Abstract
Purpose
Treatment for head and neck cancer is associated with multiple side effects, including loss of body mass, impaired physical function and reduced health-related quality of life. This study aimed to investigate the impact of treatment (radiation therapy ± concurrent chemotherapy) on (i) muscle strength, muscle cross-sectional area and patient-reported outcomes, and (ii) central and peripheral alterations during a whole-body exercise task.
Methods
Ten people with head and neck cancer (4 female; 50 ± 9 years) completed a lab visit before and after (56 ± 30 days) completion of treatment. Participants performed a neuromuscular assessment (involving maximal isometric voluntary contractions in the knee extensors and electrical stimulation of the femoral nerve) before and during intermittent cycling to volitional exhaustion. Anthropometrics and patient-reported outcomes were also assessed.
Results
From before to after treatment, maximal isometric muscle strength was reduced (P = 0.002, d = 0.73), as was potentiated twitch force (P < 0.001, d = 0.62), and muscle cross-sectional area (e.g., vastus lateralis: P = 0.010, d = 0.64). Exercise time was reduced (P = 0.008, d = 0.62) and peripheral processes contributed to a reduction in maximal force due to cycling. After treatment, the severity of self-reported fatigue increased (P = 0.041, r = − 0.65) and health-related quality of life decreased (P = 0.012, r = − 0.79).
Conclusion
Neuromuscular function was impaired in patients with head and neck cancer after treatment. Whole-body exercise tolerance was reduced and resulted in predominantly peripheral, rather than central, disturbances to the neuromuscular system. Future research should evaluate strength training after treatment for head and neck cancer, with the overall aim of reducing fatigue and improving health-related quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neoplasms that arise from different anatomic subsites in the head and neck region, and include oral cavity, laryngeal, nasopharyngeal, oropharyngeal and hypopharyngeal carcinomas are collectively known as head and neck cancer (HNC). Head and neck squamous cell carcinomas account for more than 90% of all head and neck malignancies (Wyss et al. 2013), are the sixth most common cancer worldwide (Warnakulasuriya 2009), and account for more than 830,000 new cases and 430,000 deaths annually (Cramer et al. 2019). While HNC has historically been linked to tobacco and alcohol consumption among older adults, infection with human papillomavirus (HPV) has recently become an important factor in the epidemiology of HNC, particularly of the oropharynx (Chaturvedi et al. 2011). People affected by HPV-positive HNCs have different demographic characteristics, including younger mean age at diagnosis (Gillison et al. 2015), a higher incidence in men than women (Cramer et al. 2019), and a more favourable prognosis compared to patients with HPV-negative tumours (Ang et al. 2010). Although substantial progress has been made in modern HNC disease management, approximately 60% of patients are diagnosed when the malignancy has developed into locally advanced disease and are usually treated with multimodal therapies (Adelstein et al. 2017; Uta et al. 2018). Radiation therapy remains an integral part of curative-intent treatment for HNC and is scheduled as (i) definitive curative treatment (typically delivered for 30–33 treatments over ~ 6 weeks) or (ii) after surgery, as an adjuvant treatment with/without concurrent chemotherapy (Iacovelli et al. 2018).
Given that several critical structures in the head and neck region are subject to damage from intensive treatment regimens (e.g., muscles involved in deglutition, salivary glands and taste receptors), HNC survivors often present unique late and long-term side effects, such as dysphagia (Schindler et al. 2015), xerostomia (Bressan et al. 2016), and mucositis (Mercadante et al. 2015), as well as several systemic challenges. Treatment for HNC typically results in a 6–12% loss of body mass (Adelstein et al. 1997), of which more than 70% is lean body mass (Silver et al. 2007). This severe and unintentional weight loss, which is characterized by skeletal muscle wasting with or without fat mass loss (Evans et al. 2008), is the primary clinical parameter for the diagnosis of cancer cachexia (Fearon et al. 2011), and can occur as a consequence of treatment side effects or directly from tumour burden (Der-Torossian et al. 2013). This weight loss is associated with impaired muscle strength and functional performance (Couch et al. 2015), reduced health-related quality of life (HRQL) (Orell-Kotikangas et al. 2017), and an increased perception of fatigue (Couch et al. 2007). Together, these impairments can impact physical capabilities, substantially diminish functional independence and may be accompanied by several neuromuscular complications associated with cancer (for a review, see Grisold et al. 2016).
Neuromuscular function can be measured using force measurements combined with electrical stimulation paradigms [for a review, see (Millet et al. 2012)]. Alterations in neuromuscular function can also be measured during a fatiguing exercise task to assess the central and peripheral contributions to a reduction in the ability to generate maximal force. Such measurements of objective changes in task or motor performance can also be referred to as performance fatigability (Kluger et al. 2013). Few studies have assessed the measurement of neuromuscular function or performance fatigability in cancer populations (Twomey et al. 2017). Initial studies suggest that central mechanisms may have a greater contribution than peripheral mechanisms (i.e., muscle contractile properties) in the impaired performance of a motor task in cancer survivors (Yavuzsen et al. 2009; Kisiel-Sajewicz et al. 2013; Cai et al. 2014). However, to our knowledge, no studies have used whole-body (dynamic, bilateral, large muscle group) exercises such as cycling, and no studies have been conducted in HNC survivors despite the high prevalence of cancer cachexia, which may lead to impairments in neuromuscular function. A better understanding of the potential alterations in neuromuscular function, as a crucial aspect of functional well-being and therefore independence, is a priority for future research and may lead to a more informed exercise prescription to enhance HRQL after treatment for HNC.
Thus, the aims of this study were twofold. First, we aimed to investigate the impact of treatment (radiation therapy ± concurrent chemotherapy) for HNC on muscle strength, muscle cross-sectional area (CSA), and patient-reported outcomes, including HRQL and fatigue. Second, we aimed to investigate central and peripheral aspects of performance fatigability during and after an intermittent cycling test to volitional exhaustion. First, it was hypothesized that muscle strength, muscle CSA and HRQL would decrease after HNC treatment, whereas fatigue severity would increase. Second, we hypothesized that cycling exercise time would decrease, and the loss of force for a given amount of external work would be more pronounced after treatment for HNC.
Methods
Ethics approval
All experimental procedures were conducted in accordance with the Declaration of Helsinki, with the exception of registration in a database. This study was approved by the Health Research Ethics Board of Alberta—Cancer Committee (ID HREBA.CC-16-0744). Written informed consent was provided by all volunteers prior to participation.
Participants and eligibility
Ten people diagnosed with HNC (mean ± SD; age 50 ± 9 years; stature 172 ± 9 cm; 4 female) were recruited through patient-education sessions at the head and neck tumour clinic at the Tom Baker Cancer Center (Calgary, AB), in collaboration with a senior radiation oncologist. A member of the research team gave a short presentation about the study and interested patients gave permission to be contacted with additional study information. Potential participants were able to discuss the study and any questions or concerns with a member of the research team over the phone and/or email. Participants were eligible if they met the following criteria: (1) a verified clinical diagnosis of HNC (stages I–IV) with the primary tumor in the oral cavity, pharynx, larynx, paranasal sinuses, or salivary glands; (2) due to receive radiation therapy ± concurrent chemotherapy; (3) aged 18–75 years; (4) able to walk without assistance; (5) received Certified Exercise Physiologist (Canadian Society for Exercise Physiology) approval via The Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and/or physician approval; and (6) willing/able to travel to the University of Calgary. Exclusion criteria were: (1) comorbidities that could confound the ability to participate in laboratory tests (e.g., other malignancies, neuromuscular, musculoskeletal or vascular conditions affecting the lower extremities, such as radiculopathy or myopathy (where the research team was consulted for individual cases); (2) presence of a percutaneous endoscopic gastrostomy; (3) unable to follow verbal instructions in English. Participants were instructed to refrain from consuming both caffeine and alcohol for a minimum of 12 h prior to experimental sessions and participating in intense exercise for 24 h.
Experimental design
Participant enrollment began in March 2017 and ended in February 2019. Participants (n = 10; 6–8 weeks postoperative: n = 7 of 10; radiation therapy with concurrent chemotherapy: n = 8 of 10) visited the laboratory on two separate occasions to complete a neuromuscular assessment protocol before (5 ± 5 days) and after (56 ± 30 days) treatment. Participants were not provided with specific physical activity guidelines as part of this study or as part of standard care. Participants were free to engage in self-directed physical activity during cancer treatment, but no further direction was provided. Individual laboratory visits were scheduled at the same time of day (± 2 h) to account for diurnal variations [e.g., in muscle contractile properties (Tamm et al. 2009)]. During the first lab visit, an in-person discussion was conducted by the study coordinator to inform participants of the purpose of the investigation, testing procedures, associated risks and potential benefits. Informed consent was reviewed such that the information presented was comprehensible and participants understood that their participation was entirely voluntary.
Health screening
Participants completed a PAR-Q+ and were screened for contraindications that restricted maximal effort in knee extensor contractions or cycling performance. Participants were also screened for hypertension and cardiac abnormalities, determined by resting blood pressure and electrocardiography measurements, respectively. If the participant displayed a resting blood pressure ≤ 144/94 mmHg, resting heart rate ≤ 90 bpm and normal sinus rhythm, respectively, and no further concerns that warranted physician approval, then they were cleared and continued the procedures described below, in the order in which they were completed.
Patient-reported outcomes
Perception of fatigue was measured using the Functional Assessment of Chronic Illness Therapy—Fatigue, Version 4 (FACIT-F) questionnaire where higher scores indicate lower fatigue. The FACIT-F scale consists of a 13-item, unidimensional measure of fatigue (Yellen et al. 1997) with each item answered on a five-point scale, where the total score ranges from 0–52. A score of ≤ 34 was used to classify ‘clinically significant fatigue’ (Van Belle et al. 2005; Alexander et al. 2009), and a change of > 4 to identify those with a minimal clinically important difference (MCID) (Elting et al. 2008). The FACIT-F scale has been previously used to investigate patient-reported outcomes among HNC survivors (Rogers et al. 2013). HRQL was measured using the Functional Assessment of Cancer Therapy—Head & Neck, Version 4 (FACT-H&N) questionnaire. The FACT-H&N questionnaire consists of a 38-item instrument with an 11-item subscale specific to head and neck concerns, and 27 questions in four other domains: physical well-being (7), social/family well-being (7), emotional well-being (6), and functional well-being (7) (Ringash et al. 2008). Each item was answered on a five-point scale, where the total score ranges from 0 (worst possible HRQL) to 144 (best possible HRQL). A clinically significant change in FACT-H&N score was represented by a difference of ≥ 6 to rate feeling better, and ≤ 12 as feeling worse (Ringash et al. 2004).
Anthropometry
A Detecto-Medic scale (Detecto Scales Inc., Brooklyn, NY) was used to measure body mass and height, respectively. Skinfold thicknesses were measured via a Holtain Tanner Skinfold Caliper (Holtain Ltd., Crymych, UK) to estimate relative body fat percent (BF%) using the Jackson & Pollock 4-site skinfold technique (Jackson and Pollock 1978; Jackson et al. 1980), on the right side of the body to the nearest 0.2 mm, and measured in triplicates unless two of three skinfolds were ≤ 0.4 mm apart at the following sites: (1) triceps, halfway between the acromion- and olecranon-process; (2) suprailiac, superior to the iliac crest at the mid-axillary line; (3) abdominal, 5 cm lateral of the umbilicus; and (4) thigh, halfway between the inguinal crease and proximal patella.
Muscle CSA
Using B-mode ultrasonography (GE Medical Systems LOGIQ E9, Wauwatosa, WI), muscle CSA was captured in the axial plane of the vastus lateralis and rectus femoris with a 13-MHz linear array transducer. During the measurements, participants were instructed to adopt a fully relaxed, supine position with their legs extended. One axial perpendicular line was marked with semi-permanent ink at 50% of the distance between the greater trochanter and the lateral epicondyle of the knee. A liberal amount of water-soluble gel was applied to a probe to help maintain consistent pressure and avoid compression of the muscle to ensure clear images were captured. To obtain muscle CSA, consecutive two-dimensional (2-D) images were acquired with the probe placed perpendicular to the skin while following a lateral-to-medial direction positioned over the previously marked line. A minimum of three acceptable images were acquired (i.e., the image displayed identifiable borders for the vastus lateralis and rectus femoris). This technique of ultrasound imaging and manual tracing for image analysis is a valid and reliable method for assessing quadriceps atrophy and hypertrophy (Scott et al. 2017).
Neuromuscular assessment (isometric chair)
Before the intermittent cycling test, the determination of supramaximal femoral nerve electrical stimulation (FNES) intensity took place on an isometric chair (see “Experimental set-up”). Participants performed a preparatory set of contractions that involved five submaximal voluntary isometric knee extension contractions of approximately 5 s in duration, with 5-s rest between contractions. Participants were instructed to gradually increase the force of subsequent contractions, working up to one ‘near-maximal’ contraction based on perceived effort and by referencing real-time visual feedback displayed on a large monitor positioned ~ 1 m in front of the participant. Next, participants performed a neuromuscular assessment beginning with two MIVCs with no stimulations. Where two MIVCs differed by ≥ 5%, a third was performed. Subsequently, two additional MIVCs were performed with single pulse FNES when a plateau in maximal force was reached and then at rest within 2 s after a single MIVC to determine voluntary activation (VA) (Merton 1954), and potentiated knee extensor twitch force, respectively. Each of the maximal contractions were separated by 60-s rest and performed under strong verbal encouragement. Participants were then transferred to the cycle ergometer (see next section).
Intermittent cycling test and performance fatigability
This investigation employed a cycling ergometer to perform whole body (cycling) exercise that allowed for the instantaneous and intermittent assessment of neuromuscular alterations during and after cycling (Doyle-Baker et al. 2018). Participants began on the cycle ergometer by completing a series of MIVCs identical to those performed on the isometric chair before beginning the intermittent cycling test (Fig. 1b). Briefly, the intermittent cycling test consisted of 3-min stages performed at pre-determined power outputs that were scaled to individual body mass measured at the initial laboratory visit. The increments in power output increased 0.3 W kg−1 for the first four stages, 0.4 W kg−1 for the following five stages and by 0.5 W kg−1 for any subsequent stages. Participants cycled at a self-selected cadence (≥ 60 rpm, mean ± SD 77 ± 7 rpm) on the first lab visit, and this was replicated in the second lab visit. Cadence was the only real-time feedback participants received during cycling. During cycling, participants were permitted to drink water ad libitum (this was important after treatment due to xerostomia). At the end of each 3-min stage, the pedals were locked so that the participant's knee (right, unless there was a previous injury) was at a 90º angle allowing for an intermediate neuromuscular assessment (~ 20-s duration) consisting of a single MIVC with FNES (Fig. 1a). The pedals were unlocked to allow the participant to resume cycling at their target cadence at the pre-determined higher power output. Following volitional exhaustion from cycling performance (rpm < 60 for ≥ 5 s), a post-exercise neuromuscular assessment was performed immediately after cycling (Fig. 1b). Heart rate was measured continuously, and ratings of perceived exertion (RPE) on Borg’s PRE scale (Borg 1985) and dyspnea on the Borg CR-10 scale (Mador et al. 1995) were administered and recorded in the last 30 s of each 3-min stage according to published instructions (Borg 1998).
The ergometer used for maximal incremental cycling and neuromuscular assessments. (a). The position of the pedal is locked in the isometric mode where the arrow indicates the direction of force applied by the knee extensors at ~ 90º. Description of the incremental maximal cycling test showing when neuromuscular assessments were measured. (b). The neuromuscular assessments involved a knee extensor MIVC with FNES delivered during and within 2 s after MIVC to determine voluntary activation and peripheral fatigue. FNES femoral nerve electrical stimulation, MIVC maximal isometric voluntary contraction
Experimental set-up
Force and electromyography recordings
On the isometric chair (custom-built from a Kin-Com dynamometer frame), a calibrated load cell (LC101-2K, Omegadyne, Sunbury, OH) was used to measure knee extensor force during voluntary and evoked contractions. The load cell was fixed to the isometric chair and connected to a non-compliant cuff attached to the ipsilateral testing leg, superior to the malleoli, that was individually adjusted to be positioned directly behind the point of applied force. Participants sat upright in the chair, with the hips and knees at 90º flexion. Two non-compliant straps that were fastened diagonally across the thorax and one across the abdomen constrained participants from extraneous movements of the upper body. Participants were encouraged to place their hands across their chest, grasping the opposite shoulder strap for support.
On the ergometer, voluntary and evoked force was measured using a wireless pedal force analysis system (Power-Force Model PF1.0.0, Radlabor GmbH, Freiburg, Germany) located between the pedal and crank. The ergometer permitted the pedals to be locked instantly in a fixed position such that on the testing limb, the angle at the hip was ~ 100º, the angle at the ankle and knee were both ~ 90º, with the crank parallel to the ground. This allowed participants to perform a contraction of the knee extensors, whereby the force was measured in line with the crank (Fig. 1a). Participants were secured with non-compliant straps across the trunk to limit extraneous movements of the upper body. Force was sampled at 500 Hz and recorded using Imago Record (version 8.50, Radlabor GmbH). To provide real-time visual force feedback, the PowerForce signal was transmitted to a PowerLab system (16/35, ADInstruments, Bella Vista, Australia) using a 16-bit A/D card (NI PCI-6229, National Instruments, Austin, TX) and connector block (BNC-2111, National Instruments).
Surface electromyography (EMG) activity was recorded from the muscle bellies of the vastus lateralis (VL), rectus femoris (RF), and the long head of the biceps femoris with pairs of single-use self-adhesive electrodes (10-mm diameter, Meditrace, Covidien, Mansfield, MA) according to SENIAM guidelines (Hermens et al. 2000), and a reference electrode over the patella. Electrode placement was reinforced with hypoallergenic surgical tape (3M Transpore, St. Paul, MN) to ensure they would remain in contact for the duration of the experiment. Where possible, the environment was optimized for EMG measurement (e.g., only the minimal electronic devices were running). The skin was shaven, lightly abraded and cleaned with isopropyl alcohol swabs to ensure low impedance (< 10 kΩ). The electrodes were used to measure the compound muscle action potential (M-wave) elicited by FNES. Raw EMG signals were analog-to-digitally converted, amplified with an octal bio-amplifier (ML138, ADInstruments; common-mode rejection ratio = 85db, gain = 500), band-pass filtered (5–500 Hz), sampled (2000 Hz), acquired, and later analyzed off-line (LabChart v8 software, ADInstruments).
Femoral nerve electrical stimulation
Single transcutaneous electrical muscle stimuli (1 ms pulse width) were delivered to the knee extensors of the ipsilateral testing limb using a constant-current stimulator (DS7AH, Digitimer, Ltd., Hertfordshire, UK). The cathode (10 mm diameter, Meditrace 100) was positioned high in the femoral triangle and secured with gauze and hypoallergenic tape to apply pressure. The anode (50 × 90 mm rectangular electrode; Durastick Plus, DJO Global, Vista, CA) was placed midway between the greater trochanter and iliac crest. Single stimuli were delivered to the relaxed muscle beginning at 10 mA and increasing by 10 mA until plateaus occurred in twitch peak force and VL M-wave amplitude. Supramaximal FNES was ensured by increasing the intensity at this plateau by 30% to ensure full spatial recruitment of knee extensor motor units (mean current, before treatment 152 ± 76 mA; after treatment 139 ± 47 mA).
Data analysis
Neuromuscular parameters
Muscle strength was determined as the highest force produced from any of the three maximum voluntary contractions performed prior to the MIVCs with the twitch interpolation technique. Force for all MIVCs during neuromuscular assessments was recorded as the highest 500 ms plateau before stimulation. Muscle contractility from each single electrical stimulus was assessed for potentiated twitch amplitude (Qpot). VA measured through the twitch interpolation technique was assessed by normalizing the amplitude of the superimposed twitch (SIT) during MIVC to the amplitude of the corresponding Qpot using the equation: VA (%) = [1 − (SIT/Qpot) × \(100\) (Merton 1954). When the electrical stimulation was not delivered at peak force, a correction was applied according to Strojnik and Komi (1998). Electrically evoked EMG responses (M-wave) in the VL and RF were analyzed from the peak-to-peak amplitude following each supramaximal FNES and were determined from the selection of data encompassing the biphasic wave. Following any stimulation artifacts, data selection of M-wave began at the first deviation from zero and ended on the return to zero after the biphasic wave. The EMG root mean square (RMS-EMG) from VL and RF was measured as the highest 500 ms average before stimulation. All parameters were assessed on the isometric chair pre-cycling, and on cycling ergometer pre-cycling, after each completed 3 min stage, and immediately post-cycling (at volitional exhaustion). To assess alterations after a given amount of external work, we compared the neuromuscular assessment after the last common stage (the last stage completed both before and after treatment) for each individual participant. For example, if someone completed three stages before treatment, but was only able to complete two stages after treatment, the neuromuscular assessment after the second stage was used as the ‘last common stage.’
Muscle CSA
Muscle CSA of the VL and RF images was measured using a computerized, public domain planimetry software program (ImageJ, National Institutes of Health, Bethesda, MD) by following the muscle fascia with an 800-dpi mouse (Magic Mouse, Apple, Cupertino, CA). The planimetry software was calibrated with fixed distance scales displayed in the ultrasound images, and three acceptable CSA images were measured three times each, in sequence. Representative images are presented in Fig. 2. The mean of the CSA from each image was used in subsequent analysis.
Statistical analysis
An a priori sample size estimation was performed using G*Power 3, v3.1.9.4 (Franz et al. 2007) for a two-tailed paired-samples t test. For an anticipated large effect size of d = 0.8, with α = 0.05 and a 1 − β = 0.80, the sample size required was calculated as 15. Data are reported as mean ± standard deviation (SD), median and interquartile ranges (IQRs), or frequency and percentages, as appropriate. All continuous data were tested for normal distribution using box plots, q–q plots and histograms. Paired-samples t tests were performed to assess differences from before to after HNC treatment in neuromuscular function, anthropometric parameters and time to volitional exhaustion during cycling. Nonparametric Wilcoxon signed-ranks tests were used for patient-reported outcomes (HRQL and fatigue). Differences in responses to intermittent cycling were analyzed using a two-way repeated measure analysis of variances (ANOVA), i.e. time (3: pre-test, last common stage completed, post-test) × treatment (2: before receiving treatment, after receiving treatment). Prior to ANOVA, the assumptions of normality and sphericity were tested using a Shapiro–Wilk test and Mauchly’s test, respectively. If the assumption of Sphericity was violated, the Greenhouse–Geisser correction was applied when necessary. Bonferroni corrections for post hoc analyses were used if ANOVA indicated significant main or interaction effects. Effect sizes were calculated to provide a quantitative measure of the magnitude of the reported effects. Partial eta squared (ηp2) was used as an estimate of effect size for the main and interaction effects of ANOVA. Interpretation of the size of the effect was cautiously considered as ηp2 = 0.01 is small, ηp2 = 0.06 is medium and ηp2 = 0.14 is large (Cohen 1988). Cohen’s dav was used to describe the standardized mean difference of an effect paired-samples t tests. Interpretation of the size of the effect was considered as d = 0.2 is small, d = 0.5 is medium and d = 0.8 is large (Cohen 1988). Finally, the effect size for the Wilcoxon signed-ranks tests were calculated as r = Z/SQR(n). Statistical significance was set at P < 0.05. All statistical procedures were conducted using SPSS software version 25.0 (IBM Corp., Armonk, NY).
Results
Flow of participants
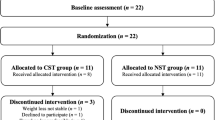
Participant characteristics are summarized in Table 1. The flow of participants is illustrated in Fig. 3. Unfortunately, recruitment was lower than anticipated, likely due to the unique and debilitating consequences associated with HNC and its treatment. During the initial lab visit, four individuals were excluded due to contradicted health screenings. Six participants who completed the lab visit before treatment did not complete testing after receiving treatment (Fig. 3).
Neuromuscular function
From before to after HNC treatment, maximal voluntary force decreased by 18 ± 13% (T(9) = 4.33, P = 0.002, d = 0.73; Fig. 4d), and evoked force decreased by 16 ± 8% (T(9) = 6.47, P < 0.001, d = 0.62; Fig. 4e). However, there was no difference in VA (T(9) = − 1.90, P = 0.09, d = 0.93; Fig. 4f). No differences were found for RMS-EMG in the VL or RF, or for M-wave amplitude in the VL (Table S1), but M-wave amplitude was reduced in the RF by 36 ± 30% (T(9) = 3.57, P = 0.006, d = 1.22).
Body mass (a); body mass index (b); percent body fat (c); maximal isometric knee extensor (MIVC) force (d); potentiated twitch force (e); voluntary activation (f); vastus lateralis (VL) muscle cross-sectional area (g); and rectus femoris (RF) muscle cross-sectional area (panel H) before and after treatment for head and neck cancer. *Denotes time effect (P < 0.05)
CSA and body composition
Muscle CSA decreased from before to after treatment by 21 ± 18% in the VL (T(8) = 3.38, P = 0.01, d = 0.64, Fig. 4g) and by 20 ± 14% in the RF (T(8) = 3.99, P = 0.004, d = 0.66; Fig. 4h). Body mass decreased by 10 ± 6% (T(9) = 4.52, P = 0.001, d = 0.47) and nine out of ten participants met the criteria for cancer cachexia [≥ 5% decrease in body mass (Fearon et al. 2011)]. BMI and estimated BF% also decreased significantly from before to after treatment by 10 ± 7% (T(9) = 3.93, P = 0.003, d = 0.54), and 12 ± 10% (T(9) = 3.30, P = 0.009, d = 0.47), respectively (Fig. 4).
HRQL and perceived fatigue
As shown in Table 2, the total FACT-H&N score decreased by 16 ± 14 points (Z = − 2.50, P = 0.012, r = − 0.79) from before to after treatment, which exceeded the MCID in seven of ten participants. The Physical Well-being (Z = − 2.55, P = 0.011, r = − 0.81) and HNC-specific (Z = − 2.69, P = 0.007, r = − 0.85) subscales showed a statistically significant worsening from baseline, while social/family well-being (Z = − 0.92, P = 0.359, r = − 0.29), emotional well-being (Z = − 1.02, P = 0.309, r = − 0.32), and functional well-being (Z = − 1.23, P = 0.219, r = − 0.39) domains did not differ. The FACIT-F score decreased by a mean of 9 ± 11 points (Z = − 2.04, P = 0.041, r = − 0.65) from before to after treatment (participants ≥ MCID: n = 7 of 10).
Intermittent cycling test
Exercise time
Time to volitional exhaustion during cycling decreased from before to after treatment by 15 ± 12%, from 966 ± 269 s to 823 ± 239 s (T(9) = 3.41, P = 0.008, d = 0.62). The corresponding mean power output achieved at volitional exhaustion was 146 ± 40 W before treatment and 123 ± 38 W after treatment (T(9) = 1.85, P = 0.098, d = 0.62).
Physiological and perceptual responses
At volitional exhaustion, maximal heart rate and ratings of perceived exertion and dyspnea were not different from before to after treatment. However, during the last common stage, ratings of dyspnea and ratings of perceived exertion were both two points higher after treatment, respectively (Table 3).
Muscle contractile responses
As a result of cycling, maximal voluntary force decreased (Fig. 5a; main effect of time: F2,18 = 15.35, P < 0.001, ηp2 = 0.63). Post hoc analysis indicated a decrease in MIVC of 28 ± 24% from pre-cycling to post-cycling (P = 0.003, d = 1.15). There was a main effect of time in the decrease of potentiated evoked force (F2,18 = 31.56, P < 0.001, ηp2 = 0.78), and post hoc analysis revealed a change from pre-cycling to the last common stage (–29 ± 30%; P = 0.015, d = 0.86), and from pre-cycling to post-cycling (− 55 ± 17%; P < 0.001, d = 1.66). No main effect of treatment or time × treatment interaction effect was detected for MIVC and potentiated evoked force. The M-wave amplitude in the VL did not change with cycling (Table S2 and S3). However, the M-wave amplitude in the RF was significantly reduced (main effect of time: F1.04,6.21 = 7.23, P = 0.034, ηp2 = 0.55) with a treatment × time interaction (F2,12 = 5.24, P = 0.023, ηp2 = 0.47). However, all Bonferroni-corrected post-hoc comparisons were nonsignificant.
Voluntary activation
No main or interaction effects was found for VA (Fig. 5c; Table S2 and S3).
Exploratory analyses
The change in patient-reported outcomes (FACT-H&N and FACIT-F), characteristics of muscle function (MIVC, Qpot and muscle CSA), and exercise time was calculated by comparing the post-treatment values to the pre-treatment values (post/pre), where a value over 1 indicates an increase, and a value under 1 indicates a decrease. We examined the effect of patient-reported outcomes on muscle function characteristics and patient-reported outcomes on exercise time, respectively, using bivariate correlations. There was a significant correlation between the reduction in overall HRQL (FACT-H&N total score) and the decrease in maximal force generation in the knee extensors (Fig. 6b; r = 0.63, P = 0.049). More so, there were significant correlations between the decrease in HRQL and the decrease in exercise time (Fig. 6h; r = 0.65, P = 0.041), and the increase in fatigue severity (decreased FACIT-F) and the decrease in exercise time (Fig. 6g; r = 0.77, P = 0.009).
Correlation between: FACIT-F and MIVC force (a); FACT-H&N and MIVC force (b); FACIT-F and potentiated twitch force (c); FACT-H&N and potentiated twitch force (d); FACIT-F and cross-sectional area (e); FACT-H&N and cross-sectional area (f); FACIT-F and exercise time (g); FACT-H&N and exercise time (h). FACIT-F Functional Assessment of Chronic Illness Therapy—Fatigue Questionnaire, FACT-H&N Functional Assessment of Cancer Therapy—Head and Neck Questionnaire, MIVC maximal isometric voluntary contraction
Discussion
The present study aimed to examine the changes in maximal force, muscle mass and performance fatigability, as well as patient-reported outcomes in people who completed treatment for HNC. To our knowledge, this is the first study to investigate contractile and electromyographic changes during and immediately after a maximal cycling test to explore performance fatigability in participants treated for HNC. Alongside a substantial reduction in muscle strength and muscle CSA after radiation therapy ± concurrent chemotherapy, we found that evoked force was reduced, with no changes in VA of the knee extensors. We also found that the decrease in the force-generating capacity of the knee extensors with cycling exercise was mainly due to peripheral disturbances (evidenced by a progressive decline in evoked force), which may have contributed to the earlier cessation of whole-body exercise after HNC treatment performed at the same power outputs. This study also showed that following treatment for HNC, participants experienced increased fatigue severity and reduced HRQL, evidenced by a mean decrease of 9 ± 11 points, and 16 ± 14 points on the FACIT-F and FACT-H&N questionnaires, respectively. This decrease was also correlated with a reduction in cycling performance following treatment (Fig. 6).
Neuromuscular function
There was a substantial reduction in the maximal force-generating capacity of the knee extensors, where, to our knowledge, decreases in muscle strength have previously only been shown in the upper limb after HNC treatment [e.g., (Jager-Wittenaar et al. 2011)]. Although grip-strength measurements provide a surrogate for limb strength (Bohannon et al. 2012), the knee extensors are a large muscle group relevant to everyday tasks including locomotion. We used muscle stimulation paradigms and EMG to investigate the potential mechanisms responsible for a loss in maximal force-generating capacity of the knee extensors following anti-neoplastic treatment for HNC. The twitch interpolation method, which estimates the amount of neural drive to the muscle, has previously shown validity to assess the maximal VA of the knee extensors (Taylor et al. 2009). Utilizing this technique in the current study elicited no change in VA from before to after HNC treatment (Fig. 4f). We note that a relatively high VA of 90–95% was observed in a rested state before and after treatment, which is similar to VA in the knee extensors in healthy individuals (Goodall et al. 2009). The lack of change in VA combined with a substantial reduction in evoked force (potentiated twitch, a measure of muscle contractile ability), and ~ 20% reduction in muscle CSA suggests that peripheral, rather than central alterations contributed to the reduction in MIVC force. Interestingly, RF M-wave amplitude was also reduced after HNC treatment, and this may suggest a decrease in muscle membrane excitability occurred, though precise mechanisms for this are unknown. Due to the substantial loss of muscle strength (voluntary and evoked) and muscle mass, interventions (specifically, carefully designed strength training) should be considered to address these deleterious consequences of treatment for HNC.
Nine of ten participants experienced cancer cachexia as a result of HNC treatment [a reduction of ≥ 5% body mass (Fearon et al. 2011)]. Although we did not capture the nutritional intake of these patients, difficulties with swallowing associated with radiation therapy (Langendijk et al. 2008) mean that these patients were unable to meet their daily nutritional needs during/after cancer treatment. Cancer cachexia is a debilitating wasting syndrome characterized by decreased muscle anabolism and increased catabolism, and results in muscle atrophy, with mounting evidence that skeletal muscle depletion results in neuromuscular impairments (Larsson et al. 2018). Cachexia is also predictive of survival in HNC (Orell-Kotikangas et al. 2017), so identifying this as an issue to address is of utmost importance. We used ultrasonography to measure muscle CSA as a surrogate for muscle size, although this technique is not a precise measure of total skeletal muscle mass. In line with the reduction we observed in muscle CSA, Sandmæl et al. (2017) assessed skeletal muscle mass before the start and at the end of radiotherapy-treated HNC patients and found a significant reduction in muscle mass at the third lumbar region. This measurement is highly correlated with total body skeletal mass (Shen et al. 2004) and can provide precise estimates of muscle wasting (Sandmæl et al. 2017). In previous investigations, the loss of lean body mass after radiation therapy with concurrent chemotherapy was significantly associated with reduced physical function and functional independence in patients with HNC (Silver et al. 2007). However, physical function was assessed as a patient-reported outcome and although this is of clinical importance, there were no objective measures to support this finding. In the present study, the reduction in muscle CSA occurred alongside a reduction in both self-reported physical well-being and objective measures of performance fatigability, including a reduced whole-body exercise tolerance.
Perceptions of fatigue and HRQL
As hypothesized, we found that anti-neoplastic treatment for patients with HNC resulted in statistically and clinically significant changes in both the severity of perceived fatigue and worse HRQL following treatment. A reduction in > 4 units is considered clinically significant with the FACIT-F scale (Elting et al. 2008), which occurred in the present study (Table 2). Additionally, a reduction of about 12 units with the FACT-H&N scale is considered as a clinically significant change (Ringash et al. 2008), and an overall difference of 16 points after treatment was observed in the current study (Table 2). These results align with previous pilot studies that have identified similar increases in symptoms and reductions in HRQL in patients with HNC (Sawada et al. 2012). Although we did not directly measure the influence of specific treatment-related toxicities on perceived fatigue and HRQL, the current study did find a correlation between the decrease in overall HRQL (total FACT-H&N score) and the reduction of maximal knee extensor muscle strength (Fig. 6b). Likewise, a significant correlation was identified between the decrease in HRQL and exercise time to volitional exhaustion, and between increased perceived fatigue and exercise time to volitional exhaustion (Fig. 6). Due to our low sample size; however, these exploratory results should be interpreted with caution until replicated in more extensive studies.
Intermittent cycling test
After treatment for HNC, participants stopped cycling ~ 2 min earlier in comparison to an identical test performed before treatment and (on average) were, therefore, unable to reach the same maximal power output. At the same power output during the last common stage, ratings of perceived exertion and dyspnea were higher after treatment, i.e. the same exercise load felt harder/heavier, and participants were more out of breath. Using a cycle ergometer equipped with the ability to measure force instantaneously with the pedals in a locked position, we were able to measure neuromuscular function within and immediately after the intermittent cycling test (Fig. 1). For the first time, we assessed (i) measures of voluntary and mechanically evoked force; (ii) VA; and (iii) EMG parameters of the knee extensors in response to a whole-body exercise after treatment for HNC. We hypothesized that after a given amount of external work (i.e., after the last common stage that was completed both before and after treatment), the loss of force would be more pronounced after treatment for HNC. However, this was not the case for peak voluntary force (MIVC, Fig. 5a). Given the small sample size, we may not have had the necessary power to detect a time × treatment interaction in this analysis. There was considerable variation in MIVC force from baseline to the last common stage (e.g., 2 ± 36% before treatment), and this may reflect the diversity of this sample (which includes HPV-positive and HPV-negative participants). Nevertheless, the magnitude of the reduction in maximal voluntary force at volitional exhaustion did not differ from before to after treatment, despite the substantially lower total exercise time between these conditions, and despite similar ratings of exertion and dyspnea at the end of the exercise.
Previous research has reported an association between irradiation of discrete structures of the central nervous system (CNS) and perceived fatigue (Ferris et al. 2017). Speculatively, unintended radiation doses to the CNS could damage the integrity of the pathway between the brain and the muscle, and lead to a reduction in VA after treatment for HNC. However, in the present study, there was no evidence of central fatigue (a reduction in VA) after the last common stage completed or post-cycling. This is also supported by the lack of change in RMS-EMG activity, which can also be used to infer changes in neural drive (Bigland-Ritchie et al. 1986). Rather, the contribution of peripheral factors influenced changes in voluntary force production both in the resting state and during intermittent cycling.
Percutaneous electrical stimulation of the motor nerve bypasses the CNS and is, therefore, used to obtain information about localized changes in muscle contractility. The mechanical twitch force and M-wave are recorded and used to determine potential sites of peripheral fatigue within the neuromuscular system (Place et al. 2010). Evoked force decreased from pre-cycling to the last common stage (by 29 ± 30%), and from pre- to post-cycling (by 55 ± 17%). However, similarly to MIVC, after a given amount of external work, the loss of evoked force was not more pronounced after HNC treatment, and the magnitude of peripheral fatigue was not different despite a reduced total exercise time. From pre-cycling to the last common stage, the reduction in MIVC force is likely the result of changes in Ca2+ release, Ca2+ sensitivity or force produced by the cross-bridges engaged because Qpot was reduced with no changes in M-waves (Place et al. 2010). However, the reduced M-wave amplitude in the RF at volitional exhaustion suggests that disturbances in muscle membrane excitability may also be involved. However, it should be noted that EMG measures can be less reliable than force outcomes (Doyle-Baker et al. 2018) such that they are susceptible to contamination by other parameters like change in temperature and sweat (Bell 1993).
Limitations
A separate familiarization session to acquaint participants with the test procedures and equipments was not feasible due to time constraints between recruitment and the beginning of cancer treatment. Although two trained research personnel were present at all sessions to instruct participants through the techniques, a familiarization session is recommended, and we recognize this as a study limitation. Although there was no significant difference in VA from pre- to post-treatment, increases for some participants (Fig. 4f) could be due to the lack of familiarization, or measurement error. The main limitation is that due to unanticipated difficulties with recruitment despite an extended recruitment period, only ten participants completed both lab visits. As such, the study may have been underpowered to detect smaller effects, although we note that many before versus after treatment effects were large due to the severity of anti-cancer therapy for HNC and its associated side effects.
Conclusion
Maximal voluntary and evoked force is substantially reduced in a major lower-body muscle group after radiation ± concurrent chemotherapy for HNC, and this occurs alongside cancer cachexia (loss of body mass, and muscle wasting indicated by a reduction in CSA), reduced HRQL and increased fatigue severity. From before to after treatment, the reduced capacity to produce maximal force in the knee extensors was explained by a decrease in evoked force in response to percutaneous electrical stimulation rather than a reduction in the neural drive to the muscle. Whole-body exercise tolerance was impaired after HNC treatment, and cycling to volitional exhaustion results in predominantly peripheral, rather than central, disturbances to the neuromuscular system. Future directions in this population should focus on exercise interventions that target muscle strength and muscle mass, with the overall aim of improving physical function and HRQL.
Abbreviations
- ANOVA:
-
Analysis of variance
- BF%:
-
Body fat percent
- BMI:
-
Body mass index
- CNS:
-
Central nervous system
- CSA:
-
Cross-sectional area
- EMG:
-
Electromyography
- FACIT-F:
-
Functional assessment of chronic illness therapy—fatigue
- FACT-H&N:
-
Functional assessment of cancer therapy—head & neck
- FNES:
-
Femoral nerve electrical stimulation
- HRQL:
-
Health-related quality of life
- HPV:
-
Human papillomavirus
- HNC:
-
Head and neck cancer
- IQR:
-
Interquartile range
- M-wave:
-
Compound muscle action potential
- MIVC:
-
Maximal isometric voluntary contraction
- PAR-Q+:
-
The Physical Activity Readiness Questionnaire for Everyone
- Qpot :
-
Potentiated twitch amplitude
- RF:
-
Rectus femoris
- RPE:
-
Rating of perceived exertion
- RPM:
-
Revolutions per minute
- RMS-EMG:
-
Root means square of electromyography
- SD:
-
Standard deviation
- SIT:
-
Superimposed twitch
- VA:
-
Voluntary activation
- VL:
-
Vastus lateralis
- ηp2 :
-
Partial eta squared
References
Adelstein DJ, Saxton JP, Lavertu P et al (1997) A phase III randomized trial comparing concurrent chemotherapy and radiotherapy with radiotherapy alone in resectable stage III and IV squamous cell head and neck cancer: preliminary results. Head Neck J Sci Spec Head Neck 19:567–575. https://doi.org/10.1002/(sici)1097-0347(199710)19:7%3c567:aid-hed2%3e3.0.co;2-5
Adelstein D, Gillison ML, Pfister DG et al (2017) NCCN Guidelines Insights: Head and Neck Cancers, Version 2.2017. J Natl Compr Canc Netw 15:761–770. https://doi.org/10.6004/jnccn.2017.0101
Alexander S, Minton O, Andrews P, Stone P (2009) A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer 45:384–392. https://doi.org/10.1016/j.ejca.2008.09.010
Ang KK, Harris J, Wheeler R et al (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35. https://doi.org/10.1056/nejmoa0912217
Bell DG (1993) The influence of air temperature on the EMG/force relationship of the quadriceps. Eur J Appl Physiol Occup Physiol 67:256–260. https://doi.org/10.1007/BF00864225
Van Belle S, Paridaens R, Evers G et al (2005) Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: proposal for use as a screening tool. Support Care Cancer 13:246–254. https://doi.org/10.1007/s00520-004-0734-y
Bigland-Ritchie B, Furbush F, Woods JJ (1986) Fatigue of intermittent submaximal voluntary contractions: central and peripheral factors. J Appl Physiol 61:421–429. https://doi.org/10.1152/jappl.1986.61.2.421
Bohannon RW, Magasi SR, Bubela DJ et al (2012) Grip and Knee extension muscle strength reflect a common construct among adults. Muscle Nerve 46:555–558. https://doi.org/10.1002/mus.23350
Borg G (1985) An introduction to Borg’s RPE-scale. Mouv Publ, Paris
Borg G (1998) Borg’s perceived exertion and pain scales. Human Kinetics, Leeds
Bressan V, Stevanin S, Bianchi M et al (2016) The effects of swallowing disorders, dysgeusia, oral mucositis and xerostomia on nutritional status, oral intake and weight loss in head and neck cancer patients: a systematic review. Cancer Treat Rev 45:105–119. https://doi.org/10.1016/j.ctrv.2016.03.006
Cai B, Allexandre D, Rajagopalan V et al (2014) Evidence of significant central fatigue in patients with cancer-related fatigue during repetitive elbow flexions till perceived exhaustion. PLoS ONE. https://doi.org/10.1371/journal.pone.0115370
Chaturvedi AK, Engels EA, Pfeiffer RM et al (2011) Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 29:4294–4301. https://doi.org/10.1200/JCO.2011.36.4596
Cohen J (1988) Statistical power analysis for the behavioral sciences. Erlbaum, Hillsdale
Couch ME, Lai V, Cannon T et al (2007) Cancer cachexia syndrome in head and neck cancer patients: Part I. Diagnosis, impact on quality of life and survival, and treatment. Head Neck 29:401–411. https://doi.org/10.1002/hed.20447
Couch ME, Dittus K, Toth MJ et al (2015) Cancer cachexia update in head and neck cancer: definitions and diagnostic features. Head Neck 37:594–604. https://doi.org/10.1002/hed.23599
Cramer JD, Burtness B, Le QT, Ferris RL (2019) The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol. https://doi.org/10.1038/s41571-019-0227-z
Der-Torossian H, Asher SA, Winnike JH et al (2013) Cancer cachexia’s metabolic signature in a murine model confirms a distinct entity. Metabolomics 9:730–739. https://doi.org/10.1007/s11306-012-0485-6
Doyle-Baker D, Temesi J, Medysky ME et al (2018) An innovative ergometer to measure neuromuscular fatigue immediately after cycling. Med Sci Sports Exerc 50:375–387. https://doi.org/10.1249/MSS.0000000000001427
Elting LS, Keefe DM, Sonis ST et al (2008) Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer 113:2704–2713. https://doi.org/10.1002/cncr.23898
Evans WJ, Morley JE, Argilés J et al (2008) Cachexia: a new definition. Clin Nutr 27:793–799. https://doi.org/10.1016/j.clnu.2008.06.013
Fearon K, Strasser F, Anker SD et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12:489–495. https://doi.org/10.1016/S1470-2045(10)70218-7
Ferris MJ, Zhong J, Switchenko JM et al (2017) Brainstem dose is associated with patient-reported acute fatigue in head and neck cancer radiation therapy. Radiother Oncol. https://doi.org/10.1016/j.radonc.2017.08.008
Franz F, Erdfelder E, Lang A-G, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical science. Behav Res Methods 39:175–191. https://doi.org/10.3758/BF03193146
Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C (2015) Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 33:3235–3242. https://doi.org/10.1200/JCO.2015.61.6995
Goodall S, Romer LM, Ross EZ (2009) Voluntary activation of human knee extensors measured using transcranial magnetic stimulation. Exp Physiol 94:995–1004. https://doi.org/10.1113/expphysiol.2009.047902
Grisold W, Grisold A, Löscher WN (2016) Neuromuscular complications in cancer. J Neurol Sci 367:184–202. https://doi.org/10.1016/j.jns.2016.06.002
Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G (2000) Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10:361–374. https://doi.org/10.1016/S1050-6411(00)00027-4
Iacovelli NA, Galaverni M, Cavallo A et al (2018) Prevention and treatment of radiation-induced acute dermatitis in head and neck cancer patients: a systematic review. Futur Oncol. https://doi.org/10.2217/fon-2017-0359
Jackson AS, Pollock ML (1978) Generalized equations for predicting body density of men. Br J Nutr 40:497. https://doi.org/10.1079/BJN19780152
Jackson AS, Pollock ML, Ward A (1980) Generalized equations for predicting body density of women. Med Sci Sports Exerc 12:175–181. https://doi.org/10.1079/BJN19780152
Jager-Wittenaar H, Dijkstra PU, Vissink A et al (2011) Changes in nutritional status and dietary intake during and after head and neck cancer treatment. Head Neck 33:863–870. https://doi.org/10.1002/hed.21546
Kisiel-Sajewicz K, Siemionow V, Seyidova-Khoshknabi D et al (2013) Myoelectrical manifestation of fatigue less prominent in patients with cancer related fatigue. PLoS ONE. https://doi.org/10.1371/journal.pone.0083636
Kluger BM, Krupp LB, Enoka RM (2013) Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology 80:409–416. https://doi.org/10.1212/WNL.0b013e31827f07be
Langendijk JA, Doornaert P, Verdonck-de Leeuw IM et al (2008) Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol 26:3770–3776. https://doi.org/10.1200/JCO.2007.14.6647
Larsson L, Degens H, Li M et al (2018) Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev 99:427–511. https://doi.org/10.1152/physrev.00061.2017
Mador MJ, Rodis A, Magalang UJ (1995) Reproducibility of Borg scale measurements of dyspnea during exercise in patients with COPD. Chest 107:1590–1597. https://doi.org/10.1378/chest.107.6.1590
Mercadante S, Aielli F, Adile C et al (2015) Prevalence of oral mucositis, dry mouth, and dysphagia in advanced cancer patients. Support Care Cancer 23:3249–3255. https://doi.org/10.1007/s00520-015-2720-y
Merton PA (1954) Voluntary strength and fatigue. J Physiol 123:553–564. https://doi.org/10.1113/jphysiol.1954.sp005070
Millet GY, Bachasson D, Temesi J et al (2012) Potential interests and limits of magnetic and electrical stimulation techniques to assess neuromuscular fatigue. Neuromuscul Disord 22:S181–S186. https://doi.org/10.1016/j.nmd.2012.10.007
Orell-Kotikangas H, Osterlund P, Makitie O et al (2017) Cachexia at diagnosis is associated with poor survival in head and neck cancer patients. Acta Otolaryngol 137:778–785. https://doi.org/10.1080/00016489.2016.1277263
Place N, Yamada T, Bruton JD, Westerblad H (2010) Muscle fatigue: from observations in humans to underlying mechanisms studied in intact single muscle fibres. Eur J Appl Physiol 110:1–15. https://doi.org/10.1007/s00421-010-1480-0
Ringash J, Bezjak A, O’Sullivan B, Redelmeier DA (2004) Interpreting differences in quality of life: the FACT-H&N in laryngeal cancer patients. Qual Life Res 13:725–733. https://doi.org/10.1023/B:QURE.0000021703.47079.46
Ringash J, Lockwood G, O’Sullivan B et al (2008) Hyperfractionated, accelerated radiotherapy for locally advanced head and neck cancer: quality of life in a prospective phase I/II trial. Radiother Oncol 87:181–187. https://doi.org/10.1016/j.radonc.2007.12.028
Rogers L, Anton PM, Fogleman A et al (2013) Pilot, randomized trial of resistance exercise during radiation therapy for head and neck cancer. Head Neck 35:1178–1188. https://doi.org/10.1002/hed.23118
Sandmæl JA, Bye A, Solheim TS et al (2017) Feasibility and preliminary effects of resistance training and nutritional supplements during versus after radiotherapy in patients with head and neck cancer: a pilot randomized trial. Cancer 123:4440–4448. https://doi.org/10.1002/cncr.30901
Sawada NO, De Paula JM, Sonobe HM et al (2012) Depression, fatigue, and health-related quality of life in head and neck cancer patients: a prospective pilot study. Support Care Cancer 20:2705–2711. https://doi.org/10.1007/s00520-012-1390-2
Schindler A, Denaro N, Russi EG et al (2015) Dysphagia in head and neck cancer patients treated with radiotherapy and systemic therapies: literature review and consensus. Crit Rev Oncol Hematol 96:372–384. https://doi.org/10.1016/j.critrevonc.2015.06.005
Scott JM, Martin DS, Ploutz-Snyder R et al (2017) Panoramic ultrasound: a novel and valid tool for monitoring change in muscle mass. J Cachexia Sarcopenia Muscle 8:475–481. https://doi.org/10.1002/jcsm.12172
Shen W, Punyanitya M, Wang Z et al (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 97:2333–2338. https://doi.org/10.1152/japplphysiol.00744.2004.-A
Silver HJ, Dietrich MS, Murphy BA (2007) Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head Neck 29:893–900. https://doi.org/10.1002/hed.20607
Strojnik V, Komi PV (1998) Neuromuscular fatigue after maximal stretch-shortening cycle exercise. J Appl Physiol 84:344–350. https://doi.org/10.1152/jappl.1998.84.1.344
Tamm AS, Lagerquist O, Ley AL, Collins DF (2009) Chronotype influences diurnal variations in the excitability of the human motor cortex and the ability to generate torque during a maximum voluntary contraction. J Biol Rhythms 24:211–224. https://doi.org/10.1177/0748730409334135
Taylor JL, De Haan A, Gerrits KHL, De Ruiter CJ (2009) Point:Counterpoint: the interpolated twitch does/does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol 107:354–357. https://doi.org/10.1152/japplphysiol.91220.2008
Twomey R, Aboodarda SJ, Kruger R et al (2017) Neuromuscular fatigue during exercise: methodological considerations, etiology and potential role in chronic fatigue. Neurophysiol Clin 47:95–110. https://doi.org/10.1016/j.neucli.2017.03.002
Uta R, Laurent M, Axel L et al (2018) Immunotherapy in head and neck cancer—scientific rationale, current treatment options and future directions. Swiss Med Wkly. https://doi.org/10.4414/smw.2018.14625
Warnakulasuriya S (2009) Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45:309–316. https://doi.org/10.1016/j.oraloncology.2008.06.002
Wyss A, Hashibe M, Chuang SC et al (2013) Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: Pooled analysis in the international head and neck cancer epidemiology consortium. Am J Epidemiol 178:679–690. https://doi.org/10.1093/aje/kwt029
Yavuzsen T, Davis MP, Ranganathan VK et al (2009) Cancer-related fatigue: central or peripheral? J Pain Symptom Manag 38:587–596. https://doi.org/10.1016/j.jpainsymman.2008.12.003
Yellen SB, Cella DF, Webster K et al (1997) Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manag 13:63–74. https://doi.org/10.1016/S0885-3924(96)00274-6
Author information
Authors and Affiliations
Contributions
RT and GYM conceived and designed the study. CL and RT were responsible for recruitment, data collection, statistical analysis and interpretation of the data. CL drafted the manuscript. RT, GYM, NCR, HL and GF provided critical revisions of the manuscript and contributed important intellectual contents. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Communicated by Nicolas Place.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lavigne, C., Lau, H., Francis, G. et al. Neuromuscular function and fatigability in people diagnosed with head and neck cancer before versus after treatment. Eur J Appl Physiol 120, 1289–1304 (2020). https://doi.org/10.1007/s00421-020-04362-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-020-04362-0