Abstract
Purpose
The applicability of laparoscopic-assisted radical gastrectomy for elderly patients with gastric cancer is still not well clarified. The aim of this double-center study was to explore the feasibility and effectiveness of laparoscopic-assisted radical gastrectomy on elderly patients with gastric cancer.
Methods
We prospectively collected data of patients who underwent gastrectomy for cancer in two centers from June 2016 to December 2019. Propensity score matching was performed at a ratio of 1:1 to compare the laparoscopic-assisted radical gastrectomy group and open radical gastrectomy group. Univariate analyses and multivariate logistic regression analyses evaluating the risk factors for total, surgical, and medical complications were performed.
Results
A total of 481 patients with gastric cancer met the inclusion criteria and were included in this study. After propensity score analysis, 258 patients were matched each other (laparoscopic-assisted radical gastrectomy (LAG) group, n = 129; open radical gastrectomy (OG) group, n = 129). LAG group had lower rate of surgical complications (P = 0.009), lower rate of severe complications (P = 0.046), shorter postoperative hospital stay (P = 0.001), and lower readmission rate (P = 0.039). Multivariate analyses revealed that anemia, Charlson comorbidity index, and combined resection were independent risk factors in the LAG group, whereas body mass index and American Society of Anesthesiology grade in the OG group.
Conclusion
Laparoscopic-assisted radical gastrectomy was relative safe even effective in elderly gastric cancer patients. We should pay attention to the different risk factors when performing different surgical procedures for gastric cancer in elderly patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As one of the means of minimally invasive surgery, laparoscopic surgery has many advantages, such as lower intraoperative blood loss, faster postoperative gastrointestinal function recovery, lower incidence of postoperative complication, and shorter length of postoperative hospital stays [1, 2]. Laparoscopic surgery has been widely used to treat various kinds of malignant tumors. In 1994, Kitano et al. introduced the first case of using laparoscopic-assisted radical gastrectomy for a patient with early gastric cancer in the world [3]. From then on, this advanced operation method was widely spread in the oriental countries. Many studies reported the efficacy and safety of laparoscopic-assisted radical gastrectomy compared with the traditional open radical resection for gastric cancer [4, 5]. To date, laparoscopic radical gastrectomy has been recognized as a minimally invasive treatment for early gastric cancer because of its several advantages over traditional open gastrectomy, such as faster and less painful recovery, and lower rate of postoperative complications [6]. Also, it has been proven to be performed by experienced surgeons for advanced gastric cancer [7].
Over the past century, human’s life expectancy has nearly doubled. The world is currently in the stage of rapid aging. China is one of the fastest aging countries. The incidence of cancer is increasing in patients over the age of 65 years [8, 9]. Elderly patients with gastric cancer often company with many chronic diseases such as cardiovascular disease or diabetes mellitus. They also had poor nutritional status and slower recovery from surgical trauma [10]. All of these conditions may damage the body function and increase the difficulty of the tumor treatment. Some studies reported that the patients’ age is the most important factor for predicting surgical complications [11, 12]. It seems more important to focus on how to treat elderly patients with gastric cancer effectively.
Although elderly patients may benefit from laparoscopic surgery, there remain a lot of problems, such as the effects of carbon dioxide pneumoperitoneum and operative time [13, 14]. Recently, there were many researches focusing on the application of laparoscopic-assisted radical gastrectomy in elderly patients with gastric cancer [15,16,17,18]. Most of these studies exist choose migration, and the level of evidence is not very high. In this double-center study, we adopted the propensity score matching method to explore the feasibility of laparoscopic-assisted radical gastrectomy on elderly patients with gastric cancer.
Material and methods
Patients and grouping
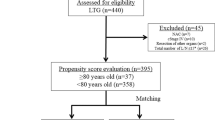
This study was performed using data from a prospectively maintained database of patients in two centers from June 2016 to December 2019. All patients who underwent radical gastrectomy in 1 of 2 centers: Gastrointestinal Surgical Department, the First Affiliated Hospital of Wenzhou Medical University in Wenzhou, and Gastrointestinal Surgical Department, Tenth People’s Hospital Affiliated to TongJi University in Shanghai, were included. The inclusion criteria included patients who (1) were ≥ 65 years old, (2) had an accurate preoperative diagnosis of gastric adenocarcinoma on the basis of histological evidence, (3) had ASA grade ≤ III, and (4) planned to receive elective radical gastrectomy for gastric cancer. Exclusion criteria included patients with a presence of cancer metastasis that could not be cured by radical surgery or patients undergoing palliative gastrectomy or emergency surgery or patients receiving neoadjuvant chemotherapy or radiotherapy. We performed standard open and laparoscopic-assisted gastrectomy with D2 lymphadenectomy for advanced stage or < D2 lymphadenectomy for early stage based on guidelines [19, 20]. Laparoscopic-assisted surgery was performed on the patients who met all of the following 3 criteria: estimated tumor size ≤ 5 cm, T stage ≤ 3, and N stage ≤ 1. All operations were performed by 6 experienced surgeons who had abundant experience with more than 100 cases of radical gastrectomy for gastric cancer. The patients were divided into two groups according to the different types of operation: laparoscopic-assisted radical gastrectomy (LAG) group and traditional open radical gastrectomy (OG) group. All patients gave written informed consent for participation in this study, and the study was approved by the ethics committee of The First Affiliated Hospital of Wenzhou Medical University and Tenth People’s Hospital Affiliated to TongJi University.
Data collection
For each patient, the following data were prospectively collected: (1) patient characteristics, including age, gender, body mass index (BMI), American Society of Anesthesiology (ASA) grade, hemoglobin concentration; anemia; plasma albumin concentration; hypoalbuminemia; comorbidity (calculated by Charlson comorbidity index score) [21], nutritional risk screening 2002 (NRS 2002) scores, history of previous abdominal surgery, histologic type, tumor location, and TNM stage; (2) operative details, including type of resection, extent of node dissection, laparoscopic-assisted operation, number of lymph nodes harvested, combined resection, and surgical duration; and (3) postoperative short-term outcomes, including postoperative complications within 30 days after surgery, postoperative hospital stays, hospitalization costs, and readmissions within 30 days of discharge.
All patients regularly received telephone interviews or outpatient visits. Follow-up investigations were scheduled every 3 months for the first 2 years after surgery and every 6 months thereafter. The last follow-up date was February 2020.
Definition
Total postoperative complications were defined as those meeting the criterion of grade II or higher according to the Clavien-Dindo classification [22]. Grade III or above complications were defined as severe complications. Surgical complications included gastrointestinal dysfunction (including delayed gastric emptying and prolonged postoperative ileus), wound infection, bleeding, intra-abdominal abscess, anastomotic leakage, intestinal obstruction, lymphorrhagia, and pancreatic fistula. Medical complications included pneumonia, pleural effusion, pulmonary atelectasis, cardiac complications, venous thrombosis, persistent hypoalbuminemia, hepatic dysfunction, cerebral infarction, urinary infection, thrombocythemia, and respiratory failure. Anemia was defined as hemoglobin concentration < 120 g/L in men or < 100 g/L in women. Hypoalbuminemia was defined as a plasma albumin concentration < 35 g/L.
Propensity score analysis
To minimize bias due to the nonrandom allocation of treatments among patients, propensity score matched (PSM) analyses between LAG group and OG group were performed using multiple logistic regression. In brief, a propensity score for each patient was calculated. LAG and OG patients were then paired 1:1 on these propensity scores using neighbor matching algorithm without replacement, with a caliper width equal to 0.2 of the standard deviation of the logit of the propensity scores. Following 1:1 propensity score-matching, some statistical analyses of postoperative outcomes were performed [23].
Statistical analysis
Statistical analyses were performed using SPSS statistics version 22.0 (IBM, Armonk, New York, USA). For normally distributed continuous variables, data were presented as the mean and standard deviation (SD); otherwise, for non-normally distributed variables, data were presented as median and interquartile range (IQR). Categorical variables are presented as numbers and percentages. Student’s t test was performed for normally distributed data, Pearson’s chi-square test or Fisher’s exact test was performed for categorical data, and the Mann–Whitney U test was used for non-normally distributed continuous data and ranked data. Univariate analysis was used to identify potential risk factors of postoperative complications. Kaplan–Meier method was used to analyze overall survive (OS) and disease-free survival (DFS). Variables with a P < 0.10 were included into subsequent multivariate (logistic regression or Cox proportional hazards regression) analyses. All of the tests were two-sided, and P < 0.05 were considered to be statistically significant.
Results
Patients’ characteristics
From June 2016 to December 2019, a total of 481 patients met the inclusion criteria and constituted the original dataset. A total of 367 (76.3 %) patients were male, and 114 (23.7 %) were female. All of patients received complete tumor resection. The median age was 72 years, and the mean BMI was 22.61 kg/m2. The median preoperative plasma albumin concentration and hemoglobin concentration of the original dataset was 39.1 g/L and 119 g/L, respectively. Median NRS2002 score was 2. Laparoscopic-assisted radical gastrectomy was performed in 133 (27.7%) patients. Comparison of the baseline characteristics of the two groups is showed in Table 1. Between LAG and OG group, gender, BMI, preoperative serum hemoglobin, Charlson comorbidity index, history of previous abdominal surgery, histologic type, tumor location or combined resection did not differ significantly. However, patients in the OG group were older (P = 0.019), had higher tumor stage (P < 0.001), higher NRS2002 score (P = 0.002), higher ASA grade (P = 0.009), and lower preoperative serum albumin (P = 0.001). After propensity score analysis, there were 129 matched patients in each group. The standardized differences in the demographic and preoperative variables of interest disappeared when matched patients were compared (Table 2).
The 1:1 matched dataset included 258 patients, with 199 males (77.1%) and 59 females (22.9%). The median age was 71 years, and the mean BMI was 22.82 kg/m2. The median preoperative plasma albumin concentration and hemoglobin concentration of the original dataset was 38.1 g/L and 121.5 g/L, respectively. Median NRS2002 score was 2. The distribution of the TNM stages in the patients was 108 (41.9 %), 68 (26.3 %), and 82 (31.8 %) for TNM stages I, II, and III, respectively.
Postoperative outcomes
As shown in Table 3, in the 258 matched patients, the overall incidence of postoperative complications was 25.2%. The most common postoperative complications were pneumonia, gastrointestinal dysfunction, bleeding, and intra-abdominal abscess. Patients in the LAG group had lower rate of surgical complications (9.3% vs 20.9%, P = 0.009), lower rate of severe complications (9.3% vs 17.8%, P = 0.046), shorter postoperative hospital stay (median, 13 days vs 14 days, P = 0.001), lower readmission rate (3.1% vs 9.3%, P = 0.039), higher hospitalization costs (median, 65458.48 yuan vs 58719.07 yuan, P = 0.003), and longer surgical durations (median, 210 min vs 195 min, P < 0.001). The median number of lymph nodes harvested in this study was 16. There was no significant difference in numbers of lymph nodes harvested between two groups. The combined resected organ or tissue included partial transverse colon or mesentery, partial liver, spleen, and distal pancreas.
Subgroup analysis
In the subgroup of 65–75 years old, patients in the LAG group had lower rate of surgical complications (9.8% vs 20.6%, P = 0.039), lower rate of severe complications (8.7% vs 18.6%, P = 0.049), and longer surgical durations (median, 214 min vs 195 min, P = 0.008). In the subgroup of older than 75 years old, there was no significant difference in short-term outcomes between two groups (Table 4).
Oncologic outcome
The survival curves for patients in LAG and OG group are shown in Fig. 1. For patients in LAG group, the 1- and 3-year OS rates were 89.5% and 70.1%. The 1- and 3-year DFS rates were 88.6% and 67.0%. For patients in OG group, the 1- and 3-year OS rates were 91.4% and 70.8%. The 1- and 3-year DFS rates were 90.7% and 69.5%. There was no significant difference in long-term outcomes between two groups (OS: P = 0.864; DPS: P = 0.841).
Risk factors of total complications
Results of univariate and multivariate analyses for risk factors of total postoperative complications in elderly patients with gastric cancer were showed in Table 5. In univariate analysis, BMI (P = 0.005), hypoalbuminemia (P = 0.017), anemia (P = 0.012), ASA grade (P = 0.006), and Charlson comorbidity index (P = 0.008) were associated with postoperative complications. In the multivariate logistic regression analysis after controlling for potential confounders, BMI (< 18.5; OR 3.994 (1.303–12.240); P = 0.015; > 24; OR 1.969 (1.048–3.700); P = 0.035), anemia (OR 2.140 (1.175–3.897); P = 0.013) and ASA grade (≥ III, OR 2.392 (1.176–4.865); P = 0.016) remained as the independent risk factors for postoperative complications after gastrectomy in elderly patients with gastric cancer.
To compare risk factors of total complications in the LAG and OG groups, subgroup analyses were performed according to surgical approaches. In the LAG group, anemia (OR 2.917 (1.160–7.338); P = 0.023), Charlson comorbidity index (≥ 2, OR 5.336 (1.553–18.332); P = 0.008), and combined resection (OR 6.096 (1.165–31.900); P = 0.032) were proven as independent risk factors for total postoperative complications (Table 6). In the OG group, BMI (< 18.5; OR 14.392 (1.515–136.730); P = 0.020) and ASA grade (≥ III, OR 3.805 (1.412–10.251); P = 0.008) were demonstrated to be independent factors associated with total postoperative complications (Table 7).
Discussion
In the coming decades, it is expected that the worldwide population of patients with gastric cancer will continue to grow, especially in East Asia, Eastern Europe, and South America, where the number of elderly patients diagnosed with gastric cancer is expected to increase [24]. Although some elderly patients with gastric cancer have no obvious comorbidity, their mortality risk, postoperative complication rate, and length of hospital stay are expected to increase in comparison to young patients [25, 26]. In addition, for patients with gastric cancer aged > 70 years, the risk of death increases by 10% for every 1-year increase in age [27]. Therefore, surgeons often recommend localized surgery for elderly patients with gastric cancer in order to reduce the incidence of postoperative complications.
Nowadays, laparoscopic-assisted radical gastrectomy is becoming well known, and studies have confirmed that laparoscopic-assisted radical gastrectomy with D2 lymph node dissection performed by experienced surgeons is safe and effective [28, 29]. Since laparoscopic-assisted surgery is relatively less traumatic, elderly patients with gastric cancer may be able to benefit from its minimal invasion. However, whether these benefits equally apply to elderly patients remain to be determined. One problem in laparoscopic-assisted radical gastrectomy is that it requires carbon dioxide pneumoperitoneum, which can be harmful to elderly patients. In addition, elderly patients with gastric cancer have more comorbidities and reduced physical functions, indicating that they may suffer from higher incidence of complications and death postoperatively [30]. Several recent multicenter clinical trials have reported conflicting results regarding the effects of age on the outcomes of laparoscopic-assisted radical gastrectomy. Therefore, the applicability of laparoscopic-assisted radical gastrectomy for elderly patients with gastric cancer is still not well clarified.
This study utilized propensity score matching analysis method to overcome the bias in clinical data, balance the baseline data of each variable between the two groups, and obtain more reliable results [31, 32]. Based on the results of this study, prior to the propensity score matching analysis, differences in age, TNM stage, NRS2002 score, ASA grade, and preoperative serum albumin were observed between two groups. However, after the propensity score matching analysis, the difference disappeared between two groups. These observations suggest that the general information after matching is more uniform and comparable.
Compared with open gastric cancer surgery, laparoscopic-assisted radical gastrectomy is characterized by its longer operation time [33], which is due to the lack of tactile sensation and longer time to conduct delicate operations in a narrow space and clean a series of lymph nodes during laparoscopic surgery. In addition, the surgeon’s experience, the size and malignancy of the tumor, and the complexity of the abdominal cavity during laparoscopic surgery also lead to the extension of surgery time [7, 34]. Kitano et al. have reported that the average length of laparoscopic gastric cancer surgery is 271 min, which is longer than the average length of traditional open gastric cancer surgery [35]. The results of the current study found that the median operative time of laparoscopic-assisted radical gastrectomy was 210 min, while the median operative time of open radical gastrectomy was 195 min, which were statistically different from each other and consistent with the results of earlier studies. Studies have also found that the operation time of laparoscopic-assisted surgery is gradually decreasing and may be shorter than traditional open surgery in the future.
Elderly patients with gastric cancer are often associated with comorbidities such as diabetes, chronic obstructive pulmonary disease, heart disease, arthritis, and hypertension; all of which can affect the surgical outcomes and long-term survival [36]. The results of present study showed that the proportion of elderly patients with gastric cancer who had preoperative comorbidities was 34.1%, and the most common comorbidity was respiratory disease. It has been reported that the probability of incision problems, intestinal obstruction, or respiratory complications after laparoscopic-assisted radical gastrectomy is higher in patients with preoperative cardiopulmonary function-related comorbidities [37]. This may be related to the requirement of carbon dioxide pneumoperitoneum during laparoscopic-assisted surgery. On one hand, laparoscopic pneumoperitoneum increases the pressure inside the abdominal cavity. On the other hand, the abdominal cavity absorbs a large amount of carbon dioxide into the circulation system, resulting in a series of hemodynamic changes. Therefore, elderly patients with gastric cancer who also have respiratory dysfunction may suffer from hypercapnia, acidosis, and respiratory-related complications after laparoscopic-assisted radical gastrectomy. As a result, some doctors suggest using low-pressure pneumoperitoneum or laparoscopic surgery without gas generation for elderly patients with cardiopulmonary comorbidities [38].
The incidence of postoperative complications in laparoscopic-assisted radical gastrectomy is between 11.0 and 25.3% [39, 40]. Results from the current study showed that the incidence of postoperative complications in elderly patients who underwent laparoscopic-assisted radical gastrectomy was 21.7%. Especially, the incidence of postoperative surgical complications and severe complications after laparoscopic-assisted radical gastrectomy was significantly lower than those of open radical gastrectomy. Results from this study also showed that after laparoscopic-assisted radical gastrectomy, the length of hospital stays significantly shortened and readmission rate significantly decreased. However, hospital costs increased, which is likely caused by higher prices of laparoscopic surgery relative to open surgery. Overall, these results demonstrated that the applicability of laparoscopic-assisted radical gastrectomy was safe and effective for elderly patients with gastric cancer. In addition, in our study, laparoscopic-assisted radical gastrectomy was conducted mostly on advantaged stages of gastric cancer among the elderly population. So future studies may focus on the effect of laparoscopic-assisted radical gastrectomy on elderly patients with advantaged gastric cancer. Moreover, the prevention of complications can extend their postoperative survival time [41]. At last, the result of oncologic outcomes also suggested that laparoscopic-assisted radical gastrectomy was not inferior to open surgery in terms of long-term outcomes.
The results of the current study showed that pneumonia, bleeding, and hypoproteinemia were the most common complications experienced by elderly patients with gastric cancer after laparoscopic-assisted radical gastrectomy. Pneumonia is especially common. A meta-analysis reported that the probability of lung-related complications following laparoscopic-assisted radical gastrectomy was lower compared with open radical gastrectomy. This may be due to the smaller incision of laparoscopic surgery. Small incision can cause less pain, and thus, patients can leave bed and exercise earlier, which helps prevent atelectasis and pneumonia caused by long-term bedrest [42].
Risk factors for total postoperative complications in the LAG group were different from those in the OG group. Factors such as anemia, Charlson comorbidity index, BMI, and ASA grade were found to be significant in the LAG group or OG group. These factors were all related to patient’s physical status. In addition, factor related to technical difficulty such as combined resection was found to be significant in the LAG group. More emphasis should be placed on patients with these risk factors for total postoperative complications. When performing surgical procedures in elderly patients, especially when attempting laparoscopic-assisted radical gastrectomy, the authors recommend selecting patients carefully.
This study has some limitations. First, although the overall sample size was large, the sample size after using the propensity score matching analysis was relatively small. Therefore, the applicability and effectiveness of laparoscopic-assisted radical gastrectomy in elderly patients with gastric cancer require further confirmation with future randomized controlled studies. Second, due to the limited duration, this study did not analyze the 5- or 10-year outcomes, or postoperative therapy. Future studies are required to extend the follow-up time to explore the long-term prognosis.
In conclusion, through the analysis of the propensity score matching, laparoscopic-assisted radical gastrectomy was relative safe even effective in elderly gastric cancer patients, which can decrease postoperative surgical complication and severe complication rate, shorten the length of hospital stay, and decrease the rate of readmission after discharge. In the population of elderly gastric cancer patients, anemia, Charlson comorbidity index, and combined resection were independent risk factors for total postoperative complications after laparoscopic-assisted radical gastrectomy, while BMI and ASA grade were independent factors for total postoperative complications after open radical gastrectomy.
References
Currie AC, Malietzis G, Jenkins JT, Yamada T, Ashrafian H, Athanasiou T, Okabayashi K, Kennedy RH (2016) Network meta-analysis of protocol-driven care and laparoscopic surgery for colorectal cancer. Br J Surg 103(13):1783–1794. https://doi.org/10.1002/bjs.10306
Lee JH, Yom CK, Han HS (2009) Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg Endosc 23(8):1759–1763. https://doi.org/10.1007/s00464-008-0198-0
Kitano S, Iso Y, Moriyama M, Sugimachi K (1994) Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 4(2):146–148
Chung KH, Lee SH, Park JM, Lee JM, Shin CM, Ahn SH, Park Do J, Kim HH, Ryu JK, Kim YT (2016) Partially covered self-expandable metallic stent for postoperative benign strictures associated with laparoscopy-assisted gastrectomy. Gastric Cancer 19(1):280–286. https://doi.org/10.1007/s10120-014-0450-3
Kosuga T, Hiki N, Nunobe S, Noma H, Honda M, Tanimura S, Sano T, Yamaguchi T (2014) Feasibility and nutritional impact of laparoscopy-assisted subtotal gastrectomy for early gastric cancer in the upper stomach. Ann Surg Oncol 21(6):2028–2035. https://doi.org/10.1245/s10434-014-3520-1
Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL (2012) Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique a systematic review and meta-analysis. Ann Surg 255(6):1048–1059. https://doi.org/10.1097/SLA.0b013e318251ee09
Hu Y, Ying M, Huang C, Wei H, Jiang Z, Peng X, Hu J, Du X, Wang B, Lin F, Xu J, Dong G, Mou T, Li G, Chinese Laparoscopic Gastrointestinal Surgery Study G (2014) Oncologic outcomes of laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective cohort study from China. Surg Endosc 28(7):2048–2056. https://doi.org/10.1007/s00464-014-3426-9
Saif MW, Makrilia N, Zalonis A, Merikas M, Syrigos K (2010) Gastric cancer in the elderly: an overview. Eur J Surg Oncol 36(8):709–717. https://doi.org/10.1016/j.ejso.2010.05.023
Terret C, Castel-Kremer E, Albrand G, Droz JP (2009) Effects of comorbidity on screening and early diagnosis of cancer in elderly people. Lancet Oncol 10(1):80–87. https://doi.org/10.1016/S1470-2045(08)70336-X
Marosi C, Koller M (2016) Challenge of cancer in the elderly. ESMO Open 1(3):e000020. https://doi.org/10.1136/esmoopen-2015-000020
Jeong O, Park YK, Ryu SY, Kim YJ (2010) Effect of age on surgical outcomes of extended gastrectomy with D2 lymph node dissection in gastric carcinoma: prospective cohort study. Ann Surg Oncol 17(6):1589–1596. https://doi.org/10.1245/s10434-010-0916-4
Zaimi I, Sparreboom CL, Lingsma HF, Doornebosch PG, Menon AG, Kleinrensink GJ, Jeekel J, Wouters M, Lange JF, Dutch ColoRectal Audit G (2018) The effect of age on anastomotic leakage in colorectal cancer surgery: a population-based study. J Surg Oncol 118:113–120. https://doi.org/10.1002/jso.25108
Bates AT, Divino C (2015) Laparoscopic surgery in the elderly: a review of the literature. Aging Dis 6(2):149–155. https://doi.org/10.14336/AD.2014.0429
Carlomagno N, Tammaro V, Scotti A, Candida M, Calogero A, Santangelo ML (2016) Is day-surgery laparoscopic cholecystectomy contraindicated in the elderly? Results from a retrospective study and literature review. Int J Surg 33(Suppl 1):S103–S107. https://doi.org/10.1016/j.ijsu.2016.06.024
Suzuki S, Nakamura T, Imanishi T, Kanaji S, Yamamoto M, Kanemitsu K, Yamashita K, Sumi Y, Tanaka K, Kuroda D, Kakeji Y (2015) Carbon dioxide pneumoperitoneum led to no severe morbidities for the elderly during laparoscopic-assisted distal gastrectomy. Ann Surg Oncol 22(5):1548–1554. https://doi.org/10.1245/s10434-014-4182-8
Lu J, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M (2015) Short- and Long-term outcomes after laparoscopic versus open total gastrectomy for elderly gastric cancer patients: a propensity score-matched analysis. J Gastrointest Surg 19(11):1949–1957. https://doi.org/10.1007/s11605-015-2912-2
Kim MG, Kim HS, Kim BS, Kwon SJ (2013) The impact of old age on surgical outcomes of totally laparoscopic gastrectomy for gastric cancer. Surg Endosc 27(11):3990–3997. https://doi.org/10.1007/s00464-013-3073-6
Shimada S, Sawada N, Oae S, Seki J, Takano Y, Ishiyama Y, Nakahara K, Maeda C, Hidaka E, Ishida F, Kudo SE (2018) Safety and curability of laparoscopic gastrectomy in elderly patients with gastric cancer. Surg Endosc 32(10):4277–4283. https://doi.org/10.1007/s00464-018-6177-1
Worhunsky DJ, Ma Y, Zak Y, Poultsides GA, Norton JA, Rhoads KF, Visser BC (2015) Compliance with gastric cancer guidelines is associated with improved outcomes. J Natl Compr Cancer Netw 13(3):319–325. https://doi.org/10.6004/jnccn.2015.0044
Japanese Gastric Cancer A (2017) Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20(1):1–19. https://doi.org/10.1007/s10120-016-0622-4
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196. https://doi.org/10.1097/SLA.0b013e3181b13ca2
Yasunaga H, Horiguchi H, Kuwabara K, Matsuda S, Fushimi K, Hashimoto H, Ayanian JZ (2013) Outcomes after laparoscopic or open distal gastrectomy for early-stage gastric cancer: a propensity-matched analysis. Ann Surg 257(4):640–646. https://doi.org/10.1097/SLA.0b013e31826fd541
Ueno D, Matsumoto H, Kubota H, Higashida M, Akiyama T, Shiotani A, Hirai T (2017) Prognostic factors for gastrectomy in elderly patients with gastric cancer. World J Surg Oncol 15(1):59. https://doi.org/10.1186/s12957-017-1131-6
Nienhueser H, Kunzmann R, Sisic L, Blank S, Strowitzk MJ, Bruckner T, Jager D, Weichert W, Ulrich A, Buchler MW, Ott K, Schmidt T (2015) Surgery of gastric cancer and esophageal cancer: does age matter? J Surg Oncol 112(4):387–395. https://doi.org/10.1002/jso.24004
Hikage M, Tokunaga M, Makuuchi R, Irino T, Tanizawa Y, Bando E, Kawamura T, Terashima M (2018) Surgical outcomes after gastrectomy in very elderly patients with gastric cancer. Surg Today 48(8):773–782. https://doi.org/10.1007/s00595-018-1651-x
Story DA (2008) Postoperative complications in elderly patients and their significance for long-term prognosis. Curr Opin Anaesthesiol 21(3):375–379. https://doi.org/10.1097/ACO.0b013e3282f889f8
Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Chen P, Liu H, Zheng C, Liu F, Yu J, Li Z, Zhao G, Chen X, Wang K, Li P, Xing J, Li G (2016) Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol 34(12):1350–1357. https://doi.org/10.1200/JCO.2015.63.7215
Lee HH, Son SY, Lee JH, Kim MG, Hur H, Park DJ (2017) Surgeon’s experience overrides the effect of hospital volume for postoperative outcomes of laparoscopic surgery in gastric cancer: multi-institutional study. Ann Surg Oncol 24(4):1010–1017. https://doi.org/10.1245/s10434-016-5672-7
Cho GS, Kim W, Kim HH, Ryu SW, Kim MC, Ryu SY (2009) Multicentre study of the safety of laparoscopic subtotal gastrectomy for gastric cancer in the elderly. Br J Surg 96(12):1437–1442. https://doi.org/10.1002/bjs.6777
Andrei AC (2017) Invited Commentary: Some considerations on matching in observational studies. J Am Coll Surg 224(5):814–815. https://doi.org/10.1016/j.jamcollsurg.2017.02.009
Austin PC, Jembere N, Chiu M (2016) Propensity score matching and complex surveys. Stat Methods Med Res 27:1240–1257. https://doi.org/10.1177/0962280216658920
Fujisaki M, Shinohara T, Hanyu N, Kawano S, Tanaka Y, Watanabe A, Yanaga K (2016) Laparoscopic gastrectomy for gastric cancer in the elderly patients. Surg Endosc 30(4):1380–1387. https://doi.org/10.1007/s00464-015-4340-5
Jeong O, Jung MR, Kim GY, Kim HS, Ryu SY, Park YK (2013) Comparison of short-term surgical outcomes between laparoscopic and open total gastrectomy for gastric carcinoma: case-control study using propensity score matching method. J Am Coll Surg 216(2):184–191. https://doi.org/10.1016/j.jamcollsurg.2012.10.014
Mochiki E, Toyomasu Y, Ogata K, Andoh H, Ohno T, Aihara R, Asao T, Kuwano H (2008) Laparoscopically assisted total gastrectomy with lymph node dissection for upper and middle gastric cancer. Surg Endosc 22(9):1997–2002. https://doi.org/10.1007/s00464-008-0015-9
Yamamoto K, Nagatsuma Y, Fukuda Y, Hirao M, Nishikawa K, Miyamoto A, Ikeda M, Nakamori S, Sekimoto M, Fujitani K, Tsujinaka T (2017) Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer 20(5):913–918. https://doi.org/10.1007/s10120-016-0683-4
Ozyuvaci E, Demircioglu O, Toprak N, Topacoglu H, Sitilci T, Akyol O (2012) Comparison of transcutaneous, arterial and end-tidal measurements of carbon dioxide during laparoscopic cholecystectomy in patients with chronic obstructive pulmonary disease. J Int Med Res 40(5):1982–1987. https://doi.org/10.1177/030006051204000540
Park SJ, Kim BG, Oh AH, Han SH, Han HS, Ryu JH (2016) Effects of intraoperative protective lung ventilation on postoperative pulmonary complications in patients with laparoscopic surgery: prospective, randomized and controlled trial. Surg Endosc 30(10):4598–4606. https://doi.org/10.1007/s00464-016-4797-x
Miki Y, Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M (2014) Perioperative risk assessment for gastrectomy by surgical apgar score. Ann Surg Oncol 21(8):2601–2607. https://doi.org/10.1245/s10434-014-3653-2
Lee JH, Park DJ, Kim HH, Lee HJ, Yang HK (2012) Comparison of complications after laparoscopy-assisted distal gastrectomy and open distal gastrectomy for gastric cancer using the Clavien-Dindo classification. Surg Endosc 26(5):1287–1295. https://doi.org/10.1007/s00464-011-2027-0
Jiang N, Deng JY, Ding XW, Zhang L, Liu HG, Liang YX, Liang H (2014) Effect of complication grade on survival following curative gastrectomy for carcinoma. World J Gastroenterol 20(25):8244–8252. https://doi.org/10.3748/wjg.v20.i25.8244
Kawamura H, Yokota R, Homma S, Kondo Y (2010) Comparison of respiratory function recovery in the early phase after laparoscopy-assisted gastrectomy and open gastrectomy. Surg Endosc 24(11):2739–2742. https://doi.org/10.1007/s00464-010-1037-7
Funding
This study was funded by the Natural Science Foundation of Zhejiang Province (No. Y15H160190).
Author information
Authors and Affiliations
Contributions
Study conception and design: X.L. Chen and Z. Yu. Acquisition of data: W.Z. Chen, Q.T. Dong and H.Y. Cai. Analysis and interpretation of data: F.M. Zhang, J.Y. Yan and C.L. Zhuang. Drafting of manuscript: W.Z. Chen and Q.T. Dong. Critical revision of manuscript: X.L. Chen.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethics committee of The First Affiliated Hospital of Wenzhou Medical University and Tenth People’s Hospital Affiliated to TongJi University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, WZ., Dong, QT., Zhang, FM. et al. Laparoscopic versus open resection for elderly patients with gastric cancer: a double-center study with propensity score matching method. Langenbecks Arch Surg 406, 449–461 (2021). https://doi.org/10.1007/s00423-020-01978-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-020-01978-w




