Abstract
Purpose
Fatigue is one of the most commonly reported side effects during treatment for breast cancer and can persist following treatment completion. Cancer-related fatigue after treatment is multifactorial in nature, and one hypothesized mechanism is cardiorespiratory and neuromuscular deconditioning. The purpose of this study was to compare cardiorespiratory and neuromuscular function in breast cancer survivors who had completed treatment and met the specified criteria for cancer-related fatigue and a control group of breast cancer survivors without fatigue.
Methods
Participants in the fatigue (n = 16) and control group (n = 11) performed a maximal exercise test on a cycle ergometer for determination of peak power, power at lactate threshold, and VO2 peak. Neuromuscular fatigue was induced with a sustained submaximal contraction of the right quadriceps. Central fatigue (failure of voluntary activation) was evaluated using twitch interpolation, and peripheral fatigue was measured with an electrically evoked twitch.
Results
Power at lactate threshold was lower in the fatigue group (p = 0.05). There were no differences between groups for power at lactate threshold as percentage of peak power (p = 0.10) or absolute or relative VO2 peak (p = 0.08 and 0.33, respectively). When adjusted for age, the fatigue group had a lower power at lactate threshold (p = 0.02) and absolute VO2 peak (p = 0.03). There were no differences between groups in change in any neuromuscular parameters after the muscle-fatiguing protocol.
Conclusions
Findings support the hypothesis that cardiorespiratory deconditioning may play a role in the development and persistence of cancer-related fatigue following treatment. Future research into the use of exercise training to reduce cardiorespiratory deconditioning as a treatment for cancer-related fatigue is warranted to confirm these preliminary findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
As survival in women diagnosed with early stage breast cancer improves, addressing the long-term and late effects of breast cancer and its treatment is of great importance to the health and quality of life of survivors. During adjuvant treatment (chemotherapy and/or radiation), fatigue is one of the most commonly reported side effects[1, 2]. Fatigue usually diminishes following treatment completion; however, in a subgroup of breast cancer survivors, fatigue may persist for months or years following completion of adjuvant treatment, which has a negative impact on quality of life [3–5]. Termed cancer-related fatigue due to its relation to cancer and/or its treatment, the etiology is largely unknown. It is most likely multifactorial, and the result of a combination of both physiological and psychological factors [4, 6, 7].
Deconditioning, a loss of functional capacity due to lack of physical activity, of the cardiorespiratory and muscular systems [8] is one potential contributor to persistent cancer-related fatigue posttreatment. Physical activity levels tend to decline during treatment for breast cancer and remain lower even after treatment is completed [9]. Low levels of physical activity combined with systemic effects of adjuvant treatment leads to a decrease in cardiorespiratory fitness, making both structured physical activity and everyday physical tasks more difficult [8]. A maximal exercise test is the gold standard assessment of cardiorespiratory fitness via evaluation of peak oxygen consumption (VO2 peak). A second component of cardiorespiratory fitness is lactate threshold. At workloads below an individual’s lactate threshold, lactate is produced and metabolized at equal rates, and blood lactate concentration is relatively stable. As exercise intensity surpasses an individual’s lactate threshold, blood lactate concentration increases rapidly. The exercise intensity at lactate threshold is regarded as the upper border of physical effort that can be sustained for extended periods of time, as the energy that can be generated using the anaerobic system is limited [10, 11]. A rightward shift of this blood lactate curve (i.e., threshold reached at higher absolute exercise intensity) indicates an increase in cardiorespiratory fitness (for a recent review of lactate threshold concepts, see Faude et al. [12]). A lower VO2 peak has been shown to correlate to levels of fatigue levels in cancer survivors posttreatment [13]. Lactate threshold, which may have a more functional impact on daily submaximal tasks, has not been studied in breast cancer survivors who complain of persistent fatigue posttreatment.
In addition to cardiorespiratory fitness, changes in neuromuscular function have also been examined as potential contributors to cancer-related fatigue [14, 15]. Causes of neuromuscular fatigue can be classified as being centrally or peripherally mediated. Twitch interpolation has been used to determine whether central mechanisms are limiting performance [16]. Central fatigue, quantified by measuring voluntary activation, is the result of a loss of voluntary muscle activation due so mechanisms proximal to the neuromuscular junction, such as reduced neural drive, motor unit recruitment, or firing rates [16–18]. Peripheral fatigue, characterized by a slowing of the contraction and quantified by the half-relaxation time of the resting twitch, includes failure of excitation–contraction coupling or metabolic changes within the muscle tissue itself [16–18]. It has been hypothesized that altered neuromuscular function due to breast cancer treatment may contribute to cancer-related fatigue [6, 14]. Yavuzsen et al. [14] used twitch interpolation to compare fatigued cancer patients entering palliative care to noncancer controls, reporting greater central fatigue and impaired neuromuscular junction conduction in the cancer patient group. Neuromuscular function has not been examined as a contributor to cancer-related fatigue using a control group who had also undergone cancer treatment.
The purpose of this study was to compare cardiorespiratory and neuromuscular components of fatigue in breast cancer survivors who were experiencing persistent cancer-related fatigue (fatigued group) and breast cancer survivors who have also undergone breast cancer treatment but do not report experiencing persistent cancer-related fatigue (control group) to determine whether breast cancer survivors with fatigue are more deconditioned posttreatment than women who do not report fatigue. We hypothesized that the fatigued group would have a lower lactate threshold (in terms of power output and as a percentage of peak power output), lower VO2 peak, and greater decline in voluntary activation (central fatigue) following a sustained isometric contraction, compared to the control group.
Materials and methods
Population
Women previously diagnosed with stage I–IIIa breast cancer and who had completed adjuvant treatment (including radiation, with or without chemotherapy) for ≥3 months but <5 years were recruited. The fatigued group self-reported persistent fatigue following completion of treatment, and during an initial phone screen, each participant was determined to have met the criteria developed by the Fatigue Coalition [19] for cancer-related fatigue of six or more of the listed symptoms nearly every day during the same 2-week period in the previous month. Those who self-reported fatigue but did not meet the defined criteria were excluded. The control group had undergone breast cancer treatment and reported not experiencing persistent fatigue after treatment and did not meet the defined criteria. Participants were ineligible if they responded yes to a diagnosis of or current treatment for clinical depression or responded yes to further prompting related to potentially undiagnosed depression. They were also excluded if they reported any comorbid condition that may contribute to fatigue or prevent completion of exercise testing (e.g., uncontrolled thyroid disorder, musculoskeletal injuries, previous myocardial infarction, or stroke). Participants were screened prior to exercise for any potential contraindications for exercise testing and received consent from their oncologist or family doctor prior to the study visit.
Participants were recruited through oncologist referral, poster advertising, and survivor groups in the Greater Vancouver area. Date of birth, type of treatment received, date of treatment completion, and current medications were collected by self-report. The University of British Columbia’s Clinical Research Ethics Board provided ethical approval and all participants provided written informed consent.
Testing day 1
Participants came to the lab for a 90-min visit on day 1. As a final screening step, 1.0 mL of venous blood was drawn to screen for anemia and analyzed using the I-Stat system (Abbott Laboratories, Abbott Park, IL, USA) [20]. Participants were excluded if they met the World Health Organization’s definition of anemia as a hemoglobin level of <12.0 dg/L [21].
Height and weight were recorded. Resting heart rate (Polar Electro, Lake Success, NY, USA) and blood pressure were assessed after a 5-min rest period in an upright, seated position. Resting blood lactate was measured via a prick of the distal tip of the third finger and analyzed by the Lactate Pro analyzer (Arkray, KDK Corporation, Kyoto, Japan) [22]. The finger was cleaned using an alcohol wipe and pricked using the Arkray Multi-Lancet II (KDK Corporation, Kyoto, Japan), and the first drop was swabbed away to remove any alcohol before the blood was collected for analysis.
Cycle ergometer test
A maximal incremental cycle ergometer test was performed using a protocol adapted from Klika et al. [23]. Participants warmed up with no resistance for 5 min. After warm-up, heart rate returned to resting level before the test began. During the warm-up, participants were asked how hard they felt they were working as well as their previous cycling experience to determine the appropriate rate of increase in watts during the test. The majority of participants (n = 11) followed the planned protocol of an increase of 20 W per stage; however, several were deemed to be less fit and better suited to an increase of 15 W (n = 4), or more fit and better suited to an increase of 25 W per stage (n = 8), to reduce test time as recommended by the American College of Sports Medicine [24]. The test consisted of two parts, i.e., part 1 to reach lactate threshold and part 2 to reach VO2 peak, in order to efficiently obtain both values from one incremental exercise test. Heart rate was recorded every 30 s and rating of perceived exertion recorded during the final 30 s of each stage. During part 1, stages were 3-min long to obtain an accurate measure of blood lactate, taken during the last minute of each stage. Part 2 of the test began once the measured blood lactate concentration had increased >1.0 mmol/L from baseline, followed by a subsequent increase of >1.0 mmol/L. During part 2 of the test, stages were reduced to 1-min duration. Blood lactate was recorded during the last 30 s of every other stage, and the test continued to volitional exhaustion, or when participants met two of three criteria for VO2 peak [24]. VO2 was measured by collection of expired gases using a Sensormedics metabolic cart (Yorba Linda, CA, USA). Lactate threshold was determined according to Coyle et al. [25] by plotting values of blood lactate versus power output and confirmed using a step-wise logistical regression [23]. A second investigator, blinded to group allocation, independently confirmed lactate threshold.
Testing day 2
Participants reported to the lab for a second testing visit, which lasted 45 min. Day 2 was scheduled at least 3 days but no longer 2 weeks after day 1. During this session, participants were secured in sitting on a Biodex dynamometer (Shirley, NY, USA). The right leg was placed in approximately 85° of hip flexion and 90° of knee flexion with the axis of the dynamometer aligned with the lateral epicondyle of the femur and the force transducer pad positioned against the anterior tibia. Electrical stimulation was applied through lead stimulating electrodes (10 × 5 cm) covered with gauze and soaked in saline solution. Stimulating electrodes were positioned over the motor points of the proximal rectus femoris and the distal vastus medialis. Stimulation was produced by a constant current stimulator (Digitimer DS7AH, Hertfordshire, UK) externally triggered to produce a doublet with frequency of 100 Hz. Each pulse was a rectangular pulse with 50-μs duration. The intensity of electrical stimulation used was determined for each individual participant before the experiment using a series of doublets of progressively greater current at rest until a maximal twitch force was obtained and adding 10 % to ensure supramaximal stimulation.
After familiarization with the protocol and two submaximal practice trials, participants performed a 3-s isometric maximum voluntary contraction of the quadriceps muscle. Next, a doublet was applied during a second maximal voluntary contraction once peak force had been obtained, followed by a doublet applied to the resting muscle. Participants then completed a muscle fatiguing protocol, consisting of a sustained isometric contraction at 30 % of maximum voluntary contraction force until volitional exhaustion. The target force level was displayed on the screen, and participants’ were verbally encouraged to maintain contraction as long as possible. Endurance time was recorded from the initiation of the submaximal contraction until voluntary exhaustion or failure to maintain target force for more than 5 s. Immediately following the termination of the sustained contraction, maximum voluntary contraction, interpolated twitch, and resting twitch were repeated. Force was digitized at 500 Hz and recorded using Spike2 data acquisition and analysis system (1401 Plus, Cambridge Electronic Design, Ltd., Cambridge, UK). Voluntary activation (to assess central fatigue) was calculated from the peak amplitude of the twitch force using the equation [voluntary activation = 100 (1 − interpolated twitch/resting twitch)] [16]. Maximum voluntary contraction peak force and half-relaxation time of the resting twitch (to quantify peripheral fatigue) were calculated offline.
Questionnaires
Participants were given the following questionnaires to complete at the end of day 1 testing and were asked to return completed questionnaires on day 2: the Functional Assessment of Cancer Therapy—Breast (FACT-B) to assess quality of life [26]; FACT-F (Fatigue) [27] to quantify magnitude of fatigue; and the Beck Depression Inventory (BDI-II) [28] to assess level of depression. Participants were administered a modified version of the Minnesota Leisure Time Physical Activity Questionnaire [29] to quantify usual physical activity.
Statistical analysis
Data were analyzed using SPSS 19.0 (IBM, Chicago, IL, USA), and the accepted level of significance was set at p < 0.05. Fisher’s exact test was used to test between group differences in treatment type (chemotherapy + radiation vs. radiation alone) and current use of antihormonal medication. A two-sided independent samples t test was performed to assess differences between groups on participant characteristics, cardiorespiratory outcomes, and neuromuscular outcomes. Secondary analysis was planned a priori, in which power at lactate threshold, lactate threshold as a percentage of peak power, and VO2 peak were plotted against potential covariates (age, body weight, time since treatment, and current levels of physical activity) to determine whether a relationship existed. If a relationship was evident, covariates were entered into an analysis of covariance (ANCOVA). Two-sided paired t tests were performed to determine within-group differences in voluntary activation, half-relaxation time of the resting twitch, and maximum voluntary contraction before and after muscle fatiguing exercise within each group.
Results
Participant characteristics
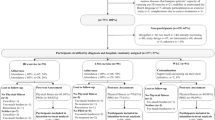
A convenience sample of 27 women were enrolled into the fatigued (n = 16) and nonfatigued (n = 11) groups (Fig. 1). Two participants (one from each group) were unable to attend the second visit, and one participant (control) did not complete the interpolated twitch measurements due to discomfort. No adverse events were reported from exercise or neuromuscular testing. While there were no statistically significant differences in physical characteristics, current use of antihormonal medication, or treatment between the groups, our small sample size does not allow enough statistical power to detect a true difference if one exists (Table 1). The fatigued group self-reported fewer metabolic equivalent (MET) hours and minutes of moderate to vigorous exercise per week, as well as a lower quality of life and higher levels of depression and fatigue. Scores for measures of fatigue were correlated with depression (r = −0.61) and quality of life (r = 0.88). While scores for depression were statistically different between the two groups, the mean scores for both groups were below the suggested cut-point for mild depression of 3. Four participants in the fatigued group and one in the control group fell within the category of mild depression [28].
Cardiorespiratory outcomes
Power at lactate threshold was lower in the fatigued group (61.9 ± 16.5 vs. 78.2 ± 25.2 W, p = 0.05). No significant differences were found between groups for lactate threshold as a percentage of peak power, or absolute (L/min) or relative (mL kg−1 min−1) VO2 peak (Table 2). Secondary analysis was performed to determine if age, body weight, exercise levels, or time since treatment had an effect on the cardiorespiratory outcomes. Older age was associated with a lower power at lactate threshold (r = −0.35) and lower absolute VO2 peak (r = −0.31). When age was adjusted for using ANCOVA, there was a difference between groups for power at lactate threshold (fatigued 60.5 ± 5.0 vs control 80.2 ± 6.1 W, p = 0.02) and absolute VO2 peak (fatigued 1.50 ± 0.09 vs. control 1.83 ± 0.11 L/min, p = 0.03).
Neuromuscular outcomes
Both groups demonstrated impaired voluntary activation before the sustained contraction. The control group demonstrated a significantly lower voluntary activation (central fatigue) before and after the sustained contraction than in the fatigue group, but groups had a similar percent change in voluntary activation following sustained contraction (Table 3). There were no significant differences between groups in half-relaxation time (peripheral fatigue) before and after the sustained contraction or percent change within each group. Groups experienced a comparable amount of neuromuscular fatigue as evidenced by a significant decline in the maximum voluntary contraction peak force following the sustained contraction and similar endurance time.
Discussion
These findings suggest that breast cancer survivors with persistent fatigue after treatment experience more cardiorespiratory deconditioning in the posttreatment period, but we found no differences in neuromuscular parameters between the two groups. When adjusted for age, the fatigued group had a lower VO2 peak than controls. A low VO2 peak is a sign of cardiorespiratory deconditioning, and individuals who are more deconditioned may experience more physical difficulty with daily tasks, which may contribute to fatigue. Exercise to improve VO2 peak has been used as an intervention to prevent and/or reduce fatigue [4, 30]; however, few randomized controlled trials of exercise have been conducted to specifically target breast cancer survivors who are experiencing persistent cancer-related fatigue in the post-treatment period [31]. Power at lactate threshold was significantly lower in breast cancer survivors who reported persistent cancer-related fatigue compared to those who did not.
We hypothesize that persistent fatigue may be the result of an individual reaching or exceeding their lactate threshold during their activities of daily living, as has been suggested for individuals with chronic fatigue syndrome [32]. While results from this study suggest that women in the fatigued group do exhibit a leftward shift of the blood lactate curve compared to controls, one must consider whether the magnitude of the shift is large enough to conclude that these women are exceeding their lactate threshold during activities of daily living. A rough estimate to assess this can be undertaken using an equation for energy expenditure during cycle ergometry [24] and the compendium of physical activities [33]. Using the age-adjusted results for power output at lactate threshold for each group and mean body weight of all participants, the fatigued group would reach lactate threshold during activities of approximately 3.6 METs and controls at activities of approximately 4.5 METs. Most daily household activities are given a MET score of 2.0–4.0, such as sweeping floors (3.3 METS), and multiple household tasks, moderate–vigorous effort (4.0 METs) [33]. Examples of physical activity in the range of 4.0–5.0 METs include walking briskly carrying objects <25 pounds (4.5 METS), and playing with children (5.0 METS). Based on these estimates, it is possible that fatigued individuals exceed their lactate threshold during activities of daily living. However, actual energy expenditure depends on body mass, adiposity, age, sex, environmental conditions, as well as personal effort, and therefore, individual differences in the actual energy cost of an activity can be large [33], and in order to determine whether our hypothesis is correct, we would need to assess intensity of activity in these women directly.
To date, the majority of studies have focused on cardiorespiratory fitness or the effect of aerobic exercise on cancer-related fatigue [34]; however, it has been hypothesized that neuromuscular parameters that may also be affected by cancer and/or its treatment. No difference in neuromuscular fatigue was noted between groups. Based on previous findings [14], we hypothesized that central mechanisms (reduced voluntary activation) following the muscle fatiguing protocol would be present in the fatigue group. However, there was no difference in percent change in voluntary activation between groups, and both groups had evidence of failure of voluntary activation. Previous research has shown that healthy adults (mean age, 56 years) are able to achieve voluntary activation of 92 % [35], which is substantially higher than the voluntary activation in the fatigued (87.5 %) and control (75 %) groups. As the target intensity for the sustained contraction was based on values obtained during the initial maximal voluntary contraction, the presence of impaired voluntary activation may have precipitated short endurance times and consequently only a tendency toward peripheral fatigue (p = 0.06 and 0.14, in the control and fatigue group, respectively). In the study by Yavuzsen et al. [14], the patient group included individuals undergoing treatment for various types of palliative cancer, and the control group was healthy individuals who had never undergone cancer treatment. Our findings suggest that the central fatigue found in other studies may be related to diagnosis and/or treatment for cancer but unrelated to cancer-related fatigue per se.
Of note are the low values for cardiorespiratory fitness (VO2 peak, peak power, and power at lactate threshold) in both groups despite high self-reported physical activity. Both groups exceeded the current physical activity guidelines for cancer survivors of 150 min a week of moderate–vigorous physical activity [36], although the control group self-reported almost twice as many minutes per week of exercise. Due to the cross-sectional design used in this study, it is not possible to determine if the differences found between the two groups are due to fatigue or due to differences in physical activity. This difference in levels of physical activity between groups may contribute as both a cause and/or effect of persistent fatigue with those who participate in regular moderate-vigorous exercise being less deconditioned, having higher energy levels and therefore not experiencing fatigue. Alternatively, those who experience cancer-related fatigue may be less likely to exercise, becoming further deconditioned, thus perpetuating the cycle of fatigue.
The study had two main strengths. First, it included an assessment of both cardiorespiratory and neuromuscular aspects of deconditioning, using gold standard techniques. Second, a clear definition for cancer-related fatigue was used, and individuals with other potential clinical causes of fatigue (i.e., anemia, clinical depression, and thyroid disorder) were excluded. This resulted in two distinct groups who were closely matched on other variables that may impact cancer-related fatigue.
This study has some limitations. First, the interpretation of our findings is limited by both a small sample size and cross-sectional study design. As this is one of the first investigations into the link between both cardiorespiratory and neuromuscular deconditioning as it relates to fatigue after breast cancer, it provides important information about the potential association between deconditioning and cancer-related fatigue and provides the rationale for future research in this area. Second, the assumptions regarding lactate threshold and physiological implications for activity at or above that threshold are based on healthy individuals. It is unknown whether breast cancer and/or its treatment have any effect on the physiological processes related to blood lactate during exercise. Additionally, measurement of neuromuscular outcomes may be limited by participants’ motivation to achieve a maximum muscle contraction or maximal endurance time. Some participants were apprehensive about the interpolated twitch and, as a result, may not have maximally contracted in anticipation of the twitch. While there may have been inherent differences between groups contributing to cancer-related fatigue, the fatigue group may have been more motivated as they were recruited to participate in a study to help determine potential underlying causes or contributors to cancer-related fatigue. In future studies, further familiarization may be warranted to ensure maximal voluntary contraction during the interpolated twitch.
In addition, interpretations of the results of this study are limited by the multifactorial nature of cancer-related fatigue. While deconditioning may be a primary cause of fatigue in some, psychological factors, presence of inflammatory cytokines, or other unknown variables may also play a greater or lesser role depending on the individual. For example, while those who had been diagnosed with clinical depression were excluded, scores on the BDI demonstrated higher scores for depression in the fatigued group, which approached the established cut-point of mild depression. Those who are depressed may have less motivation to engage in physical activity and thus will become further deconditioned, which speaks to the potential importance of psychological factors to cancer-related fatigue.
Our findings provide support for the relationship between cardiorespiratory deconditioning and the presence of persistent cancer-related fatigue following treatment for breast cancer. This may have implications for the use of exercise training to reduce fatigue in this population. In order to maximize the potential benefits of exercise in reducing cancer-related fatigue, the underlying mechanisms relating to the cause of fatigue and how exercise works to reduce it must be examined. These findings could be applied to future exercise interventions in breast cancer survivors, and researchers should consider incorporating an assessment of blood lactate and/or prescribing aerobic exercise relative to this threshold to ensure that participants are working at an intensity that will not worsen fatigue, but will result in an improvement in lactate threshold and VO2 peak.
References
Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR (2007) Cancer-related fatigue: the scale of the problem. Oncologist 12(Suppl 1):4–10
Holley S (2000) Cancer-related fatigue. Suffering a different fatigue. Cancer Pract 8(2):87–95
Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR (2000) Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol 18(4):743–753
Dimeo FC (2001) Effects of exercise on cancer-related fatigue. Cancer 92(6 Suppl):1689–1693
Geinitz H, Zimmermann FB, Thamm R, Keller M, Busch R, Molls M (2004) Fatigue in patients with adjuvant radiation therapy for breast cancer: long-term follow-up. J Cancer Res Clin Oncol 130(6):327–333. doi:10.1007/s00432-003-0540-9
Al-Majid S, McCarthy DO (2001) Cancer-induced fatigue and skeletal muscle wasting: the role of exercise. Biol Res Nurs 2(3):186–197
Morrow GR, Shelke AR, Roscoe JA, Hickok JT, Mustian K (2005) Management of cancer-related fatigue. Cancer Invest 23(3):229–239
Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR (2009) Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol 10(6):598–605. doi:10.1016/S1470-2045(09)70031-2
Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, Kriska A, Ballard-Barbash R (2003) Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer 97(7):1746–1757. doi:10.1002/cncr.11227
Wasserman K (1987) Determinants and detection of anaerobic threshold and consequences of exercise above it. Circulation 76(6 Pt 2):VI29–VI39
Wasserman K, Beaver WL, Whipp BJ (1990) Gas exchange theory and the lactic acidosis (anaerobic) threshold. Circulation 81(1 Suppl):II14–II30
Faude O, Kindermann W, Meyer T (2009) Lactate threshold concepts: how valid are they? Sports Med (Auckland, NZ) 39(6):469–490
Peterson BMR, Chris P, Dallow K, Hayward R, Schneider CM (2011) Cancer-related fatigue and the impact of psychological and physiological variables. Med Sci Sports Exerc 43(5):562
Yavuzsen T, Davis MP, Ranganathan VK, Walsh D, Siemionow V, Kirkova J, Khoshknabi D, Lagman R, LeGrand S, Yue GH (2009) Cancer-related fatigue: central or peripheral? J Pain Symptom Manage 38(4):587–596. doi:10.1016/j.jpainsymman.2008.12.003
Monga U, Jaweed M, Kerrigan AJ, Lawhon L, Johnson J, Vallbona C, Monga TN (1997) Neuromuscular fatigue in prostate cancer patients undergoing radiation therapy. Arch Phys Med Rehabil 78(9):961–966
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81(4):1725–1789
Enoka RM, Duchateau J (2008) Muscle fatigue: what, why and how it influences muscle function. J Physiol 586(1):11–23. doi:10.1113/jphysiol.2007.139477
Evans WJ, Lambert CP (2007) Physiological basis of fatigue. Am J Phys Med Rehabil 86(1 Suppl):S29–S46
Cella D, Davis K, Breitbart W, Curt G (2001) Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol 19(14):3385–3391
Schneider J, Dudziak R, Westphal K, Vettermann J (1997) The i-STAT analyzer. A new, hand-held device for the bedside determination of hematocrit, blood gases, and electrolytes. Anaesthesist 46(8):704–714
Patel KV (2008) Epidemiology of anemia in older adults. Semin Hematol 45(4):210–217. doi:10.1053/j.seminhematol.2008.06.006
Tanner RK, Fuller KL, Ross ML (2010) Evaluation of three portable blood lactate analysers: lactate pro, lactate scout and lactate plus. Eur J Appl Physiol 109(3):551–559. doi:10.1007/s00421-010-1379-9
Klika RJ, Golik KS, Drum SN, Callahan KE, Thorland WG (2011) Comparison of physiological response to cardiopulmonary exercise testing among cancer survivors and healthy controls. Eur J Appl Physiol 111(6):1167–1176. doi:10.1007/s00421-010-1749-3
American College of Sports M (2009) ACSM's guidelines for exercise testing and prescription, vol 8. Lippincott, Williams and Wilkins, Philadelphia, pp 91–134
Coyle EF, Martin WH, Ehsani AA, Hagberg JM, Bloomfield SA, Sinacore DR, Holloszy JO (1983) Blood lactate threshold in some well-trained ischemic heart disease patients. J Appl Physiol Respir Environ Exerc Physiol 54(1):18–23
Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G (1997) Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol 15(3):974–986
Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E (1997) Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 13(2):63–74
Beck AT, Steer RA (1984) Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol 40(6):1365–1367
Taylor HL, Jacobs DR Jr, Schucker B, Knudsen J, Leon AS, Debacker G (1978) A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 31(12):741–755
Drouin JS, Young TJ, Beeler J, Byrne K, Birk TJ, Hryniuk WM, Hryniuk LE (2006) Random control clinical trial on the effects of aerobic exercise training on erythrocyte levels during radiation treatment for breast cancer. Cancer 107(10):2490–2495. doi:10.1002/cncr.22267
Dimeo F, Schwartz S, Wesel N, Voigt A, Thiel E (2008) Effects of an endurance and resistance exercise program on persistent cancer-related fatigue after treatment. Ann Oncol 19(8):1495–1499. doi:10.1093/annonc/mdn068
Lane RJ, Barrett MC, Woodrow D, Moss J, Fletcher R, Archard LC (1998) Muscle fibre characteristics and lactate responses to exercise in chronic fatigue syndrome. J Neurol Neurosurg Psychiatry 64(3):362–367
Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR Jr, Schmitz KH, Emplaincourt PO, Jacobs DR Jr, Leon AS (2000) Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 32(9 Suppl):S498–S504
Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH (2010) An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv 4(2):87–100. doi:10.1007/s11764-009-0110-5
Hurley MV, Rees J, Newham DJ (1998) Quadriceps function, proprioceptive acuity and functional performance in healthy young, middle-aged and elderly subjects. Age Ageing 27(1):55–62
Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL (2010) American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42(7):1409–1426. doi:10.1249/MSS.0b013e3181e0c112
Acknowledgements
We wish to thank Cheryl So for her assistance with data collection and Tanya Ivanova for her assistance with the Biodex equipment and data collection. We would also like to thank the participants their time in participating in this study.
Funding
SN was supported by a Canada Graduate Student Scholarship Master’s Award from Canadian Institutes of Health Research; infrastructure support by Canadian Breast Cancer Foundation BC/Yukon and Canadian Cancer Society BC/Yukon was given to KC.
Conflict of interest
The authors declare they have no conflict of interest to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neil, S.E., Klika, R.J., Garland, S.J. et al. Cardiorespiratory and neuromuscular deconditioning in fatigued and non-fatigued breast cancer survivors. Support Care Cancer 21, 873–881 (2013). https://doi.org/10.1007/s00520-012-1600-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-012-1600-y