementary DNA cloning and pharmacological characterization. Life Sci 74:1911–1924. https://doi.org/10.1016/j.lfs.2003.08.035S0024320503011020[pii]
Papademetriou V, Doumas M, Faselis C, Tsioufis C, Douma S, Gkaliagkousi E, Zamboulis C (2011) Carotid baroreceptor stimulation for the treatment of resistant hypertension. Int J Hypertens 2011:964394. https://doi.org/10.4061/2011/964394
Pelosi GG, Resstel LB, Correa FM (2007) Dorsal periaqueductal gray area synapses modulate baroreflex in unanesthetized rats. Auton Neurosci 131:70-76. https://doi.org/10.1016/j.autneu.2006.07.002
Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, Dedic N, Schumacher M, von Wolff G, Avrabos C, Touma C, Engblom D, Schutz G, Nave KA, Eder M, Wotjak CT, Sillaber I, Holsboer F, Wurst W, Deussing JM (2011) Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science 333:1903-1907. https://doi.org/10.1126/science.1202107
Resstel LB, Correa FM (2006) Injection of l-glutamate into medial prefrontal cortex induces cardiovascular responses through NMDA receptor - nitric oxide in rat. Neuropharmacology 51:160-167. https://doi.org/10.1016/j.neuropharm.2006.03.010
Resstel LB, Fernandes KB, Correa FM (2004) Medial prefrontal cortex modulation of the baroreflex parasympathetic component in the rat. Brain Res 1015:136–144. https://doi.org/10.1016/j.brainres.2004.04.065
Resstel LB, Fernandes KB, Correa FM (2005) Alpha-adrenergic and muscarinic cholinergic receptors are not involved in the modulation of the parasympathetic baroreflex by the medial prefrontal cortex in rats. Life Sci 77:1441-1451. doi:S0024-3205(05)00369-3 https://doi.org/10.1016/j.lfs.2005.03.012
Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, Johnson JE, Cerbone A, Malaspina D (2003) Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry 54:1382-1388. https://doi.org/10.1016/s0006-3223(03)00571-7
Tan H, Zhong P, Yan Z (2004) Corticotropin-releasing factor and acute stress prolongs serotonergic regulation of GABA transmission in prefrontal cortical pyramidal neurons. J Neurosci 24:5000-5008. https://doi.org/10.1523/JNEUROSCI.0143-04.200424/21/5000[pii]
Tavares RF, Correa FM, Resstel LB (2009) Opposite role of infralimbic and prelimbic cortex in the tachycardiac response evoked by acute restraint stress in rats. J Neurosci Res 87:2601–2607. https://doi.org/10.1002/jnr.22070
Vale W, Spiess J, Rivier C, Rivier J (1981) Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213:1394–1397. https://doi.org/10.1126/science.6267699
Verberne AJ, Owens NC (1998) Cortical modulation of the cardiovascular system. Progress in neurobiology 54:149-168. doi:S0301-0082(97)00056-7 https://doi.org/10.1016/s0301-0082(97)00056-7
Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58. https://doi.org/10.1002/syn.10279
Zhang JJ, Swiergiel AH, Palamarchouk VS, Dunn AJ (1998) Intracerebroventricular infusion of CRF increases extracellular concentrations of norepinephrine in the hippocampus and cortex as determined by in vivo voltammetry. Brain Res Bull 47:277-284. https://doi.org/10.1016/s0361-9230(98)00117-8
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The present research was also supported by the Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da FMRP-USP (FAEPA) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES);
Author information
Authors and Affiliations
Contributions
The authors wish to thank Resstel for the technical help. João P.T. Brufatto, Davi C. Lagatta, Daniela L. Uliana, Egidi M. S. Firmino, and Anna A. Borges contributed to the conception and design of the study; João P.T. Brufatto and Davi C. Lagatta organized the database and performed the statistical analysis. All authors contributed to the manuscript revision and read and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All experiments were performed in accordance with Ethical Principles for Animal Experimentation followed by the Brazilian Committee for Animal Experimentation (COBEA) and approved by the Committee of Ethics in Animal Research of the School of Medicine of Ribeirão Preto, University of São Paulo (number 101/09//2015).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brufatto, J.P.T., Lagatta, D.C., Uliana, D.L. et al. Ventromedial prefrontal cortex CRF1 receptors modulate the tachycardic activity of baroreflex. Pflugers Arch - Eur J Physiol 473, 697–709 (2021). https://doi.org/10.1007/s00424-020-02512-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02512-z