Abstract
Purpose
Light-load blood flow restriction exercise (BFRE) may provide a novel training method to limit the effects of age-related muscle atrophy in older adults. Therefore, the purpose of this study was to compare the haemodynamic response to resistance and aerobic BFRE between young adults (YA; n = 11; 22 ± 1 years) and older adults (OA; n = 13; 69 ± 1 years).
Method
On two occasions, participants completed BFRE or control exercise (CON). One occasion was leg press (LP; 20 % 1-RM) and the other was treadmill walking (TM; 4 km h−1). Haemodynamic responses (HR, \(\dot{Q}\), SV and BP) were recorded during baseline and exercise.
Result
At baseline, YA and OA were different for some haemodynamic parameters (e.g. BP, SV). The relative responses to BFRE were similar between YA and OA. Blood pressures increased more with BFRE, and also for LP over TM. \(\dot{Q}\) increased similarly for BFRE and CON (in both LP and TM), but with elevated HR and reduced SV (TM only).
Conclusion
While BFR conferred slightly greater haemodynamic stress than CON, this was lower for walking than leg-press exercise. Given similar response magnitudes between YA and OA, these data support aerobic exercise being a more appropriate BFRE for prescription in older adults that may contribute to limiting the effects of age-related muscle atrophy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Training using heavy-load resistance exercise (HLRE) is a primary intervention for limiting muscle atrophy (Marcell 2003; Janssen et al. 2004; Hurley and Roth 2000). However, older adults and some clinical populations, particularly those unaccustomed to resistance exercise, may be contraindicated to perform HLRE according to traditional training loads and repetitions and due to the high mechanical/musculoskeletal stress and increased risk of a cardiovascular event (Thompson et al. 2007; Frankel et al. 2006). While these adverse outcomes may initially be overcome by prescribing fewer repetitions and/or reducing the exercise intensity, this limits the stimuli that aim to maximise beneficial gains in muscle strength and muscle size/growth in response to the resistance training programme (Thompson et al. 2007; Frankel et al. 2006; Karlsen et al. 2009; Pollock et al. 2000). Consequently, it is recommended that older adults, especially those that are deconditioned, undertake a larger proportion of light-intensity exercise that is more aerobic in nature with significantly reduced prescriptions of HLRE (Thompson et al. 2007; Frankel et al. 2006). While this light-intensity exercise still provides benefits to cardiorespiratory health, mobility and functional independence (Fletcher et al. 1996; Frankel et al. 2006), large gains in muscle size and/or strength are not expected (Kraemer et al. 2002; Loenneke and Pujol 2009).
Blood-flow restriction exercise (BFRE) is a potential alternative mode of exercise training for older adults that may contribute to limiting the progression of skeletal muscle atrophy and/or produce muscle hypertrophy. Despite utilising light loads [20–30 % one repetition maximum (1-RM)], BFRE develops muscle size and strength, the magnitude of which can be in the range of that also reported for HLRE (Clark et al. 2011; Karabulut et al. 2011; Takarada et al. 2000). In addition, significant gains in muscle size and strength with aerobic walking BFRE have also been reported (Abe et al. 2006; Sakamaki et al. 2011). While these gains in muscle size and strength have largely been the primary focus of interventional studies of BFRE (Abe et al. 2005, 2006, 2010; Yasuda et al. 2011; Karabulut et al. 2010), the acute haemodynamic and perceptual responses to BFRE are less well characterised (Hollander et al. 2010; Loenneke et al. 2011; Vieira et al. 2013). These would, however, seem essential to evaluate if BFRE is to be more widely prescribed.
Prior investigations in young healthy populations demonstrate increased haemodynamic stress with BFRE in comparison to light-load resistance exercise (LLRE) (Brandner et al. 2015; Takano et al. 2005). At a minimum, this suggests that older adults will also likely be exposed to a greater haemodynamic stress during BFRE. However, to date few investigations of BFRE haemodynamics have been made in older adults (Sakamaki et al. 2008), with only one study making direct comparisons between young and older adults within the same study design (Vieira et al. 2013). While this report demonstrated similar haemodynamic responses to BFRE between young and older adults, this study used bicep curl exercise and so only targeted a small muscle mass (Vieira et al. 2013). A comparison of the acute haemodynamic responses to BFRE using large muscle groups (e.g. leg press) or even aerobic exercise (e.g. walking) has not been made between young and older adults within the same study design. This is despite these muscle groups being used frequently in older adults for mobility, activities of daily living and exercise. Similarly, while perceptual responses have been examined for resistance BFRE (Hollander et al. 2010; Lagally et al. 2002; Loenneke et al. 2011), little is known for aerobic BFRE, with no study comparing these responses between young and older adults despite being relevant to further understanding exercise tolerance and programme adherence (Loenneke et al. 2011).
Therefore, this study aimed to assess the acute haemodynamic and perceptual responses to both resistance (leg-press) and aerobic (walking) modes of BFRE between young and older male participants utilising large muscle groups, which are highly applicable for use in activities of daily living, mobility and exercise. Furthermore, we compared these responses with equivalent (and more commonly prescribed) light-intensity exercises.
It was hypothesised that BFRE would elevate the perceptual and acute haemodynamic responses over those for equal-intensity non-BFRE, with these responses being greater for resistance exercise compared with aerobic exercise. Finally, it was hypothesised that the perceptual and acute haemodynamic responses to BFRE between young and older participants would be similar.
Materials and methods
Subjects
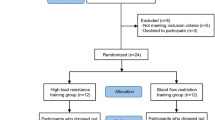
Young male adults (YA, n = 11, 23 ± 2 year, 180.7 ± 6.7 cm, 76.5 ± 8.8 kg, mean ± SD) and older male adults (OA, n = 13, 70 ± 5 year, 176.7 ± 8.3 cm, 83.1 ± 12.9 kg, mean ± SD) were recruited to participate in this study. All participants were non-smokers, had not undertaken any heavy-load strength exercise within the previous 6 months and did not present with any musculoskeletal, neurological, or vascular disease/injury. Prior to inclusion, participants provided written informed consent and underwent a pre-screening procedure that comprised a Mini Mental State Examination and a health questionnaire. Briefly, participants were excluded if presenting with pre-existing diagnosed diabetes mellitus or hypertension, or if currently taking prescribed medication for blood pressure control. While otherwise healthy, participants were classified as inactive/sedentary because none indicated participation in a consistent, structured, progressive training programme, and all participants reported undertaking less than 150 min of physical activity per week. This study was approved by the Human Ethics Advisory Group, Deakin University.
Experimental design
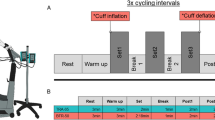
Participants attended the laboratory on three occasions. The first was a familiarisation session, with a further two experimental trials conducted in random balanced order. One experimental trial comprised two bouts of resistance exercise (45° double leg press, LP), the other trial comprised two bouts of aerobic exercise (0° incline/decline treadmill walking, TM) (Fig. 1). In each trial, one bout was light-intensity exercise without blood flow restriction (CON), the other bout being of equal light-intensity BFRE (BFR). Within each trial, exercise bouts were also conducted in a random balanced order. A minimum of 7 days separated each experimental trial.
Familiarisation session
Participants were instructed on the correct technique to undertake a rebreathing manoeuvre using a closed circuit metabolic system to measure cardiac output (\(\dot{Q}\)) (Innocor, Innovision A/S, Odense, Denmark). This included providing instruction about the breathing rate, depth and timing required to successfully conduct the test. Following this, an assessment of 1-RM leg-press strength was conducted to determine the load required for the LP trial, according to procedures we described previously (Brandner et al. 2015). Briefly, repetitions were controlled, with good posture, starting at full knee and hip extension, lowering to 90° knee flexion then returning to full leg extension. 1-RM was defined as the final load that could be successfully lifted with proper technique where an additional 2.5 kg could not be successfully lifted. Rest intervals between 1-RM attempts were dependent on participant readiness, but ranged from 3 to 5 min. In addition, participants underwent a short exposure to BFR at a pressure estimated to be in the range of that to be determined for the BFR experimental trial.
Experimental trials
Each bout comprised four sets of exercise separated by 1-min rest periods. For the LP trial, the first set comprised 30 repetitions and was followed by three sets of 15 repetitions with the load equivalent to 20 % 1-RM. Repetitions were performed at a fixed cadence guided by a metronome (2-s eccentric; 2-s concentric). For the TM trial, sets were 2 min duration performed at a velocity of 4 km h−1 (Abe et al. 2006; Renzi et al. 2010; Sakamaki et al. 2011).
Rest periods between bouts were 20–40 min and determined by initially ensuring that heart rate (HR) and blood lactate had returned to baseline (tested every 5 min from 15 min into the rest period), whereby subsequent (every 5 min) testing of blood pressure (BP) and \(\dot{Q}\) was performed to ensure these measures had also returned to baseline prior to the commencement of the next exercise bout. Rest periods in all cases did not exceed 40 min.
Blood-flow restriction
For all BFR bouts, blood flow restriction was applied using an automatic tourniquet system (ATS 3000, Zimmer Inc., OH, USA) connected to inflatable pneumatic cuffs (86-cm long, 10.5-cm wide; bladder width 8 cm). Cuffs were placed around the most proximal portion of each thigh. Blood flow restriction was applied for the entire duration of the bout (i.e. throughout all sets and rest periods) and released at the conclusion of the bout, immediately prior to the rest period. To provide an individualised cuff pressure, the restriction pressure was set at 60 % of the participant’s pre-determined limb occlusion pressure (LOP) (Table 2). This was within the range of absolute pressures used previously and that have been shown to increase muscle strength and size when used throughout a BFRE training programme (Yasuda et al. 2011; Takarada et al. 2000; Sumide et al. 2009).
LOP was determined separately for each limb and prior to each trial. For the TM trial, this was conducted with participants standing, while for the LP trial participants lay supine. These positions were chosen to allow stable measurement of LOP and to ensure that the level of the heart relative to the restrictive cuffs was similar to that when performing exercise during each trial. With the restriction cuffs in place on the limb, a plethysmograph (LOP Sensor Kit, Zimmer Inc., OH, USA) was applied to the distal process of the second phalange of the foot (second toe). Following a 5-min rest period, the automated measurement of LOP was performed using the inbuilt LOP function (ATS 3000, Zimmer Inc., OH, USA), whereby the restriction cuffs gradually inflated to produce a continuous rise in pressure until tissue blood flow was no longer detected at the toe. Measurements of LOP were conducted twice on each limb and were typically within 20 mmHg, whereby the average was then used to set the cuff pressure for the BFR bout for that limb. LOP measurements greater than 20 mmHg apart required a third test to be conducted, after which the average of all tests was used to set the cuff pressure for the BFR bout.
Measurements
Haemodynamic parameters
For each trial, haemodynamic parameters were measured at baseline immediately prior to each bout and following a 5-min rest period while in the exercising posture. During exercise, haemodynamic measurements were also taken within the final 30 s of set 2 and set 4, with BP also taken during set 1 and set 3 (Fig. 1). To measure \(\dot{Q}\), participants completed a standard inert gas rebreathing technique, as described previously (Fontana et al. 2010; Brandner et al. 2015) (Innocor DK-5260, Innovision, Odense, Denmark). For LP, all rebreathing manoeuvres were undertaken at a breathing rate synchronised with the contraction cycle to minimise transmural pressures, provide some haemodynamic protection and a more consistent approach to haemodynamic measurements. For TM, rebreathing manoeuvres were conducted under the participant’s natural respiratory frequency and tidal volume. HR was obtained via a standard chest strap and wrist unit (RS800CX, Polar Electro, Kemple, Finland), with stroke volume (SV) subsequently derived as the quotient of \(\dot{Q}\) and HR. Brachial artery blood pressures (systolic (sBP); diastolic (dBP); mean arterial (MAP)) were recorded via a manual sphygmomanometer, with total peripheral resistance (TPR = MAP/\(\dot{Q}\)) and double product (DP = sBP × HR) derived during subsequent analysis (Nelson et al. 1974). Mean arterial pressure (MAP) was calculated according to the methods of Moran et al. (1995) (Moran et al. 1995). Age-predicted maximum HR (APHRmax) was estimated according to the formula [206.9 − (0.67 × age)] (Gellish et al. 2007).
Perceptual responses
At the completion of each bout, participants were required to provide a rating of perceived exertion (RPE) and a rating of perceived pain (RPP) (Borg 1998) on a modified Borg scale ranging from 0 (no exertion/no pain) to 10 (maximal exertion/maximal pain).
Lactate
Blood lactate via fingerprick was recorded at baseline and immediately upon completion of each exercise bout (Lactate Pro, Arkray Inc, Japan) (Fig. 1).
Data presentation and statistical analyses
An initial analysis of all haemodynamic variables showed no difference between exercising values across all sets, as such measurements across time (sets) were averaged for each parameter to provide an exercising value. These were then analysed via a multiple-factor repeated measures analysis of variance (ANOVA) for group (YA, OA), bout (BFR and CON) and time (baseline, exercise). Perceptual responses were also analysed via a multiple-factor repeated measures ANOVA for group (YA, OA), bout (BFR and CON) and trial (LP, TM).
Upon identification of a significant interaction or main effect a subsequent post hoc test (Tukey–Kramer) was used to identify specific differences. Unless otherwise stated, all data are presented as mean ± SEM. Significance was set at P < 0.05. All statistical analyses were computed using NCSS (v2007, NCSS LLC, Utah, USA). In addition, an a priori power analysis was conducted for expected outcomes for blood pressure (Takano et al. 2005; Brandner et al. 2015) with power set to 0.8. This deemed 15–18 participants in total to be suitable (G*Power v3.1.7 free software, Institute of Experimental Psychology, Heinrich Heine University, Dusseldorf, Germany).
Results
Physical characteristics
Baseline anthropometric characteristics, resting haemodynamic measurements and 1-RM leg-press strength obtained during the familiarisation session are displayed in Table 1. Of note, blood pressures (sBP, dBP and MAP) were higher in OA compared with YA, while 1-RM leg-press strength was lower. In addition, while restriction pressures were not different between limbs, these were greater in OA compared with YA (main effect) and for TM compared with LP (Table 2). Moreover, restriction pressures were similar between groups for the TM trial, but lower in YA compared with OA for the LP trial (group × trial interaction).
Haemodynamic responses
Leg-press trial
HR increased from baseline to exercise, and this was greater for BFR compared with CON (Table 3). This increase in HR during exercise was also greater in YA compared with OA independent of bout, but not when expressed relative to APHRmax. From baseline to exercise, \(\dot{Q}\) increased similarly in both CON and BFR (Table 3). However, this increase was greater in YA (group × time interaction) even though \(\dot{Q}\) was greater in YA compared with OA (main effect for group). In contrast, SV did not change from baseline to exercise and was not different between CON and BFR. However, SV was greater in YA compared with OA (main effect).
Mean and systolic blood pressures (sBP, MAP) were higher in OA compared with YA (main effect; Table 4) and increased similarly in both groups from baseline to exercise. This increase was greater for BFR compared with CON (Table 4). TPR remained unchanged from baseline to exercise (Table 4) and was not different between CON and BFR. However, TPR was greater in OA compared with YA (main effect). DP increased from baseline to exercise, with this increase being greater for YA compared with OA. In addition, the increase in DP was greater in BFR compared with CON.
Treadmill trial
HR increased similarly from baseline to exercise in both YA and OA. However, this increase was greater for BFR compared with CON. When expressed relative to APHRmax, the increase in HR from baseline to exercise was also similar between YA and OA, with this increase being larger for BFR compared with CON (Table 3). \(\dot{Q}\) increased similarly from baseline to exercise in both CON and BFR (Table 3). However, \(\dot{Q}\) was lower in OA compared with YA (main effect). SV was greater in YA compared with OA (main effect). SV increased from baseline to exercise, but to a lesser extent in BFR compared with CON for both OA and YA.
Mean and systolic blood pressures increased from baseline to exercise. This increase was greater for BFR compared with CON (Table 4). However, blood pressures were not different between OA and YA. TPR was greater for CON compared with BFR at baseline only and decreased similarly during exercise in both bouts. Additionally, TPR was greater for OA compared with YA at both baseline and exercise (main effect). DP increased similarly from baseline to exercise in both YA and OA. This increase was greater in BFR compared with CON.
Lactate
For LP, lactate increased from baseline in all bouts in both groups. However, the increase was greater for YA compared with OA. Lactate concentrations were not different between bouts in either YA or OA, but tended to be greater for BFR compared with CON (P = 0.06) (Table 5).
For TM, lactate remained unchanged from baseline in all bouts and was not different between bouts or groups at any measurement point (Table 5).
Perceptual responses
RPE and RPP scores reported by participants showed identical statistical effects (Table 5). There was a main effect for trial, such that scores in LP were greater than TM. In addition, there was a main effect for bout such that scores were greater for BFR compared with CON. Moreover, YA reported higher scores following LP compared with OA, but these were not different between groups for TM.
Discussion
Major findings
The major findings of the present study were that the acute haemodynamic responses to low-load exercise with an applied blood flow restriction (BFR) were greater than for CON and that these responses were not different between young (YA) and older adults (OA). However, these haemodynamic responses were apparently greater for leg-press resistance exercise (LP) when compared with treadmill walking (TM), which is supported by the greater blood lactate and perceptual responses also observed for LP compared with TM.
While we did not compare these haemodynamic responses to other modes of exercise (e.g. HLRE) that are more typically expected to produce gains in muscle size and strength (Yasuda et al. 2011), cross-sectional comparisons would suggest the greater responses for BFR compared with CON were not as great as those observed for bilateral heavy-load resistance (Mayo and Kravitz 1999), nor intense aerobic exercise (Gosselin et al. 2012). As such, these data provide support to the proposal that BFRE may be a viable alternative mode of exercise training in older adults, and perhaps even more so when undertaken for aerobic exercise such as walking, to elicit gains in muscle size and strength to benefit functional fitness.
LP trial
In the present study, \(\dot{Q}\), HR and SV responded similarly to the leg-press exercise between bouts (CON and BFR) in both YA and OA, despite being lower overall at baseline and during exercise in YA compared with OA. In comparison with a control exercise, the similar exercising \(\dot{Q}\) with BFRE in conjunction with an elevated HR is typically explained by a lower SV as a direct result of the applied BFR causing a reduction in venous return (Takano et al. 2005; Renzi et al. 2010). In the present study, there was no evidence of a reduction in SV in the BFR bout. Most likely, this was due to the posture/positioning of participants such that the elevation of the legs provided some assistance to venous return that is not present with other modes of BFRE performed whilst seated or standing (Renzi et al. 2010; Rossow et al. 2012; Takano et al. 2005). However, we have also shown previously that SV remains unchanged during unilateral bicep curl BFRE of small muscle groups (Brandner et al. 2015), and while wider cuffs combined with lower restriction pressures are known to reduce the haemodynamic stress of BFRE (Rossow et al. 2012), it remains untested as to whether a reduction in SV is a necessary consequence that is indicative of suitably prevailing BFRE conditions required to maximise the muscle adaptations observed with chronic BFRE training.
In contrast, blood pressure responses were similar to those observed previously, being greater for BFR compared with CON (Takano et al. 2005; Rossow et al. 2012; Renzi et al. 2010; Vieira et al. 2013). However, with sBP and MAP being greater in OA compared with YA at baseline and during exercise, the absence of an age-by-bout interaction suggests a blood pressure response to BFRE that is similar between groups.
The tendency for leg-press BFRE to induce a greater increase in blood lactate was similar in both OA and YA (P = 0.06), and similar to that observed previously in young participants (Fujita et al. 2007). However, it is expected that the level of applied restriction will somewhat determine the magnitude of the metabolic response to BFRE (Lagally et al. 2002). Given a main effect for age such that there was a greater blood lactate response to exercise in YA when compared with OA that was independent of bout, it appears this response was not due to any influence of the applied cuff pressure during the BFR bout. Instead, it is possible that the relative exercising load for OA may have been underestimated making exercise in YA relatively more difficult (Ploutz-Snyder and Giamis 2001). It is also possible that a different fibre-type proportion and/or distribution between OA and YA may have contributed given that type II muscle fibre size and number, and hence glycolytic capacity, are known to be reduced with ageing such that the capacity to produce lactate with light-load exercise may also be reduced (Deschenes 2004). This apparently different metabolic effect of age between YA and OA appears to be supported by the greater RPE in YA compared with OA that was also independent of bout. However, RPE was greater for BFR than CON, and so for BFRE this alone suggests this mode of exercise was more difficult in both YA and OA, which is aligned with the tendency for elevated metabolism (blood lactate) (P = 0.06).
TM trial
For the treadmill trial, the increase in \(\dot{Q}\) was also not different between BFR and CON. However, unlike for LP, this was driven by a greater HR in BFR combined with a lower SV when compared with CON. This haemodynamic response is typical for BFRE (Takano et al. 2005), and despite a main effect for age for \(\dot{Q}\), HR and SV with these being lower in OA when compared with YA, the response to exercise in the CON and BFR bouts was similar between groups. This response highlights that an applied blood flow restriction may limit the rise in venous return and SV during exercise and result in greater compensatory sympathetic stimulation to increase HR and maintain \(\dot{Q}\) (Takano et al. 2005; Renzi et al. 2010). The absence of this effect in LP when seated with slightly raised legs in comparison to when upright while walking highlights the importance of a postural contribution to the response to BFRE that has not previously been examined.
While the blood pressure response to TM was greater during BFR in comparison with CON, these responses were similar between YA and OA and are similar to previous observations (Renzi et al. 2010; Sakamaki et al. 2008). Indeed, the magnitude of this response in TM was also apparently lower than that for LP. This lends support for aerobic BFRE training using protocols similar to that of the present study to be an alternative to HLRE or even resistance BFRE in older adult populations given such protocols have previously been shown to increase both muscle size and strength (Abe et al. 2006; Renzi et al. 2010; Sakamaki et al. 2011).
This is also supported by the perceptual response for TM that showed similar RPE and RPP responses between OA and YA. While these were greater during the BFR bout, they were typically low, and still lower in comparison with LP as shown previously (Loenneke et al. 2011; Wernbom et al. 2006; Yasuda et al. 2011). Although we did not compare against a high-intensity bout for either LP (e.g. 80 % 1-RM) or TM (e.g. 80 % \(\dot{V}{\text{O}}_{2}\) max), it is generally observed that perceptual responses for resistance BFRE are lower than a high-intensity equivalent (Hollander et al. 2010). In addition, data from our laboratory (unpublished) not only supports this for resistance exercise, but aerobic exercise as well.
Conclusions
Walking as a form of physical activity is suitable for older adults given that the low-to-moderate intensity confers a reduction in the apparent musculoskeletal and/or cardiovascular stress. The present study shows that when BFR is applied during walking exercise, the haemodynamic and perceptual responses are lower than for light-load resistance exercise with BFR. Therefore, walking combined with blood flow restriction may provide a more favourable alternative to HLRE to increase muscle size, strength and functional capacity in older adults when undertaken chronically across a training programme. Moreover, other clinical populations may also be suited to BFRE (walking or otherwise) to gain benefits from an achievable and practical alternative to HLRE to limit the progression of age-related muscle atrophy.
Abbreviations
- BFRE:
-
Blood flow restriction exercise
- HLRE:
-
Heavy load resistance exercise
- LLRE:
-
Light load resistance exercise
- YA:
-
Young adults
- OA:
-
Older adults
- BFR:
-
Blood flow restriction exercise bout
- CON:
-
Control exercise bout
- LP:
-
Leg-press trial
- TM:
-
Treadmill walking trial
- LOP:
-
Limb occlusion pressure
- 1-RM:
-
One repetition maximum
- BP:
-
Blood pressure
- HR:
-
Heart rate
- APHRmax :
-
Age-predicted maximum heart rate
- \(\dot{Q}\) :
-
Cardiac output
- SV:
-
Stroke volume
- MAP:
-
Mean arterial pressure
- sBP:
-
Systolic blood pressure
- dBP:
-
Diastolic blood pressure
- TPR:
-
Total peripheral resistance
- DP:
-
Double product
- RPE:
-
Rating of perceived exertion
- RPP:
-
Rating of perceived pain
References
Abe T, Yasuda T, Midorikawa T, Sato Y, Kearns CF, Inoue K, Koizumi K, Ishii N (2005) Skeletal muscle size and circulating IGF-1 are increased after 2 weeks of twice daily “KAATSU” resistance training. Int J KAATSU Training Res 1(1):6–12. doi:10.3806/ijktr.1.6
Abe T, Kearns CF, Sato Y (2006) Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol 100(5):1460–1466. doi:10.1152/japplphysiol.01267.2005
Abe T, Fujita S, Nakajima T, Sakamaki M, Ozaki H, Ogasawara R, Sugaya M, Kudo M, Kurano M, Yasuda T, Sato Y, Ohshima H, Mukai C, Ishii N (2010) Effects of low-intensity cycle training with restricted leg blood flow on thigh muscle volume and VO2max in young men. J Sports Sci Med 9(3):452–458
Borg G (1998) Borg’s perceived exertion and pain scales. Human Kinetics, Champaign
Brandner CR, Kidgell DJ, Warmington SA (2015) Unilateral bicep curl hemodynamics: low-pressure continuous vs high-pressure intermittent blood flow restriction. Scand J Med Sci Sports. doi:10.1111/sms.12297 (in Press)
Clark BC, Manini TM, Hoffman RL, Williams PS, Guiler MK, Knutson MJ, McGlynn ML, Kushnick MR (2011) Relative safety of 4 weeks of blood flow-restricted resistance exercise in young, healthy adults. Scand J Med Sci Sports 21(5):653–662. doi:10.1111/j.1600-0838.2010.01100.x
Deschenes MR (2004) Effects of aging on muscle fibre type and size. Sports Med 34(12):809–824. doi:10.2165/00007256-200434120-00002
Fletcher GF, Balady G, Blair SN, Blumenthal J, Caspersen C, Chaitman B, Epstein S, Froelicher ESS, Froelicher VF, Pina IL, Pollock ML (1996) Statement on exercise: benefits and recommendations for physical activity programs for all Americans—a statement for health professionals by the committee on exercise and cardiac rehabilitation of the council on clinical cardiology, American Heart Association. Circulation 94(4):857–862
Fontana P, Boutellier U, Toigo M (2010) Non-invasive haemodynamic assessments using Innocor during standard graded exercise tests. Eur J Appl Physiol 108(3):573–580. doi:10.1007/s00421-009-1252-x
Frankel JE, Bean JF, Frontera WR (2006) Exercise in the elderly: research and clinical practice. Clin Geriatr Med 22(2):239–256. doi:10.1016/j.cger.2005.12.002
Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB (2007) Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol 103(3):903–910. doi:10.1152/japplphysiol.00195.2007
Gellish RL, Goslin BR, Olson RE, McDonald A, Russi GD, Moudgil VK (2007) Longitudinal modeling of the relationship between age and maximal heart rate. Med Sci Sports Exerc 39(5):822–829. doi:10.1097/mss.0b013e31803349c6
Gosselin LE, Kozlowski KF, DeVinney-Boymel L, Hambridge C (2012) Metabolic response of different high-intensity aerobic interval exercise protocols. J Strength Cond Res 26(10):2866–2871. doi:10.1519/JSC.0b013e318241e13d
Hollander DB, Reeves GV, Clavier JD, Francois MR, Thomas C, Kraemer RR (2010) Partial occlusion during resistance exercise alters effort sense and pain. J Strength Cond Res 24(1):235–243. doi:10.1519/JSC.0b013e3181c7badf
Hurley BF, Roth SM (2000) Strength training in the elderly: effects on risk factors for age-related diseases. Sports Med 30(4):249–268
Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R (2004) The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 52(1):80–85. doi:10.1111/j.1532-5415.2004.52014.x
Karabulut M, Abe T, Sato Y, Bemben MG (2010) The effects of low-intensity resistance training with vascular restriction on leg muscle strength in older men. Eur J Appl Physiol 108(1):147–155. doi:10.1007/s00421-009-1204-5
Karabulut M, Bemben DA, Sherk VD, Anderson MA, Abe T, Bemben MG (2011) Effects of high-intensity resistance training and low-intensity resistance training with vascular restriction on bone markers in older men. Eur J Appl Physiol 111(8):1659–1667. doi:10.1007/s00421-010-1796-9
Karlsen T, Helgerud J, Stoylen A, Lauritsen N, Hoff J (2009) Maximal strength training restores walking mechanical efficiency in heart patients. Int J Sports Med 30(5):337–342. doi:10.1055/s-0028-1105946
Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, Fleck SJ, Franklin B, Fry AC, Hoffman JR, Newton RU, Potteiger J, Stone MH, Ratamess NA, Triplett-McBride T, American College of Sports M (2002) American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 34(2):364–380
Lagally KM, Robertson RJ, Gallagher KI, Goss FL, Jakicic JM, Lephart SM, McCaw ST, Goodpaster B (2002) Perceived exertion, electromyography, and blood lactate during acute bouts of resistance exercise. Med Sci Sports Exerc 34(3):552–559
Loenneke JP, Pujol TJ (2009) The use of occlusion training to produce muscle hypertrophy. Strength Cond J 31(3):77–84. doi:10.1519/Ssc.0b013e3181a5a352
Loenneke JP, Balapur A, Thrower AD, Barnes JT, Pujol TJ (2011) The perceptual responses to occluded exercise. Int J Sports Med 32(3):181–184. doi:10.1055/s-0030-1268472
Marcell TJ (2003) Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci 58(10):M911–M916. doi:10.1093/gerona/58.10.M911
Mayo JJ, Kravitz L (1999) A review of the acute cardiovascular responses to resistance exercise of healthy young and older adults. J Strength Cond Res 13(1):90–96
Moran D, Epstein Y, Keren G, Laor A, Sherez J, Shapiro Y (1995) Calculation of mean arterial pressure during exercise as a function of heart rate. Appl Human Sci 14(6):293–295
Nelson RR, Gobel FL, Jorgensen CR, Wang K, Wang Y, Taylor HL (1974) Hemodynamic predictors of myocardial oxygen consumption during static and dynamic exercise. Circulation 50(6):1179–1189. doi:10.1161/01.cir.50.6.1179
Ploutz-Snyder LL, Giamis EL (2001) Orientation and familiarization to 1RM strength testing in old and young women. J Strength Cond Res 15(4):519–523
Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B, Limacher M, Piña IL, Stein RA, Williams M, Bazzarre T (2000) Resistance exercise in individuals with and without cardiovascular disease : benefits, rationale, safety, and prescriptionan advisory from the committee on exercise, rehabilitation, and prevention, council on clinical cardiology, American Heart Association. Circulation 101(7):828–833. doi:10.1161/01.cir.101.7.828
Renzi CP, Tanaka H, Sugawara J (2010) Effects of leg blood flow restriction during walking on cardiovascular function. Med Sci Sports Exerc 42(4):726–732. doi:10.1249/MSS.0b013e3181bdb454
Rossow LM, Fahs CA, Loenneke JP, Thiebaud RS, Sherk VD, Abe T, Bemben MG (2012) Cardiovascular and perceptual responses to blood-flow-restricted resistance exercise with differing restrictive cuffs. Clin Physiol Funct Imaging 32(5):331–337. doi:10.1111/j.1475-097X.2012.01131.x
Sakamaki M, Fujita S, Sato Y, Bemben MG, Abe T (2008) Blood pressure response to slow walking combined with KAATSU in the elderly. Int J KAATSU Training Res 4(1):17–20. doi:10.3806/ijktr.4.17
Sakamaki M, Bemben MG, Abe T (2011) Legs and trunk muscle hypertrophy following walk training with restricted leg muscle blood flow. J Sports Sci Med 10(2):338–340
Sumide T, Sakuraba K, Sawaki K, Ohmura H, Tamura Y (2009) Effect of resistance exercise training combined with relatively low vascular occlusion. J Sci Med Sport 12(1):107–112. doi:10.1016/j.jsams.2007.09.009
Takano H, Morita T, Iida H, Asada K, Kato M, Uno K, Hirose K, Matsumoto A, Takenaka K, Hirata Y, Eto F, Nagai R, Sato Y, Nakajima T (2005) Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol 95(1):65–73. doi:10.1007/s00421-005-1389-1
Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N (2000) Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 88(6):2097–2106
Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NA 3rd, Fulton JE, Gordon NF, Haskell WL, Link MS, Maron BJ, Mittleman MA, Pelliccia A, Wenger NK, Willich SN, Costa F (2007) Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 115(17):2358–2368. doi:10.1161/CIRCULATIONAHA.107.181485
Vieira PJ, Chiappa GR, Umpierre D, Stein R, Ribeiro JP (2013) Hemodynamic responses to resistance exercise with restricted blood flow in young and older men. J Strength Cond Res 27(8):2288–2294. doi:10.1519/JSC.0b013e318278f21f
Wernbom M, Augustsson J, Thomee R (2006) Effects of vascular occlusion on muscular endurance in dynamic knee extension exercise at different submaximal loads. J Strength Cond Res 20(2):372–377. doi:10.1519/R-16884.1
Yasuda T, Ogasawara R, Sakamaki M, Ozaki H, Sato Y, Abe T (2011) Combined effects of low-intensity blood flow restriction training and high-intensity resistance training on muscle strength and size. Eur J Appl Physiol 111(10):2525–2533. doi:10.1007/s00421-011-1873-8
Acknowledgments
This research was supported only by local funds made available by the School of Exercise and Nutrition Sciences, Faculty of Health, Deakin University, Victoria, Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Carsten Lundby.
Rights and permissions
About this article
Cite this article
Staunton, C.A., May, A.K., Brandner, C.R. et al. Haemodynamics of aerobic and resistance blood flow restriction exercise in young and older adults. Eur J Appl Physiol 115, 2293–2302 (2015). https://doi.org/10.1007/s00421-015-3213-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3213-x