Abstract
Purpose
Cancer-related fatigue (CRF) is the most reported side effect of cancer and its treatments. Mechanisms of CRF are multidimensional, including neuromuscular alterations leading to decreased muscle strength and endurance (i.e., fatigability). Recently, exercise fatigability and CRF have been related, while fatigability mechanisms remain unclear. Traditionally, fatigability is assessed from maximal voluntary contractions (MVC) decrease, but some authors hypothesized that the rate of force development (RFD) determined during a rapid contraction could also be an interesting indicator of functional alterations. However, to our knowledge, no study investigated RFD in cancer patients. The purpose of this study was to determine whether RFD, fatigability amplitude, and etiology are different between fatigued and non-fatigued cancer patients.
Methods
Eighteen participants with cancer, divided in fatigued or non-fatigued groups according their CRF level, completed a 5-min all-out exercise in ankle plantar flexor muscles composed of 62 isometric MVC of 4 s with 1 s rest, to assess fatigability amplitude as the force–time relationship asymptote (FA). Before and after exercise, fatigability etiologies (i.e., voluntary activation (VA) and evoked forces by electrical stimulation (Db100)) were assessed as well as RFD in 50 and 100 ms (RFD50 and RFD100, respectively) during rapid contractions.
Results
FA is significantly lower in fatigued group. Significant differences were found between pre- and post-exercise VA, Db100, RFD50, and RFD100 for both groups, with no statistical difference between groups.
Conclusion
During treatments, fatigability is higher in fatigued patients; however, the mechanisms of fatigability and RFD alterations are similar in both groups.
Trial registration
ClinicalTrials.gov, NCT04391543, May 2020.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer-related fatigue (CRF) is the most reported side effect of cancer and its treatments (Weis 2011). CRF is defined as “a distressing persistent subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity that interferes with usual functioning” (Berger et al. 2015). Numerous parameters have been evidenced to be related to CRF among biological, physical, behavioral, psychological, and/or social dimensions (Bower 2014). Within the physical dimension, alterations of the peripheral nervous system and muscle induced by cancer and its treatments can be noted (Grisold et al. 2016), sometimes leading to fatigability, a decrease in the force-generating capacity of the neuromuscular system during exercise. Fatigability can be defined as “a response that is less than the expected or anticipated contractile response, for a given stimulation” (MacIntosh and Rassier 2002) and involves central and peripheral mechanisms, with interactions between both (Gandevia 2001). If increased exercise fatigability is one of the mechanisms involved in the onset of CRF, it would be possible to envisage interventions targeting a reduction in fatigability (through physical activity) in an attempt to reduce CRF, as suggested by the results of de Lima et al. (2020). One way to evaluate fatigability amplitude is to compare the maximum force that can be produced during maximal voluntary contractions (MVC) before and after an exercise. Different exercise modalities are frequently used such as fixed-intensity exercises until exhaustion, or do a given number of muscle contractions (Place and Millet 2020). Nevertheless, these modalities may only partly describe the fatigability phenomenon. Indeed, the pre–post-exercise comparison masks the fatigue kinetic information. Based on the critical force concept (Monod and Scherrer 1965), Burnley (2009) proposed to perform repeated MVC until reaching a force plateau, representing maximum fatigability amplitude that can be reached during an exercise. Initially validated for knee extensors muscles, such all-out protocols have been extended to fingers flexors (Chartogne et al. 2020; Veni et al. 2019) and ankle plantar flexors (Abdalla et al. 2018). The latter are not only recognized as representative of activities of daily living (Millet et al. 2023), but also enable a valid assessment of central and peripheral mechanisms of fatigability in both resting and fatigued states using electrical muscle stimulation (Neyroud et al. 2015).
Significant correlation between CRF and fatigability amplitude was reported in three studies, with fatigability being knee extensor MVC decrease after an incremental cycling test to task failure (Brownstein et al. 2021) and in fingers flexor MVC decrease during a 60 MVC all-out exercise (Chartogne et al. 2020; Veni et al. 2019). This suggests that the more fatigued a cancer patient is, the more fatigable he/she is. This also indicates that there may be a link between a perceived feeling of exhaustion and an objective performance decrease after an exercise, while being two distinct phenomenon (Kluger et al. 2013). This rational was developed by Twomey et al. (2017), who hypothesized that this link is supported by a deteriorated resistance to acute exercise in daily physical tasks (e.g., house cleaning, climbing stairs) leading to greater functional capacity reductions, in turn associated to longer recovery time. Furthermore, only three studies, to our knowledge, investigated differences in terms of fatigability amplitude and etiologies between fatigued and non-fatigued cancer patients and reported inconsistent results. First, Neil et al. (2013) found no differences between fatigued and non-fatigued patients in MVC decrease and fatigability etiologies during sustained contractions of the biceps brachii. Then although they did not find differences in MVC decrease, Prinsen et al. (2015) concluded that non-fatigued cancer survivors exhibited more peripheral mechanisms implication in fatigability compared to fatigued ones. Finally, Brownstein et al. (2022) demonstrated that MVC decrease was more important in fatigued than in non-fatigued patients during and after an incremental cycling test with no differences in central mechanisms implication. Fatigability is well-known for being task dependant (Enoka and Stuart 1992), so these differences could be explained by different fatiguing exercises in the abovementioned studies.
Besides, maximum force is often reported but it only represents one characteristic of the muscle function. Some authors hypothesized that the force production during a short and rapid contraction could also be an interesting indicator of functional alterations (D’Emanuele et al. 2021). Indeed, some daily living activities are more characterized by a short time to develop a muscular force than the need to develop a maximal force (e.g., descending stairs, walking) (Aagaard et al. 2002; Izquierdo et al. 1999; Maffiuletti et al. 2010). However, fatigued cancer patients showed impaired balance (Morris and Lewis 2020; Schmitt et al. 2017), thus requiring to produce quickly a muscular force to restore balance and prevent a fall after a sudden postural perturbation. To our knowledge, either no study investigated rapid force alterations after a fatiguing exercise in cancer patients, nor whether there are differences between fatigued and non-fatigued cancer patients. Commonly used to measure rapid force, the rate of force development (RFD) is the ability to increase force as quickly as possible. RFD is calculated from the derivative of the force–time signal as a function of time recorded during a rapid voluntary contraction. Interestingly, the rapid force development is governed by different mechanisms than maximum force in both fresh (Maffiuletti et al. 2016) and fatigue conditions (Morel et al. 2015). In the early phase of contraction (first 50 ms), RFD is mainly determined by reaching a maximal frequency discharge rate while in the advanced-late phases (> 100 ms), discharge rate being already maximal, RFD depends rather on contractile factors (Maffiuletti et al. 2016). In a fatigue context, Morel et al. (2015) showed that RFD was strongly associated with average torque reduction during 160 isokinetic maximal knee extensions at moderate velocity (i.e., 240°.s−1). This demonstrates that rapid force production alteration can be also an important manifestation of fatigability.
Thus, the aim of the present study was to determine whether at the end of a 5-min all-out exercise (i), fatigability amplitude and etiology are different between fatigued and non-fatigued cancer patients during treatments and (ii) whether RFD decreases are related to CRF severity. We hypothesized that fatigued cancer patients would exhibit (i) a higher fatigability amplitude associated to exacerbated central mechanisms; and (ii) a greater RFD alteration compared to non-fatigued patients.
Methods
Participants
Twenty participants diagnosed with an early stage cancer were recruited while treatments were in progress (chemotherapy, radiotherapy, and/or hormone therapy). They were included after oncologist agreement and if no contraindication to physical exercise or comorbidities (e.g., neurological, muscular, skeletal disorders, or other conditions that would influence their sensorimotor performances) have been identified. Written informed consent was obtained from the participants before inclusion. This study is a feasibility study of the BIOCARE Factory study (ClinicalTrials.gov, NCT04391543, May 2020; (Chartogne et al. 2021a, b) and has been approved by the French ethics committee of human research CPP SUD EST VI (IDRCB: 2019-A02525-52) and will be performed according to the Declaration of Helsinki. Participants were required to answer the EORTC QLQ-C30 self-assessment questionnaire alone at home in quiet conditions. This questionnaire assesses several aspects of quality of life, notably CRF through a specific symptom scale (FA) with a score ranging from 0 to 100, with higher level indicating greater degree of CRF. A cutoff value of 39 is used for the FA score to identify clinically relevant CRF symptoms (Giesinger et al. 2016). This value has been used to divide participants in two groups (i.e., fatigued group, with FA score ≥ 39 or non-fatigued group, with FA score < 39) (Table 1).
Experimental protocol
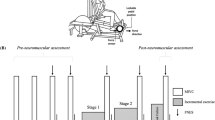
Throughout the test (Fig. 1), participants remained lie prone on a patient table, with a fully extended knee and an ankle angle of 90°, their foot securely blocked at the metatarsal level in a custom-made device enabling isometric strength measurement with a load cell (LSB350, Futek, Irvine, USA). First, optimal electrical stimulation intensity was determined to set the supramaximal intensity used during the subsequent neuromuscular assessments by progressively increasing the current (from 20 to 200 mA, with a 20-mA increment) until there was no further increase in the evoked isometric twitch response. The last intensity obtained was further increased by 20% to ensure stimulus supramaximality (optimal intensity: 150 ± 28 mA). All electrical stimulations were delivered with a constant current (Digitimer DS7A-H, Hertfordshire, UK) using squarewave stimuli of 200 µs duration with a maximal voltage of 400 V and via rectangular self-adhesive electrodes (5 × 10 cm, Compex). The cathode was placed over the gastrocnemii (~ 5 cm distal to the popliteal fossa) and the anode over the soleus (~ 10 cm proximal to the calcaneus).
Second, participants have to accomplish a standardized warm-up (ten isometric contractions of 4 s at 50% of their maximal self-estimated maximal strength with 4 s of recovery in between), followed after 2 min of rest by the MVC measurement (two MVC of 4 s separated by 2 min, if the difference between these MVCs was superior to 5%, a third one was performed). The higher peak force produced was considered as the MVC in baseline conditions (pre-fatigue MVC). Pre-fatigue neuromuscular functions were assessed on a third MVC, using a 100-Hz doublet on force plateau and a stimulation sequence on the relaxed muscles beginning 2 s after the end of contraction: a 100-Hz doublet, a 10-Hz doublet, and a simple stimulation, interspersed by 3 s.
Then participants were asked to complete a fatiguing exercise composed of 62 isometric MVC in ankle plantar flexor with the dominant leg. Each MVC last 4 s with 1 s rest, duty cycle was ensured using a metronome to provide subjects contraction and relaxation times. To avoid pacing strategies, participants were kept uninformed of the time remaining or the number of MVC performed (Tucker 2009) and investigators used verbal encouragements for participants to contract as strong as possible and maintain the maximal contraction until the end of the 4-s period during each MVC. Post-fatigue neuromuscular functions were assessed on the last MVC (i.e., 62nd) of the fatiguing exercise with the same stimulation procedure as in pre-fatigue condition. In case of missing or electrical stimulation applied improperly in the last MVC, neuromuscular assessments were also performed on the 60th MVC (i.e., 100-Hz doublet electrical stimulation during and after the 60th MVC), but finally all post-exercise data were computed from electrical stimulations applied to the 62nd MVC.
Besides, to evaluate the rapid force capacity before and after the fatiguing exercise, three explosive contractions (“flash”) were performed on 1 s period (15 s rest between each), with the instructions given to participants to produce the most important contraction as fast as possible (Maffiuletti et al. 2016). The first flash contraction started 5 s after the last electrical stimulation associated with the 62nd MVC of the fatiguing exercise.
Data analysis
Peak force (Fpeak; expressed in percentage of MVC) occurring during each 4-s MVC set was recorded and fitted using non-linear regression techniques in function of time for each participant (Eq. 1) (Hendrix et al. 2009). Fittings were performed via non-linear least-squares procedures with Matlab 2016a (The MathWorks, Natick, MA, USA), i.e., an iterative process was used to minimize the sum of squared error between the fitted function and the observed values:
where FA is the force–time asymptote relationship expressed in percentage of the pre-fatigue MVC; t the time in seconds; and τ the curvature constant in seconds, indicating the rate of force decrease.
Amplitudes of the superimposed and potentiated doublets force responses were also determined to enable the voluntary activation (VA) calculation. Since superimposed stimulations were not always elicited directly at MVC peak force, a correction factor for determination of VA was adopted (Strojnik and Komi 1998), as:
where MVC is the maximal force before the superimposed stimulation; FS the force when superimposed stimulation was applied; ST the difference between MVC and the force induced by the superimposed stimulation; and Db100 the force produced by the potentiated stimulation in resting conditions.
To investigate neuromuscular regulations, a correlation analysis of voluntary activation-contractile properties was performed, but VA being dependent on Db100 (Eq. 2), the central activation ratio (CAR) (Kent-Braun 1997) was used instead. CAR was then calculated as:
where MVC is the maximal force before the superimposed stimulation and FS the force when superimposed stimulation was applied.
The presence of post-exercise low-frequency fatigue (LFF) was evaluated from the ratio of forces evoked at low- and high-frequency stimulations (i.e., 10 Hz and 100 Hz) in resting conditions (Millet et al. 2011), as:
where Db10 is the force evoked at 10 Hz potentiated stimulation; and Db100 the force evoked at 100 Hz potentiated stimulation.
The RFD was calculated as the means slope of the force–time curve, normalized to pre-fatigue MVC, on 0–50 (RFD50) and 50–100 ms (RFD100), corresponding to the early and the intermediate/advanced phases of the contraction, respectively. The mean of the three flash contractions were computed for each condition (i.e., pre- or post-fatiguing exercise, and 0–50 or 50–100 ms interval) (Maffiuletti et al. 2016). All presented neuromuscular and RFD parameters were also expressed as pre–post-fatiguing exercise differences and normalized to pre-fatiguing exercise values (i.e., ΔVA, ΔCAR, and ΔDb100).
Statistical analysis
All data were analyzed with JASP (JASP Team, University of Amsterdam, Netherlands) and expressed as means ± standard deviations (SD). Normality of the variables of interest was tested using Shapiro–Wilk tests and homogeneity of variance was checked using Levene’s tests. To compare anthropometric and clinical data, pre-fatigue MVC, τ and FA between fatigued and non-fatigued groups, Student’s t tests for independent samples have been realized. Then an analysis of variance (ANOVA) for repeated measures with two factors (i.e., pre–post and between-group) was performed to determine whether some significant differences and interactions between factors existed in neuromuscular parameters (i.e., VA, Db100, LFF), RFD50 and RFD100. Linear correlation was performed to verify the relationship between ΔDb100 and ΔCAR variances characterized by the correlation coefficient (r). Correlation coefficient values of 0.00–0.19, 0.20–0.39, 0.40–0.59, 0.60–0.79, and > 0.79 were classified as very weak, weak, moderate, strong, and very strong, respectively (Evans 1996). A Student’s t tests for independent samples were used to compare healthy participants having performed the same fatiguing exercise in a previous study (Chartogne et al. 2020) and cancer patients from this study. For all tests, an alpha level for statistical significance of 0.05 was selected.
Results
Between-group comparison
Two patients were excluded due to incomplete data (i.e., missing questionnaires and incomplete neuromuscular tests due to discomfort of electrical stimuli). The remaining 18 participants were classified as fatigued (n = 9) or not fatigued (n = 9), to perform group comparison. Participants from fatigued group presented significant higher body mass and body mass index (BMI) (Table 1). Fatigued group had a significant lower FA than non-fatigued one (63.0 ± 11.4%MVC vs. 74.6 ± 9.6%MVC, respectively; p = 0.03) (Fig. 2 and Fig. 3a). However, no significant difference was found between fatigued and non-fatigued groups for pre-fatigue MVC (419.2 ± 82.8 N vs. 332.2 ± 103.3 N, respectively; p = 0.07), for pre-fatigue MVC normalized to body mass (5.46 ± 1.02 N.kg−1 vs. 5.74 ± 2.12 N.kg−1, respectively; p = 0.72) (Fig. 3b) and for τ (12.26 ± 4.12 s and 13.27 ± 2.45 s, respectively; p = 0.72) (Fig. 2).
Pre–post-exercise comparison
Significant decreases were found between pre- and post-exercise VA and Db100 for both groups (Table 2). ΔVA and ΔDb100 were 30.9 ± 24.6% and 9.3 ± 33.3% for fatigued group respectively, and 19.6 ± 16.5% and 11.7 ± 23.7% for non-fatigued group. Regarding RFD, significant pre–post-exercise differences were evidenced for both groups in RFD50 and RFD100 (Table 2). No significant pre–post-exercise difference has been found for LFF and no interaction between groups and pre–post-exercise factors was observed. Significant negative correlations between ΔCAR and ΔDb100 were observed when all cancer patients of this study were gathered (r = – 0.63; p = 0.005) (Fig. 4a).
Relationship between central activation ratio (ΔCAR) and evoked forces (ΔDb100) considering all cancer patients (dark grey and light grey dots represent data for non-fatigued and fatigued patients, respectively) (a). Relationship between ΔCAR and ΔDb100 for all cancer patients of this study (dark grey dots and regression line), healthy participants from a previous study (light grey dots and regression line), and all participants gathered (dashed regression line). Dark and light grey squares represent mean data for cancer patients and healthy participants, respectively (b)
Comparison of the later ΔCAR and ΔDb100 correlation analysis with one previously obtained in healthy participants (Chartogne et al. 2020) (r = – 0.82; p < 0.001) (Fig. 4b) showed no statistical difference (ΔCAR = –0.4793 ΔDb100 + 30.075; ΔCAR = – 0.5377 ΔDb100 + 29.431; for the present study and Chartogne et al. (2020), respectively). Besides, significant differences were found between ΔDb100 (13.9 ± 22.2% vs. 33.3 ± 18.5%; p < 0.01) and age (54.3 ± 11.6 years vs. 23.5 ± 5.0 years; p < 0.001) but not ΔCAR (21.9 ± 18.9% vs. 14.1 ± 10.8%; p = 0.07) for cancer and healthy participants, respectively.
Discussion
The main purpose of this study was to determine whether fatigability amplitude and etiology evaluated during a 5-min all-out effort of the ankle plantar flexors were different between fatigued and non-fatigued cancer patients during treatments. Results showed that i) FA was significantly different between fatigued and non-fatigued cancer patients; ii) no significant differences regarding fatigability mechanisms and RFD were observed; and iii) voluntary activation and evoked force decrease were negatively correlated for all cancer patients whatever their CRF condition was.
Fatigued patients showed higher fatigability amplitude in ankle plantar flexors muscles after a 5-min fatiguing exercise compared to the non-fatigued one, but with similar rate of fatigability onset (i.e., τ) (Fig. 2). These results are in accordance with those of Veni et al. (2019) and Chartogne et al. (2021a, b), showing that higher CRF level were associated with increased fingers flexors fatigability after a 5-min fatiguing exercise. A greater decrease in maximal force-generating capacities has also been reported by Brownstein et al. (2022) after an incremental cycling exercise. Together, these results are in line with the previously established link between CRF, a chronic feeling of exhaustion in daily life, and fatigability (Twomey et al. 2017), which could be explained by numerous neuromuscular complications and functional impairment (e.g., loss of muscular strength, cachexia or physical deconditioning). Regarding fatigability etiology, the present study showed significant pre–post differences in VA and Db100 for both patient groups (Table 2), emphasizing the involvement of both central and peripheral neuromuscular mechanisms. Nevertheless, no significant differences were found between both groups, as evidenced after a sustained fatiguing exercise in a previous study (Neil et al. 2013), which also reported submaximal VA in pre-fatigue condition (Table 2). Significant pre–post-exercise differences were found in RFD50 and RFD100 for both patient groups (Table 2). However, there was no difference between fatigued and non-fatigued patients; thus, RFD of fatigued patients was as much altered as that of non-fatigued patients after a fatiguing exercise, even though fatigued patients are more fatigable (i.e., lower FA). Moreover, force-generating capacities during a rapid contraction seem equally impacted in cancer patients than in healthy participants (Boccia et al. 2018; Thorlund et al. 2008). Based on the results of the present study, no link between RFD and CRF can be highlighted. Lastly, fatigued group showed significant higher body mass and body mass index (BMI) than non-fatigued group (Table 1). Moreover, mean BMI of fatigued group indicates a tendency to be overweight (Nuttall 2015), in accordance with previous studies (Bower 2014; Donovan and Jacobsen 2007), which found significant correlation between CRF and BMI during and after treatments. This increase in body mass could be part of the side effects of some treatments (e.g., chemotherapy and hormone therapy) (Thomson and Reeves 2017) or due to a reduced spontaneous physical activity during cancer (Veni et al. 2019).
Interestingly, cancer patients showed significant lower evoked forces decrease but no difference in voluntary activation decrease compared to healthy participants from a previous study with the same protocol (Chartogne et al. 2020). Several studies reported different results with predominant central mechanisms in cancer patient concluding that they are the primary cause of fatigability in cancer patients (Cai et al. 2014; Hucteau et al. 2023; Kisiel-Sajewicz et al. 2012; Yavuzsen et al. 2009). Hucteau et al. (2023), despite similar fatiguing task but involving different muscles, reported higher voluntary activation decrease for breast cancer patients associated with similar evoked forces decrease compared to age-matched healthy women. One may hypothesize that the significant difference in evoked forces decrease observed in our study between cancer and healthy participants can be due, at least in part, to the significant age difference, but studies investigating age-related differences in neuromuscular amplitude and etiology found no differences between young (< 35 years) and old (> 60 years) participants (Krüger et al. 2018; Varesco et al. 2022). We also evidenced similar negative correlation between voluntary activation and evoked forces decreases for cancer patients and healthy participants (Fig. 4). The ΔVA – ΔDb100 regression equation being not different between both populations suggests that potential regulation between central and peripheral dimensions of fatigability does not differ, but that patients are on average found to have greater central fatigability and preserved peripheral capacity. It is important to note that there is considerable individual variability, of an order of magnitude far greater than the difference between the two populations. These results echo to the sensory tolerance limit concept (Gandevia 2001; Hureau et al. 2016), a negative feedback loop integrating muscles sensory afferents and/or corollary discharge associated with the central motor command. When this hypothetical limit is reached, descending motor drive is limited to prevent intolerable peripheral perturbations.
This study, illustrating the link between CRF and fatigability, is the first, to our knowledge, investigating neuromuscular mechanisms of CRF during treatment stage. From a clinical applications point of view, this seems particularly relevant since interventions for the management of CRF have been recommended from cancer treatment period (Campbell et al. 2019; Oberoi et al. 2018) and to be tailored to patient comorbidities and treatment-induced adverse effects (Twomey et al. 2018; van der Leeden et al. 2018). Thus, it could be hypothesized that physical activity in patients with relevant fatigability could enhance the resistance to acute exercise in daily living and prevent fatigue accumulation. Thus, CRF would be alleviated during treatments and his long-term trajectory altered (Bower et al. 2021). This hypothesis is supported by de Lima et al. (2020) who observed, in breast cancer patients, a reduced fatigability after 30 maximal isokinetic knee extensions concomitant to a decreased CRF following a 10-week strength training protocol. Moreover, the link between CRF and fatigability could explain why physical activity remain the most effective, with a moderate effect, among the most commonly recommended treatments for CRF (i.e., physical activity, psychological, combined physical activity and psychological, and pharmaceutical treatment) (Mustian et al. 2017).
Conclusion
To conclude, results of the present study indicate that, during treatments, fatigability after a 5-min all-out exercise in ankle plantar flexor muscles is higher in fatigued patients, demonstrating the links between CRF and fatigability. Contrary to our hypotheses, this study has not revealed any significant differences regarding central and peripheral fatigability mechanisms between both fatigued and non-fatigued groups. However, the voluntary activation and evoked force decrease are negatively correlated when all cancer patients were gathered, so descending motor drive may be regulated to prevent peripheral perturbations. Furthermore, there were no differences in rapid force production during a short and rapid contraction between fatigued and non-fatigued cancer patients. Thus, these results, taken in association with previous studies, provide valuable information for supportive care professionals who seek to tailor interventions for CRF management, especially in physical activity treatment.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- CAR:
-
Central activation ratio
- CRF:
-
Cancer-related fatigue
- Db10 :
-
10-Hz doublet electrical stimulation
- Db100 :
-
100-Hz doublet electrical stimulation
- EORTC:
-
European Organization for Research and Treatment of Cancer
- F A :
-
Force–time relationship asymptote
- F peak :
-
Peak force occurring during a muscular contraction
- FS:
-
Force when the superimposed electrical stimulation was applied
- MVC:
-
Maximal voluntary contraction
- RFD:
-
Rate of force development
- SD:
-
Standard deviation
- ST:
-
Difference between the force produced during a maximal voluntary contraction and the force induced by a superimposed electrical stimulation applied on this later
- VA:
-
Voluntary activation
References
Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P (2002) Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol 93(4):1318–1326. https://doi.org/10.1152/japplphysiol.00283.2002
Abdalla LHP, Denadai BS, Bassan NM, Greco CC (2018) Exercise tolerance during muscle contractions below and above the critical torque in different muscle groups. Appl Physiol Nutr Metab 43(2):174–179. https://doi.org/10.1139/apnm-2017-0381
Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, National comprehensive cancer network (2015) Cancer-related fatigue, version 2.2015. J Natl Compr Cancer Netw 13(8):1012–1039. https://doi.org/10.6004/jnccn.2015.0122
Boccia G, Dardanello D, Brustio PR, Tarperi C, Festa L, Zoppirolli C, Rainoldi A (2018) Neuromuscular fatigue does not impair the rate of force development in ballistic contractions of submaximal amplitudes. Front Physiol 9:1503. https://doi.org/10.3389/fphys.2018.01503
Bower JE (2014) Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 11(10):597–609. https://doi.org/10.1038/nrclinonc.2014.127
Bower JE, Ganz PA, Irwin MR, Cole SW, Garet D, Petersen L, Crespi CM (2021) Do all patients with cancer experience fatigue? A longitudinal study of fatigue trajectories in women with breast cancer. Cancer. https://doi.org/10.1002/cncr.33327
Brownstein CG, Twomey R, Temesi J, Wrightson JG, Martin T, Medysky ME, Millet GY (2021) Physiological and psychosocial correlates of cancer-related fatigue. J Cancer Surviv. https://doi.org/10.1007/s11764-021-01115-6
Brownstein CG, Twomey R, Temesi J, Medysky ME, Culos-Reed SN, Millet GY (2022) Mechanisms of neuromuscular fatigability in people with cancer-related fatigue. Med Sci Sports Exerc 54(8):1355–1363. https://doi.org/10.1249/MSS.0000000000002919
Burnley M (2009) Estimation of critical torque using intermittent isometric maximal voluntary contractions of the quadriceps in humans. J Appl Physiol 106(3):975–983. https://doi.org/10.1152/japplphysiol.91474.2008
Cai B, Allexandre D, Rajagopalan V, Jiang Z, Siemionow V, Ranganathan VK, Yue GH (2014) Evidence of significant central fatigue in patients with cancer-related fatigue during repetitive elbow flexions till perceived exhaustion. PLoS ONE 9(12):e115370. https://doi.org/10.1371/journal.pone.0115370
Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, Schmitz KH (2019) Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 51(11):2375–2390. https://doi.org/10.1249/MSS.0000000000002116
Chartogne M, Rahmani A, Nicolon L, Jubeau M, Morel B (2020) Neuromuscular fatigability amplitude and aetiology are interrelated across muscles. Exp Physiol 105(10):1758–1766. https://doi.org/10.1113/EP088682
Chartogne M, Leclercq A, Beaune B, Boyas S, Forestier C, Martin T, Morel B (2021a) Building a biopsychosocial model of cancer-related fatigue: the BIOCARE FActory cohort study protocol. BMC Cancer 21(1):1140. https://doi.org/10.1186/s12885-021-08831-3
Chartogne M, Rahmani A, Landry S, Bourgeois H, Peyrot N, Morel B (2021b) Neuromuscular, psychological, and sleep predictors of cancer-related fatigue in cancer patients. Clin Breast Cancer 21(5):425–432. https://doi.org/10.1016/j.clbc.2020.12.002
D’Emanuele S, Maffiuletti NA, Tarperi C, Rainoldi A, Schena F, Boccia G (2021) Rate of force development as an indicator of neuromuscular fatigue: a scoping review. Front Hum Neurosci 15:701916. https://doi.org/10.3389/fnhum.2021.701916
de Lima FD, Battaglini CL, Chaves SN, Ugliara L, Sarandy J, Lima RM, Bottaro M (2020) Effect of strength training and antioxidant supplementation on perceived and performance fatigability of breast cancer survivors - a randomized, double-blinded, placebo-controlled study. Appl Physiol Nutr Metab. https://doi.org/10.1139/apnm-2020-0166
Donovan KA, Jacobsen PB (2007) Fatigue, depression, and insomnia: evidence for a symptom cluster in cancer. Semin Oncol Nurs 23(2):127–135. https://doi.org/10.1016/j.soncn.2007.01.004
Enoka RM, Stuart DG (1992) Neurobiology of muscle fatigue. J Appl Physiol 72(5):1631–1648. https://doi.org/10.1152/jappl.1992.72.5.1631
Evans JD (1996) Straightforward Statistics for the Behavioral Sciences. Brooks/Cole Pub. Co, Pacific Grove
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81(4):1725–1789. https://doi.org/10.1152/physrev.2001.81.4.1725
Giesinger JM, Kuijpers W, Young T, Tomaszewski KA, Friend E, Zabernigg A, Aaronson NK (2016) Thresholds for clinical importance for four key domains of the EORTC QLQ-C30: physical functioning, emotional functioning, fatigue and pain. Health Qual Life Outcomes 14:87. https://doi.org/10.1186/s12955-016-0489-4
Grisold W, Grisold A, Löscher WN (2016) Neuromuscular complications in cancer. J Neurol Sci 367:184–202. https://doi.org/10.1016/j.jns.2016.06.002
Hendrix CR, Housh TJ, Mielke M, Zuniga JM, Camic CL, Johnson GO, Schmidt RJ (2009) Critical torque, estimated time to exhaustion, and anaerobic work capacity from linear and nonlinear mathematical models. Med Sci Sports Exerc 41(12):2185–2190. https://doi.org/10.1249/MSS.0b013e3181ab8cc0
Hucteau E, Mallard J, Pivot X, Schott R, Pflumio C, Trensz P, Hureau TJ (2023) Exacerbated central fatigue and reduced exercise capacity in early-stage breast cancer patients treated with chemotherapy. Eur J Appl Physiol 123(7):1567–1581. https://doi.org/10.1007/s00421-023-05177-5
Hureau TJ, Romer LM, Amann M (2016) The “sensory tolerance limit”: a hypothetical construct determining exercise performance? Eur J Sport Sci 18(1):13–24. https://doi.org/10.1080/17461391.2016.1252428
Izquierdo M, Aguado X, Gonzalez R, López JL, Häkkinen K (1999) Maximal and explosive force production capacity and balance performance in men of different ages. Eur J Appl Physiol 79(3):260–267. https://doi.org/10.1007/s004210050504
Kent-Braun JA (1997) Noninvasive measures of central and peripheral activation in human muscle fatigue. Muscle Nerve Suppl 5:S98-101
Kisiel-Sajewicz K, Davis MP, Siemionow V, Seyidova-Khoshknabi D, Wyant A, Walsh D, Yue GH (2012) Lack of muscle contractile property changes at the time of perceived physical exhaustion suggests central mechanisms contributing to early motor task failure in patients with cancer-related fatigue. J Pain Symptom Manag 44(3):351–361. https://doi.org/10.1016/j.jpainsymman.2011.08.007
Kluger BM, Krupp LB, Enoka RM (2013) Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology 80(4):409–416. https://doi.org/10.1212/WNL.0b013e31827f07be
Krüger RL, Aboodarda SJ, Samozino P, Rice CL, Millet GY (2018) Isometric versus dynamic measurements of fatigue: does age matter? A meta-analysis. Med Sci Sports Exerc 50(10):2132–2144. https://doi.org/10.1249/MSS.0000000000001666
MacIntosh BR, Rassier DE (2002) What is fatigue? Can J Appl Physiol 27(1):42–55
Maffiuletti NA, Bizzini M, Widler K, Munzinger U (2010) Asymmetry in quadriceps rate of force development as a functional outcome measure in TKA. Clin Orthop Relat Res 468(1):191–198. https://doi.org/10.1007/s11999-009-0978-4
Maffiuletti NA, Aagaard P, Blazevich AJ, Folland J, Tillin N, Duchateau J (2016) Rate of force development: physiological and methodological considerations. Eur J Appl Physiol 116(6):1091–1116. https://doi.org/10.1007/s00421-016-3346-6
Millet GY, Martin V, Martin A, Vergès S (2011) Electrical stimulation for testing neuromuscular function: from sport to pathology. Eur J Appl Physiol 111(10):2489–2500. https://doi.org/10.1007/s00421-011-1996-y
Millet GY, Bertrand MF, Lapole T, Féasson L (2023) Measuring objective fatigability and autonomic dysfunction in clinical populations: how and why? Front Sports Act Living. https://doi.org/10.3389/fspor.2023.1140833
Monod H, Scherrer J (1965) The work capacity of a synergic muscular group. Ergonomics 8(3):329–338. https://doi.org/10.1080/00140136508930810
Morel B, Rouffet DM, Saboul D, Rota S, Clémençon M, Hautier CA (2015) Peak torque and rate of torque development influence on repeated maximal exercise performance: contractile and neural contributions. PLoS ONE 10(4):e0119719. https://doi.org/10.1371/journal.pone.0119719
Morris R, Lewis A (2020) Falls and cancer. Clin Oncol 32(9):569–578. https://doi.org/10.1016/j.clon.2020.03.011
Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, Miller SM (2017) Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol 3(7):961–968. https://doi.org/10.1001/jamaoncol.2016.6914
Neil SE, Klika RJ, Garland SJ, McKenzie DC, Campbell KL (2013) Cardiorespiratory and neuromuscular deconditioning in fatigued and non-fatigued breast cancer survivors. Support Care Cancer 21(3):873–881. https://doi.org/10.1007/s00520-012-1600-y
Neyroud D, Temesi J, Millet GY, Verges S, Maffiuletti NA, Kayser B, Place N (2015) Comparison of electrical nerve stimulation, electrical muscle stimulation and magnetic nerve stimulation to assess the neuromuscular function of the plantar flexor muscles. Eur J Appl Physiol 115(7):1429–1439. https://doi.org/10.1007/s00421-015-3124-x
Nuttall FQ (2015) Body mass index: obesity, BMI, and health: a critical review. Nutr Today 50(3):117–128. https://doi.org/10.1097/NT.0000000000000092
Oberoi S, Robinson PD, Cataudella D, Culos-Reed SN, Davis H, Duong N, Sung L (2018) Physical activity reduces fatigue in patients with cancer and hematopoietic stem cell transplant recipients: a systematic review and meta-analysis of randomized trials. Crit Rev Oncol Hematol 122:52–59. https://doi.org/10.1016/j.critrevonc.2017.12.011
Place N, Millet GY (2020) Quantification of neuromuscular fatigue what do we do wrong and why? Sports Med 50(3):439–447. https://doi.org/10.1007/s40279-019-01203-9
Prinsen H, van Dijk JP, Zwarts MJ, Leer JWH, Bleijenberg G, van Laarhoven HWM (2015) The role of central and peripheral muscle fatigue in postcancer fatigue: a randomized controlled trial. J Pain Symptom Manag 49(2):173–182. https://doi.org/10.1016/j.jpainsymman.2014.06.020
Schmitt AC, Repka CP, Heise GD, Challis JH, Smith JD (2017) Comparison of posture and balance in cancer survivors and age-matched controls. Clin Biomech 50:1–6. https://doi.org/10.1016/j.clinbiomech.2017.09.010
Strojnik V, Komi PV (1998) Neuromuscular fatigue after maximal stretch-shortening cycle exercise. J Appl Physiol 84(1):344–350. https://doi.org/10.1152/jappl.1998.84.1.344
Thomson ZO, Reeves MM (2017) Can weight gain be prevented in women receiving treatment for breast cancer? A systematic review of intervention studies. Obes Rev. https://doi.org/10.1111/obr.12591
Thorlund JB, Michalsik LB, Madsen K, Aagaard P (2008) Acute fatigue-induced changes in muscle mechanical properties and neuromuscular activity in elite handball players following a handball match. Scand J Med Sci Sports 18(4):462–472. https://doi.org/10.1111/j.1600-0838.2007.00710.x
Tucker R (2009) The anticipatory regulation of performance: the physiological basis for pacing strategies and the development of a perception-based model for exercise performance. Br J Sports Med 43(6):392–400. https://doi.org/10.1136/bjsm.2008.050799
Twomey R, Aboodarda SJ, Kruger R, Culos-Reed SN, Temesi J, Millet GY (2017) Neuromuscular fatigue during exercise: Methodological considerations, etiology and potential role in chronic fatigue. Neurophysiol Clinique Clin 47(2):95–110. https://doi.org/10.1016/j.neucli.2017.03.002
Twomey R, Martin T, Temesi J, Culos-Reed SN, Millet GY (2018) Tailored exercise interventions to reduce fatigue in cancer survivors: study protocol of a randomized controlled trial. BMC Cancer 18(1):757. https://doi.org/10.1186/s12885-018-4668-z
van der Leeden M, Huijsmans RJ, Geleijn E, de Rooij M, Konings IR, Buffart LM, Stuiver MM (2018) Tailoring exercise interventions to comorbidities and treatment-induced adverse effects in patients with early stage breast cancer undergoing chemotherapy: a framework to support clinical decisions. Disabil Rehabil 40(4):486–496. https://doi.org/10.1080/09638288.2016.1260647
Varesco G, Luneau E, Féasson L, Lapole T, Rozand V (2022) Very old adults show impaired fatigue resistance compared to old adults independently of sex during a knee-extensors isometric test. Exp Gerontol 161:111732. https://doi.org/10.1016/j.exger.2022.111732
Veni T, Boyas S, Beaune B, Bourgeois H, Rahmani A, Landry S, Morel B (2019) Handgrip fatiguing exercise can provide objective assessment of cancer-related fatigue: a pilot study. Support Care Cancer 27(1):229–238. https://doi.org/10.1007/s00520-018-4320-0
Weis J (2011) Cancer-related fatigue: prevalence, assessment and treatment strategies. Expert Rev Pharmacoecon Outcomes Res 11(4):441–446. https://doi.org/10.1586/erp.11.44
Yavuzsen T, Davis MP, Ranganathan VK, Walsh D, Siemionow V, Kirkova J, Yue GH (2009) Cancer-related fatigue: central or peripheral? J Pain Symptom Manag 38(4):587–596. https://doi.org/10.1016/j.jpainsymman.2008.12.003
Acknowledgements
The authors thank all participants for the time allowed and their involvement in this study.
Funding
This work was supported by La Ligue contre le Cancer (thesis scholarship from the first author) and La Région Pays de la Loire (BIOCARE FActory project).
Author information
Authors and Affiliations
Contributions
MC, AR, and BM conceived and designed research. MC and SL conducted experiments. MC, AR, and BM analyzed data. MC wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Communicated by Michalis G Nikolaidis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chartogne, M., Rahmani, A., Landry, S. et al. Comparison of neuromuscular fatigability amplitude and etiologies between fatigued and non-fatigued cancer patients. Eur J Appl Physiol 124, 1175–1184 (2024). https://doi.org/10.1007/s00421-023-05347-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05347-5