Abstract
The coexistence of different subtypes of voltage-dependent calcium channels (VDCC) within the same chromaffin cell (CC) and the marked interspecies variability in the proportion of VDCC subtypes that are present in the plasmalemma of the CCs raises the question on their roles in controlling different physiological functions. Particularly relevant seems to be the role of VDCCs in the regulation of the exocytotic neurotransmitter release process, and its tightly coupled membrane retrieval (endocytosis) process since both are Ca2+-dependent processes. This review is focused on the role of Ca2+ influx through L-type VDCC in the regulation of these two processes. It is currently accepted that the different VDCC subtypes (i.e., T, L, N, P/Q, R) contribute to exocytosis proportionally to their density of expression and gating properties. However, the pattern of stimulation defines a preferential role of the different subtypes of VDCC on exocytosis and endocytosis. Thus, L-type channels seem to control catecholamine release induced by prolonged stimuli while fast exocytosis in response to short square depolarizing pulses or action potentials is mediated by Ca2+ entering CCs through P/Q channels. The pattern of stimulation also influences the endocytotic process, and thus, electrophysiological data suggest the sustained Ca2+ entry through slow-inactivating L-type channels could be responsible for the activation of fast endocytosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The “fight or flight” response constitutes a highly coordinated and precise response physiologically generated as an attempt for maintaining the equilibrium of the internal milieu against fear or stress conflicts [15, 19]. This response is highly regulated by the sympathetic nervous system, being particularly relevant the participation of the chromaffin cells (CCs) of the adrenal gland that release the catecholamines adrenaline and noradrenaline, a response that is dependent on extracellular Ca2+ [33] that enters the CC upon opening of different voltage-dependent Ca2+ channels (VDCCs) present in their plasma membrane [43].
As it happens for other neurotransmitters and hormones, the Ca2+-dependent release of catecholamines is highly dependent on the preservation of the equilibrium between the amount of vesicular membrane that incorporates into the plasmalemma during the exocytotic process and the membrane retrieval during subsequent endocytosis. This will serve to warrant that a given number of secretory vesicles are available to participate in subsequent rounds of exocytosis during repetitive cell activation [10, 27, 50]. Both exocytosis and endocytosis processes are mediated by a rise in intracellular Ca2+ concentration ([Ca2+]i) achieved primarily by Ca2+ entry through VDCCs [16, 27, 74, 84].
The identification and characterization of the properties, the regulation, and the functional role of the different subtypes of VDCC have been possible thanks to the improvement of the patch-clamp techniques [49], the isolation, the purification and synthesis of different neurotoxins [76], and the molecular biology and genetic approaches that have led to the elucidation of the molecular structure of VDCCs [26]. The main properties of the different subtypes of VDCC, including the major pore-forming subunit and their pharmacological profile are summarized in Table 1.
By combining electrophysiological techniques and selective blockers of VDCC, we have found that the whole-cell inward ICa of bovine chromaffin cells (BCCs) is mainly mediated by Ca2+ entry through, at least, three of the subtypes of VDCCs described in neurons [76], namely, 20% L-type (α1D, Cav 1.3), 30% N-type (α1B, Cav 2.2), and 50% P/Q-type (α1A, Cav 2.1) [4, 5, 37, 40, 80]. The coexistence of these three subtypes of VDCCs within the same CC raises the question on their roles in controlling different physiological functions, particularly the implication of each VDCC subtype in the regulation of the two main Ca2+-dependent steps involved in the neurotransmitter release process, i.e., the exocytotic release of catecholamines and the subsequent endocytotic process [43, 66]. In this review, we will focus on the Ca2+ influx into the chromaffin cell through L-type VDCC that serves to regulate both the exocytosis and the endocytosis processes. In addition, growing evidence suggest that L-type (Cav1.2 and Cav1.3) channels are also directly involved in the repetitive firing of spontaneous [69, 71, 72] and evoked AP firings [89, 90].
L-type Ca2+ channels in chromaffin cells
The presence of L-type currents has been electrophysiologically characterized in bovine [4, 11, 12, 17, 18, 20, 37], rat [6, 28, 32, 35, 38, 69, 78], mouse [52, 67, 71, 72], pig [58], cat [2, 62], and human CCs [42, 55].
A comparative study has shown a high interspecies variability in the proportion of L-type VDCCs that are present in the plasmalemma of the CCs. Thus, L-type calcium channels account for near half of the whole-cell Ca2+ channel current in the cat [2], rat [38], and mouse CCs [52], while in pig [58], bovine [4, 37], and human species [42] L channels carry only 15–20% of the whole-cell Ca2+ current measured at holding voltage of about −70 to −80 mV. In addition, within the same animal species, age-dependent differences have also been described, i.e., in rat embryo CCs (RECCs) whole-cell ICa is carried 60% by L channels in comparison with 50% found in adult rat CCs [36].
At this point, it should be mentioned that the estimate of L-type channels expression based on the action of dihydropyridines (DHPs) is highly sensitive to the holding potential [67]. Thus, for instance, in a recent study conducted in human CCs, the block of Ca2+ currents by nifedipine at −80 mV is 20%, but increases to 50% at −50 mV [55]. These differences could be partially related to the voltage-dependent inactivation of non L-type VDCCs as will be discussed below.
Molecular evidence indicates that L-type currents in CCs is mediated by the expression of two subtypes of L channels, α1C and α1D [13, 46, 47, 55, 66, 92], and the most common view is that CCs express equal percentages of Cav1.2 and Cav1.3 L-type channels [68, 72]. However, on the basis of their affinities for DHPs, from RT-PCR and from single-channel recordings, it is difficult to separate the contribution of these two channel types to the total L-type current [67, 72, 88]. Also, using Cav1.3 KO mice show clearly that both isoforms are equally modulated by cAMP and cGMP [68].
At this point, we would like to comment that, in order to characterize the functional role of L-type VDCC, some characteristics that differentiate L-type channels from other VDCCs should be considered, as these could contribute to explain some of the discrepancies observed between different studies. These differences are related to (1) the different autocrine/paracrine regulation by catecholamines and other co-exocytosed vesicular components (the L current is regulated by neurotransmitters in a voltage-independent manner while N and PQ currents are regulated in a voltage-dependent manner [3, 20, 39, 51]), (2) the voltage-dependent inactivation (N and PQ channels undergo a pronounced voltage-dependent inactivation while L channels are resistant to such inactivation [53, 91]), and/or (3) the Ca2+-dependent inactivation (L-type channels undergo Ca2+-dependent inhibition at a rate slower than that of N and PQ-type channels [54, 80]). Finally, it should be noted that the number of Ca2+-channel and its distribution might be also altered by culturing conditions as result of denervation/isolation of the CCs.
L channels and exocytosis in chromaffin cells
Some discrepancies on the role of the different subtypes of VDCCs on the regulation of the exocytotic process have been published. These differences are somehow related to the different stimulation patterns used (i.e., stimulation with the physiological neurotransmitter acetylcholine, K+ depolarization, electrical stimulation, short or long stimulation, …), the preparation used (i.e., intact gland, adrenal slices, cultured cell populations, or cultured isolated cells), and/or the techniques used to quantify the catecholamine secretion (i.e., amperometry in cell populations or in single cell, cell capacitance in patch-clamped cells, …).
For instance, in the intact adrenal gland of the cat, the K+-evoked secretion of catecholamines is effectively blocked in a concentration dependent manner by DHPs and by other drugs acting on L-type VDCCs like verapamil and diltiazem [25, 41] and markedly potentiated by the DHP agonist BAY-K-8644 [44] thus suggesting that catecholamine secretion in these cells was mainly controlled by an L-type channel. However, electrophysiological experiments demonstrated that cat CCs also contained N-type channels in a similar proportion to that of L-type channels [2]. Further experiments showed that though Ca2+ entry through both channels (N- and L-type) lead to similar increments of the average [Ca2+]c, the control of K+-evoked catecholamine release response in cat chromaffin cells was dominated by Ca2+ entering through L-type VDCCs [62].
In the intact rat adrenal gland, it was reported that the L-type VDCC blocker isradipine partially inhibited electrical stimulation- and acetylcholine-induced catecholamine secretion, but potently inhibited nicotine- and K+-induced secretion in the perfused rat adrenal gland. In addition, BAY-K-8644 potentiated mildly the secretory responses to electrical stimulation and to acetylcholine, but increased threefold the responses to K+ and nicotine. These results suggested that responses mediated by high K+ or nicotinic receptors are mediated by Ca2+ entry through L-type channels, although other VDCCs also contributes to modulate the physiological adrenal catecholamine secretory process [63].
In a similar study, the catecholamine release induced by electrical field stimulation of splanchnic nerves was halved either by ω-conotoxin MVIIC (a non L-type channel blocker) and the DHP furnidipine, thus suggesting that both the L- and P/Q-types of Ca2+ channels were involved. Similar results were observed when secretion was elicited by acetylcholine. However, the K+-induced secretory responses were reduced 75% by furnidipine and 45% by ω-conotoxin MVIIC, indicating that this type of stimulation preferentially recruited L-type channels [82]. Similarly, Nagayama et al. found that L-type channels were responsible for the catecholamine secretion mediated by nicotinic receptors but not by muscarinic receptors, and that their contribution to noradrenaline secretion may be greater than that of adrenaline secretion. N-type voltage-dependent Ca2+ channels may not contribute to catecholamine secretion, and P/Q-type Ca2+ channels may control the secretion at presynaptic sites [73].
By using bovine chromaffin cell populations stimulated with K+ depolarization, it was first concluded that Ca2+ entry through both L- and P/Q-type channels controlled the K+-evoked catecholamine release responses [64], in spite that L-type channels account for only 20% of the whole-cell currents in these cells. These results led to the hypothesis that L and P/Q channels were strategically located close to the secretory machinery, thus regulating the exocytosis of catecholamines [59, 64]. In a similar study conducted in distinct populations of bovine chromaffin cells, it was described that exocytosis in noradrenaline-containing cells was regulated mainly by L-type channels, while in adrenaline-containing cells exocytosis was controlled by P/Q-type channels [61].
However, when the possible coupling between VDCCs and exocytosis was evaluated at the single-cell level by measuring membrane capacitance, no preferential role of any VDCC subtype in eliciting exocytosis has been found in rat [48, 57] or in bovine CCs [34, 85, 83, 65, 87], thus suggesting an uneven distribution of calcium channels in chromaffin cells. A possible explanation for these discrepancies could be, at least partially, related to the voltage-dependent inactivation of VDCCs that minimizes the role of N and PQ channels in the experiments conducted in intact adrenal glands or in isolated cell populations, in which the physiological resting membrane potential of the chromaffin cells might favor a partial voltage-dependent inactivation of non L channels, while L channels are more resistant to such type of inactivation [53, 91].
Some striking differences have been observed related to the role of the different VDCC subtypes in the regulation of hypoxia-induced catecholamine secretion (HIS response). Thus, during fetal and neonatal periods in which there is no functional innervation of the adrenal medulla, a non-neurogenic acute HIS response is produced that depends on Ca2+entry through VDCCs of CCs, as is proven by the fact that this response is abolished in the absence of Ca2+ [1] and blocked by cadmium [45]. Different studies have concluded that this acute HIS response is mainly controlled by L channels in fetal sheep CCs [1], embryonic rat CCs [36], and neonatal rat CCs [85, 86]; However, the study by Levitsky and López-Barneo suggests that neonatal rat CCs express relatively high levels of T-type VDCCs and that the function of these channels is required for a proper secretory response to acute hypoxia [60]. On the other hand, by using both electrophysiological and molecular biology tools, it has been demonstrated that chronic hypoxia up-regulates the expression of T-type channels in adult CCs [22, 23, 70, 83]. These data are in good agreement with the idea that hypoxia, like other stress-mimicking conditions, up-regulates T-type channels in CCs [56, 75].
Finally, it has been proposed that channel gating and the type of stimuli applied, rather than the possible co-localization of the exocytotic machinery with VDCCs, regulate the exocytosis in chromaffin cells. Thus, as commented above, the different experimental approaches used during the last 30 years, mostly based on the application of a long-lasting stimulus, i.e., prolonged stimulation with high K+ containing solutions [62] or acetylcholine [73], support the idea of a preferential coupling of L-type VDCCs to catecholamine secretion. However, a predominant role of P/Q-type channels in regulating the fast release of vesicles from the immediately releasable pool (IRP) has been proposed when short (10 ms) stimulation with square depolarizing pulses [8, 9] or trains of action potentials [29] are used to stimulate catecholamine secretion in mouse CCs. This seems to be likely due to the rapid activation of P/Q channels (Cav2.1) with respect to the other VDCCs which is more evident during stimuli of short duration since less affected by fast channel inactivation. The slow-inactivating L-type channels would be regulating the vesicular replenishment of the releasable pool, that is, the sustained or tonic release [24].
L channels and endocytosis in chromaffin cells
As commented above, the Ca2+-dependent release of catecholamines is highly dependent on the preservation of the membrane equilibrium between the amount of vesicular membrane that incorporates into the plasmalemma during the exocytotic process and the membrane retrieval during subsequent compensatory endocytosis.
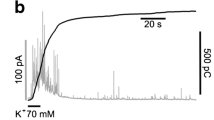
In trying to characterize the possible relationship between Ca2+ entry, exocytosis, and endocytosis by measuring changes in membrane capacitance (ΔCm) in BCCs, we found that Ca2+ entry through VDCCs induced by the application of depolarizing pulses (DPs) of increasing length (50–2000 ms) produced different patterns of exo/endocytosis. A linear relationship between exocytotic responses and DP duration was found; however, endocytotic responses were almost absent when short DPs (50–200 ms) were applied and were more pronounced with longer DPs (500–2000 ms) [31]. These data pose the question on whether the same Ca2+ entry that triggers exocytosis is also responsible to initiate subsequent endocytosis.
As far as the specific contributions of the different VDCC subtypes in controlling endocytosis are concerned, it has been proposed that, as for exocytosis, the pattern of stimulation, and therefore, the characteristics of the Ca2+ signal generated by the stimulus also influence endocytosis [24].
In bovine CCs stimulated with single DPs of long (500 ms) duration, a preferential coupling of L-type VDCCs to endocytosis has been proposed [79]. In this study, we found that, despite the small contribution of L-type VDCCs to the total global Ca2+ current, their inhibition by the DHP nifedipine almost completely abolished the endocytotic response without significantly affecting exocytosis. ω-Conotoxin GVIA (N-channel blocker) affected little the exo/endocytotic responses while ω-agatoxin IVA (P/Q-channel blocker) markedly blocked those responses in a parallel manner. These data support the hypotheses that Ca2+-entry through L channels is more effective in triggering endocytosis than exocytosis [79]. Additional experiments were performed with the isolation of L from N/PQ channels by blocking the non L channels with ω-conotoxin MVIIC (MVIIC). It was found that, in cells treated with MVIIC, superfusion with FPL64176 (an L-type VDCC agonist) increased Ca2+ entry and doubled the endo/exocytosis ratio, indicating a selective augmentation of endocytosis related to this Ca2+ entry through L-type channels [81]. Similar results were obtained by using the FM-dye methodology and long stimulations with high K+; endocytosis was inhibited by about 50% when the L-channel blocker nifedipine was present [81].
Bay et al. (2012) have also reported the implication of L-type VDCC in the membrane excess retrieval that follows a strong Ca2+ entry in mouse CCs. In this study, excess retrieval (a rapid endocytosis process that retrieves more membrane than the one fused by preceding exocytosis) was monitored with FM1.43 after the stimulation with high-K+ or cholinergic agonists lasting for 15–30 s. It was found that this excess retrieval membrane pool is associated with the generation of a non-releasable fraction of membrane co-localizing with the lysosomal compartment and is controlled by the concerted contribution of extracellular and intracellular Ca2+ sources. The blocking of the L-type VDCC with nitrendipine suppressed excess retrieval [14].
In trying to characterize if this preferential role of L-type VDCCs in controlling endocytosis was related to the existence of a close co-localization between endocytosis proteins, such as dynamin and/or clathrin, and L-type channels, we performed immunofluorescence experiments on bovine CCs that showed a practically negligible co-localization of clathrin with the three VDCC subtypes (CaV1.3, CaV2.1, and CaV2.2) studied. Also, only a mild co-localization (about 20–30%) was observed between VDCCs and dynamin. Taken together, these experiments do not support the existence of a close co-localization of VDCC subtypes with the endocytotic proteins clathrin and dynamin in bovine chromaffin cells [81].
The next issue is whether Cav 1.2 or Cav1.3 VDCC has a preferential control on endocytosis. One argument in favor of Cav1.3 is its slower and less complete time-dependent inactivation with respect to CaV 1.2 that would condition the mode of Ca2+ entry. The delayed inactivation of Cav 1.3 would favor a slow and prolonged Ca2+ entry through the less inactivating L-type channels that could be physiologically relevant for sustaining prolonged Ca2+ influxes that support normal endocytosis.
In the study by Rosa et al. (2007), upon the application of a 500-ms DP, the degree of inactivation of each Ca2+ channel subtype strongly conditioned the kinetics and the amount of Ca2+ entry. Thus, the slow-inactivating L-type channel, which contributes only by about 30% to the initial peak ICa, carried more than half of the total Ca2+ entry along the 500-ms depolarizing pulse. Conversely, the fast-inactivating N-type channel that also contributes by about 30% to the initial ICa peak, only contributed by about 24% to the total QCa. These data support the idea that a low-rate, non-inactivating Ca2+ entry might be more critical to trigger compensatory as well as excess endocytosis [30, 66, 79].
In addition, a pharmacological approach that serves to further slow-down the Ca2+ entry through the slow-inactivating L-type calcium channels is based on the use of L-channel activators such as FPL64176 and Bay-K-864. Membrane capacitance recordings and fluorescence imaging with FM-dyes in chromaffin cells have demonstrated that endocytic process is increased in the presence of both agonists without significantly altering exocytosis [14, 81]. The effect of BAY-K-8644 on endocytosis was also studied in the mouse neuromuscular junction, where the vesicle loading with FM2-10 was increased in the presence of the agonist BAY-K-8644 [77]. This finding further supports the hypothesis that L channels are preferentially coupled to the endocytic machinery than the exocytic, and that, not all calcium that enters into the cell through VDCCs have the same function.
Concluding remarks
By measuring membrane capacitance at the single-cell level, no preferential role of any VDCC subtype in eliciting exocytosis has been found in rat [57] or in bovine CCs [21, 34, 48, 65, 87]. It has been proposed that channel gating and the type of stimuli applied, rather than the possible co-localization of the exocytotic machinery with VDCCs, regulate the exocytosis in chromaffin cells. Thus, a predominant role of P/Q-type channels in regulating the fast release of vesicles when short stimulation with square depolarizing pulses [8, 9] or trains of action potentials [29] are used, while the slow-inactivating L-type channels would be regulating the sustained or tonic exocytosis when prolonged stimulations are applied [24].
As for exocytosis, it has been proposed that the pattern of stimulation, and therefore, the characteristics of the Ca2+ signal generated by the stimulus also influence endocytosis [24]. A predominant role of L-type channels on the regulation of the endocytotic process has been described, but this functional coupling between L channels and endocytosis is related neither to the co-localization of VDCCs and endocytosis proteins nor to the total amount of Ca2+ entering the cell through a given subtype of VDCC, suggesting that a low-rate, non-inactivating Ca2+ entry through L channels (Cav 1.3) might be more critical to trigger compensatory as well as excess endocytosis [30, 66, 79, 81].
References
Adams MB, Simonetta G, McMillen IC (1996) The non-neurogenic catecholamine response of the fetal adrenal to hypoxia is dependent on activation of voltage sensitive Ca2+ channels. Brain Res Dev Brain Res 94:182–189
Albillos A, Artalejo AR, López MG, Gandía L, García AG, Carbone E (1994) Calcium channel subtypes in cat chromaffin cells. J Physiol 477:197–213
Albillos A, Carbone E, Gandía L, García AG, Pollo A (1996) Opioid inhibition of Ca2+ channel subtypes in bovine chromaffin cells: selectivity of action and voltage-dependence. Eur J Neurosci 8:1561–1570
Albillos A, García AG, Gandía L (1993) Omega-Agatoxin-IVA-sensitive calcium channels in bovine chromaffin cells. FEBS Lett 336:259–262
Albillos A, García AG, Olivera B, Gandía L (1996) Re-evaluation of the P/Q Ca2+ channel components of Ba2+ currents in bovine chromaffin cells superfused with solutions containing low and high Ba2+ concentrations. Pflugers Arch 432:1030–1038
Albiñana E, Segura-Chama P, Baraibar AM, Hernández-Cruz A, Hernández-Guijo JM (2015) Different contributions of calcium channel subtypes to electrical excitability of chromaffin cells in rat adrenal slices. J Neurochem 133:511–521
Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, Spedding M, Peters JA, Harmar AJ, Collaborators C (2013) The concise guide to PHARMACOLOGY 2013/14: ion channels. Br J Pharmacol 170:1607–1651
Alvarez YD, Belingheri AV, Pérez Bay AE, Javis SE, Tedford HW, Zamponi G, Marengo FD (2013) The immediately releasable pool of mouse chromaffin cell vesicles is coupled to P/Q-type calcium channels via the synaptic protein interaction site. PLoS One 8:e54846
Alvarez YD, Ibañez LI, Uchitel OD, Marengo FD (2008) P/Q Ca2+ channels are functionally coupled to exocytosis of the immediately releasable pool in mouse chromaffin cells. Cell Calcium 43:155–164
Artalejo CR, Henley JR, McNiven MA, Palfrey HC (1995) Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proc Natl Acad Sci U S A 92:8328–8332
Artalejo CR, Mogul DJ, Perlman RL, Fox AP (1991) Three types of bovine chromaffin cell Ca2+ channels: facilitation increases the opening probability of a 27 pS channel. J Physiol 444:213–240
Artalejo CR, Perlman RL, Fox AP (1992) Omega-conotoxin GVIA blocks a Ca2+ current in bovine chromaffin cells that is not of the “classic” N type. Neuron 8:85–95
Baldelli P, Hernández-Guijo JM, Carabelli V, Novara M, Cesetti T, Andrés-Mateos E, Montiel C, Carbone E (2004) Direct and remote modulation of L-channels in chromaffin cells: distinct actions on alpha1C and alpha1D subunits? Mol Neurobiol 29:73–96
Bay AE, Belingheri AV, Alvarez YD, Marengo FD (2012) Membrane cycling after the excess retrieval mode of rapid endocytosis in mouse chromaffin cells. Acta Physiol (Oxf) 204:403–418
Bernard C (1878-1879) Leçons sur les phénomènes de la vie communs aux animaux et aux végétaux. Bailliere, Paris
Betz WJ, Mao F, Smith CB (1996) Imaging exocytosis and endocytosis. Curr Opin Neurobiol 6:365–371
Bossu JL, De Waard M, Feltz A (1991) Inactivation characteristics reveal two calcium currents in adult bovine chromaffin cells. J Physiol 437:603–620
Bossu JL, De Waard M, Feltz A (1991) Two types of calcium channels are expressed in adult bovine chromaffin cells. J Physiol 437:621–634
Cannon WB (1929) Organization for physiological homeostasis. Physiol Rev 9:399–431
Carabelli V, Carra I, Carbone E (1998) Localized secretion of ATP and opioids revealed through single Ca2+ channel modulation in bovine chromaffin cells. Neuron 20:1255–1268
Carabelli V, D'Ascenzo M, Carbone E, Grassi C (2002) Nitric oxide inhibits neuroendocrine CaV1 L-channel gating via cGMP-dependent protein kinase in cell-attached patches of bovine chromaffin cells. J Physiol 541:351–366
Carabelli V, Marcantoni A, Comunanza V, Carbone E (2007) Fast exocytosis mediated by T- and L-type channels in chromaffin cells: distinct voltage-dependence but similar Ca2+ -dependence. Eur Biophys J 36:753–762
Carabelli V, Marcantoni A, Comunanza V, de Luca A, Diaz J, Borges R, Carbone E (2007) Chronic hypoxia up-regulates alpha1H T-type channels and low-threshold catecholamine secretion in rat chromaffin cells. J Physiol 584:149–165
Cárdenas AM, Marengo FD (2016) How the stimulus defines the dynamics of vesicle pool recruitment, fusion mode, and vesicle recycling in neuroendocrine cells. J Neurochem 137:867–879
Cárdenas AM, Montiel C, Esteban C, Borges R, Garcia AG (1988) Secretion from adrenaline- and noradrenaline-storing adrenomedullary cells is regulated by a common dihydropyridine-sensitive calcium channel. Brain Res 456:364–366
Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J (2005) International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 57:411–425
Ceccarelli B, Hurlbut WP (1980) Ca2+-dependent recycling of synaptic vesicles at the frog neuromuscular junction. J Cell Biol 87:297–303
Cesetti T, Hernández-Guijo JM, Baldelli P, Carabelli V, Carbone E (2003) Opposite action of beta1- and beta2-adrenergic receptors on CaV1 L-channel current in rat adrenal chromaffin cells. J Neurosci 23:73–83
Chan SA, Polo-Parada L, Smith C (2005) Action potential stimulation reveals an increased role for P/Q-calcium channel-dependent exocytosis in mouse adrenal tissue slices. Arch Biochem Biophys 435:65–73
Comunanza V, Marcantoni A, Vandael DH, Mahapatra S, Gavello D, Carabelli V, Carbone E (2010) CaV1.3 as pacemaker channels in adrenal chromaffin cells: specific role on exo- and endocytosis? Channels (Austin) 4:440–446
de Diego AM, Arnaiz-Cot JJ, Hernández-Guijo JM, Gandía L, García AG (2008) Differential variations in Ca2+ entry, cytosolic Ca2+ and membrane capacitance upon steady or action potential depolarizing stimulation of bovine chromaffin cells. Acta Physiol (Oxf) 194:97–109
de Pascual R, Miranda-Ferreira R, Galvao KM, Lameu C, Ulrich H, Smaili SS, Jurkiewicz A, García AG, Gandía L (2013) Lower density of L-type and higher density of P/Q-type of calcium channels in chromaffin cells of hypertensive, compared with normotensive rats. Eur J Pharmacol 706:25–35
Douglas WW, Rubin RP (1961) The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol Paris 159:40–57
Engisch KL, Nowycky MC (1996) Calcium dependence of large dense-cored vesicle exocytosis evoked by calcium influx in bovine adrenal chromaffin cells. J Neurosci 16:1359–1369
Fernández-Morales JC, Cortés-Gil L, García AG, de Diego AM (2009) Differences in the quantal release of catecholamines in chromaffin cells of rat embryos and their mothers. Am J Physiol Cell Physiol 297:C407–C418
Fernández-Morales JC, Padín JF, Arranz-Tagarro JA, Vestring S, García AG, de Diego AM (2014) Hypoxia-elicited catecholamine release is controlled by L-type as well as N/PQ types of calcium channels in rat embryo chromaffin cells. Am J Physiol Cell Physiol 307:C455–C465
Gandía L, Albillos A, García AG (1993) Bovine chromaffin cells possess FTX-sensitive calcium channels. Biochem Biophys Res Commun 194:671–676
Gandía L, Borges R, Albillos A, García AG (1995) Multiple calcium channel subtypes in isolated rat chromaffin cells. Pflugers Arch 430:55–63
Gandía L, García AG, Morad M (1993) ATP modulation of calcium channels in chromaffin cells. J Physiol 470:55–72
Gandía L, Lara B, Imperial JS, Villarroya M, Albillos A, Maroto R, García AG, Olivera BM (1997) Analogies and differences between omega-conotoxins MVIIC and MVIID: binding sites and functions in bovine chromaffin cells. Pflugers Arch 435:55–64
Gandía L, López MG, Fonteriz RI, Artalejo CR, García AG (1987) Relative sensitivities of chromaffin cell calcium channels to organic and inorganic calcium antagonists. Neurosci Lett 77:333–338
Gandía L, Mayorgas I, Michelena P, Cuchillo I, de Pascual R, Abad F, Novalbos JM, Larrañaga E, García AG (1998) Human adrenal chromaffin cell calcium channels: drastic current facilitation in cell clusters, but not in isolated cells. Pflugers Arch 436:696–704
García AG, García-De-Diego AM, Gandía L, Borges R, García-Sancho J (2006) Calcium signaling and exocytosis in adrenal chromaffin cells. Physiol Rev 86:1093–1131
García AG, Sala F, Reig JA, Viniegra S, Frías J, Fonteriz R, Gandía L (1984) Dihydropyridine BAY-K-8644 activates chromaffin cell calcium channels. Nature 309:69–71
García-Fernández M, Mejias R, López-Barneo J (2007) Developmental changes of chromaffin cell secretory response to hypoxia studied in thin adrenal slices. Pflugers Arch 454:93–100
García-Palomero E, Cuchillo-Ibañez I, García AG, Renart J, Albillos A, Montiel C (2000) Greater diversity than previously thought of chromaffin cell Ca2+ channels, derived from mRNA identification studies. FEBS Lett 481:235–239
García-Palomero E, Renart J, Andrés-Mateos E, Solís-Garrido LM, Matute C, Herrero CJ, García AG, Montiel C (2001) Differential expression of calcium channel subtypes in the bovine adrenal medulla. Neuroendocrinology 74:251–261
Giancippoli A, Novara M, de Luca A, Baldelli P, Marcantoni A, Carbone E, Carabelli V (2006) Low-threshold exocytosis induced by cAMP-recruited CaV3.2 (alpha1H) channels in rat chromaffin cells. Biophys J 90:1830–1841
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391:85–100
Henkel AW, Almers W (1996) Fast steps in exocytosis and endocytosis studied by capacitance measurements in endocrine cells. Curr Opin Neurobiol 6:350–357
Hernández-Guijo JM, Carabelli V, Gandía L, García AG, Carbone E (1999) Voltage-independent autocrine modulation of L-type channels mediated by ATP, opioids and catecholamines in rat chromaffin cells. Eur J Neurosci 11:3574–3584
Hernández-Guijo JM, de Pascual R, García AG, Gandía L (1998) Separation of calcium channel current components in mouse chromaffin cells superfused with low- and high-barium solutions. Pflugers Arch 436:75–82
Hernández-Guijo JM, Gandía L, de Pascual R, García AG (1997) Differential effects of the neuroprotectant lubeluzole on bovine and mouse chromaffin cell calcium channel subtypes. Br J Pharmacol 122:275–285
Hernández-Guijo JM, Maneu-Flores VE, Ruiz-Nuño A, Villarroya M, García AG, Gandía L (2001) Calcium-dependent inhibition of L, N, and P/Q Ca2+ channels in chromaffin cells: role of mitochondria. J Neurosci 21:2553–2560
Hernández-Vivanco A, Sanz-Lazaro S, Jiménez-Pompa A, García-Magro N, Carmona-Hidalgo B, Pérez-Alvarez A, Caba-González JC, Tabernero A, Alonso YGS, Passas J, Blázquez J, González-Enguita C, de Castro-Guerín C, Albillos A (2017) Human native Cav1 channels in chromaffin cells: contribution to exocytosis and firing of spontaneous action potentials. Eur J Pharmacol 796:115–121
Hill J, Chan SA, Kuri B, Smith C (2011) Pituitary adenylate cyclase-activating peptide (PACAP) recruits low voltage-activated T-type calcium influx under acute sympathetic stimulation in mouse adrenal chromaffin cells. J Biol Chem 286:42459–42469
Kim SJ, Lim W, Kim J (1995) Contribution of L- and N-type calcium currents to exocytosis in rat adrenal medullary chromaffin cells. Brain Res 675:289–296
Kitamura N, Ohta T, Ito S, Nakazato Y (1997) Calcium channel subtypes in porcine adrenal chromaffin cells. Pflugers Arch 434:179–187
Lara B, Gandía L, Martínez-Sierra R, Torres A, García AG (1998) Q-type Ca2+ channels are located closer to secretory sites than L-type channels: functional evidence in chromaffin cells. Pflugers Arch 435:472–478
Levitsky KL, López-Barneo J (2009) Developmental change of T-type Ca2+ channel expression and its role in rat chromaffin cell responsiveness to acute hypoxia. J Physiol 587:1917–1929
Lomax RB, Michelena P, Nuñez L, García-Sancho J, García AG, Montiel C (1997) Different contributions of L- and Q-type Ca2+ channels to Ca2+ signals and secretion in chromaffin cell subtypes. Am J Phys 272:C476–C484
López MG, Albillos A, de la Fuente MT, Borges R, Gandía L, Carbone E, García AG, Artalejo AR (1994) Localized L-type calcium channels control exocytosis in cat chromaffin cells. Pflugers Arch 427:348–354
López MG, Shukla R, García AG, Wakade AR (1992) A dihydropyridine-resistant component in the rat adrenal secretory response to splanchnic nerve stimulation. J Neurochem 58:2139–2144
López MG, Villarroya M, Lara B, Martínez Sierra R, Albillos A, García AG, Gandía L (1994) Q- and L-type Ca2+ channels dominate the control of secretion in bovine chromaffin cells. FEBS Lett 349:331–337
Lukyanetz EA, Neher E (1999) Different types of calcium channels and secretion from bovine chromaffin cells. Eur J Neurosci 11:2865–2873
Mahapatra S, Calorio C, Vandael DH, Marcantoni A, Carabelli V, Carbone E (2012) Calcium channel types contributing to chromaffin cell excitability, exocytosis and endocytosis. Cell Calcium 51:321–330
Mahapatra S, Marcantoni A, Vandael DH, Striessnig J, Carbone E (2011) Are Cav1.3 pacemaker channels in chromaffin cells? Possible bias from resting cell conditions and DHP blockers usage. Channels (Austin) 5:219–224
Mahapatra S, Marcantoni A, Zuccotti A, Carabelli V, Carbone E (2012) Equal sensitivity of Cav1.2 and Cav1.3 channels to the opposing modulations of PKA and PKG in mouse chromaffin cells. J Physiol 590:5053–5073
Marcantoni A, Baldelli P, Hernandez-Guijo JM, Comunanza V, Carabelli V, Carbone E (2007) L-type calcium channels in adrenal chromaffin cells: role in pace-making and secretion. Cell Calcium 42:397–408
Marcantoni A, Carabelli V, Comunanza V, Hoddah H, Carbone E (2008) Calcium channels in chromaffin cells: focus on L and T types. Acta Physiol (Oxf) 192:233–246
Marcantoni A, Carabelli V, Vandael DH, Comunanza V, Carbone E (2009) PDE type-4 inhibition increases L-type Ca2+ currents, action potential firing, and quantal size of exocytosis in mouse chromaffin cells. Pflugers Arch 457:1093–1110
Marcantoni A, Vandael DH, Mahapatra S, Carabelli V, Sinnegger-Brauns MJ, Striessnig J, Carbone E (2010) Loss of Cav1.3 channels reveals the critical role of L-type and BK channel coupling in pacemaking mouse adrenal chromaffin cells. J Neurosci 30:491–504
Nagayama T, Matsumoto T, Kuwakubo F, Fukushima Y, Yoshida M, Suzuki-Kusaba M, Hisa H, Kimura T, Satoh S (1999) Role of calcium channels in catecholamine secretion in the rat adrenal gland. J Physiol 520:503–512
Neher E, Zucker RS (1993) Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron 10:21–30
Novara M, Baldelli P, Cavallari D, Carabelli V, Giancippoli A, Carbone E (2004) Exposure to cAMP and beta-adrenergic stimulation recruits CaV3 T-type channels in rat chromaffin cells through Epac cAMP-receptor proteins. J Physiol 558:433–449
Olivera BM, Miljanich GP, Ramachandran J, Adams ME (1994) Calcium channel diversity and neurotransmitter release: the omega-conotoxins and omega-agatoxins. Annu Rev Biochem 63:823–867
Perissinotti PP, Giugovaz Tropper B, Uchitel OD (2008) L-type calcium channels are involved in fast endocytosis at the mouse neuromuscular junction. Eur J Neurosci 27:1333–1344
Prakriya M, Lingle CJ (1999) BK channel activation by brief depolarizations requires Ca2+ influx through L- and Q-type Ca2+ channels in rat chromaffin cells. J Neurophysiol 81:2267–2278
Rosa JM, de Diego AM, Gandía L, García AG (2007) L-type calcium channels are preferentially coupled to endocytosis in bovine chromaffin cells. Biochem Biophys Res Commun 357:834–839
Rosa JM, Gandía L, García AG (2009) Inhibition of N and PQ calcium channels by calcium entry through L channels in chromaffin cells. Pflugers Arch 458:795–807
Rosa JM, Torregrosa-Hetland CJ, Colmena I, Gutiérrez LM, García AG, Gandía L (2011) Calcium entry through slow-inactivating L-type calcium channels preferentially triggers endocytosis rather than exocytosis, in bovine chromaffin cells. Am J Physiol Cell Physiol 301:C86–C98
Santana F, Michelena P, Jaén R, García AG, Borges R (1999) Calcium channel subtypes and exocytosis in chromaffin cells: a different view from the intact rat adrenal. Naunyn Schmiedeberg's Arch Pharmacol 360:33–37
Scott AL, Zhang M, Nurse CA (2015) Enhanced BDNF signalling following chronic hypoxia potentiates catecholamine release from cultured rat adrenal chromaffin cells. J Physiol 593:3281–3299
Smith C, Moser T, Xu T, Neher E (1998) Cytosolic Ca2+ acts by two separate pathways to modulate the supply of release-competent vesicles in chromaffin cells. Neuron 20:1243–1253
Takeuchi Y, Mochizuki-Oda N, Yamada H, Kurokawa K, Watanabe Y (2001) Nonneurogenic hypoxia sensitivity in rat adrenal slices. Biochem Biophys Res Commun 289:51–56
Thompson RJ, Jackson A, Nurse CA (1997) Developmental loss of hypoxic chemosensitivity in rat adrenomedullary chromaffin cells. J Physiol 498:503–510
Ulate G, Scott SR, González J, Gilabert JA, Artalejo AR (2000) Extracellular ATP regulates exocytosis in inhibiting multiple Ca2+ channel types in bovine chromaffin cells. Pflugers Arch 439:304–314
Vandael DH, Marcantoni A, Carbone E (2015) Cav1.3 channels as key regulators of neuron-like firings and catecholamine release in chromaffin cells. Curr Mol Pharmacol 8:149–161
Vandael DH, Marcantoni A, Mahapatra S, Caro A, Ruth P, Zuccotti A, Knipper M, Carbone E (2010) Cav1.3 and BK channels for timing and regulating cell firing. Mol Neurobiol 42:185–198
Vandael DH, Zuccotti A, Striessnig J, Carbone E (2012) CaV1.3-driven SK channel activation regulates pacemaking and spike frequency adaptation in mouse chromaffin cells. J Neurosci 32:16345–16359
Villarroya M, De la Fuente MT, López MG, Gandía L, García AG (1997) Distinct effects of omega-toxins and various groups of Ca2+-entry inhibitors on nicotinic acetylcholine receptor and Ca2+ channels of chromaffin cells. Eur J Pharmacol 320:249–257
Wick PF, Westenbroek RE, Holz RW (1996) Effects of expression of a mouse brain L-type calcium channel alpha 1 subunit on secretion from bovine adrenal chromaffin cells. Mol Pharmacol 49:295–302
Acknowledgements
This work was partially supported by grants SAF2013-44108-P and SAF2016-78892-R (Ministerio de Economía y Competitividad, Spain) to LG. We thank the continued support of Fundación Teófilo Hernando, Madrid, Spain.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the special issue on chromaffin cells in Pflügers Archiv —European Journal of Physiology
Rights and permissions
About this article
Cite this article
Nanclares, C., Baraibar, A.M. & Gandía, L. L-type calcium channels in exocytosis and endocytosis of chromaffin cells. Pflugers Arch - Eur J Physiol 470, 53–60 (2018). https://doi.org/10.1007/s00424-017-2064-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-017-2064-1