Abstract
Purpose
To determine the macrovascular and microvascular function responses to resistance training with blood flow restriction (BFR) compared to high-load resistance training (HLRT) control group.
Methods
Twenty-four young, healthy men were randomly assigned to BFR or HLRT. Participants performed bilateral knee extensions and leg presses 4 days per week, for 4 weeks. For each exercise, BFR completed 3 X 10 repetitions/day at 30% of 1-repetition max (RM). The occlusive pressure was applied at 1.3 times of individual systolic blood pressure. The exercise prescription was identical for HLRT, except the intensity was set at 75% of one repetition maximum. Outcomes were measured pre-, at 2- and 4-weeks during the training period. The primary macrovascular function outcome was heart-ankle pulse wave velocity (haPWV), and the primary microvascular function outcome was tissue oxygen saturation (StO2) area under the curve (AUC) response to reactive hyperemia.
Results
Knee extension and leg press 1-RM increased by 14% for both groups. There was a significant interaction effect for haPWV, decreasing − 5% (Δ−0.32 m/s, 95% confidential interval [CI] − 0.51 to – 0.12, effect size [ES] = − 0.53) for BFR and increasing 1% (Δ0.03 m/s, 95%CI − 0.17 to 0.23, ES = 0.05) for HLRT. Similarly, there was an interaction effect for StO2 AUC, increasing 5% (Δ47%・s, 95%CI − 3.07 to 98.1, ES = 0.28) for HLRT and 17% (Δ159%・s, 95%CI 108.23–209.37, ES = 0.93) for BFR group.
Conclusion
The current findings suggest that BFR may improve macro- and microvascular function compared to HLRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A major advantage of resistance training with blood flow restriction (BFR) is that skeletal muscle adaptations can be induced at low intensities (Madarame et al. 2008; Takada et al. 2012; Takarada et al. 2000), making this approach more appealing to those who are previously untrained or who have orthopedic complications (Roth et al. 2000). However, the findings are mixed when it comes to the effects of BFR on vascular function. If low-intensity resistance training with BFR can safely improve vascular function in addition to conferring increases in muscle strength and/or volume, this type of exercise may be particularly useful for populations who cannot safely perform high-intensity resistance training, such as elderly, orthopedic patients, or athletes under rehabilitation. In this regard, it would be more clinically relevant if the experiments could be conducted in these populations. However, BFR training should not be recommended for these populations until it is proven to be safe (Loenneke et al. 2011). Thus, it is pertinent to confirm that it poses no detriment to the vasculature, at least, for young, healthy populations.
Two meta-analyses, both published in 2021, have reported on the overall effect of BFR on macrovascular function (arterial stiffness or compliance, and vasodilator function), and vascular structure. Compared to high-load resistance training without BFR, one meta-analysis reported that low-load resistance training with BFR improved macrovascular function evaluated by endothelium vasodilation, but, did not affect vascular structure (Pereira-Neto et al. 2021), and the other reported that low-load resistance training with BFR showed a positive effect for arterial stiffness (or compliance), but not for vasodilator function (Liu et al. 2021). These conflicting findings make it difficult to reach a definitive conclusion. However, significant heterogeneity was reported for both meta-analyses, likely due in part to age differences across studies, and the inclusion of individuals with cardiometabolic diseases. Of note, none of the studies included in the meta-analyses investigated both macrovascular and microvascular function. While the macrovasculature is often the primary outcome of interest, microvascular function is also associated with the development of cardiovascular disease (Houben et al. 2017) and may precede macrovascular dysfunction (Krentz et al. 2009). Proposed mechanisms underlying the beneficial effects of BFR on vascualr function include the promotion of the release of vasodilator factors (e.g., nitric oxide, vascular endothelial growth factor) due to shear stress in the arterial wall (Paiva et al. 2016), potentially enhanced by reperfusion upon cuff release (Loenneke et al. 2011; Pereira-Neto et al. 2021).
Therefore, the aim of the present study was to determine the effects of resistance training, with and without BFR on macrovascular and microvascular function in young, healthy men.
Methods
This study is reported according Consolidated Standards of Reporting Trials (CONSORT) guidelines (Schulz et al. 2010). All study procedures were approved by the ethical committee of the Yamanashi Institute of Environmental Science (current name: Mount Fuji Research Institute) and were performed in accordance with the guidelines of the Declaration of Helsinki (ECHE-03-2012). All participants submitted their written informed consent prior to participating in the study.
Participants
We recruited young, healthy men between the ages of 18 and 30 years using a flyer at a local community and an adjacent university. A diagram illustrating our flow of enrollment, allocation, and analyis is shwon in Fig. 1, according to CONSORT guidelines. Young and healthy populations were selected to minimize confounding effects of age and health conditions on vascular function (Horiuchi and Stoner 2021, 2022). Additionally, only men participated in this study. This is because the duration of the training period and evaluation time points consisted four weeks (see below “Experimental design”), and thus, we sought to avoid potential effects of menstrual cycle on our outcomes of interest, which could occur even if sex was randomized within our experimental groups. The exclusion criteria were regular engagement in resistance training and moderate or vigorous physical activity, such as jogging, running, or stationary bike riding (120 > min/week) (Horiuchi and Stoner 2021, 2022), as recreationally active individuals may exercise regularly in addition to the exercise training protocol of the present study, introducing potentially effects on vascular function that may not be seen in the less active individuals. Additionally, participants were excluded if they were a current smoker, had any known musculoskeletal and cardiometabolic disorders, or used medications known to affect musculoskeletal or cardiometabolic function.
Experimental design
This intervention study randomly allocated participants to one of two experimental groups: (i) a high-load resistance training group (HLRT, n = 12) and (ii) a low-intensity resistance training with blood flow restriction group (BFR, n = 12). Participants were stratified by age, height, and body mass and randomly assigned to the HLRT or BFR group using a lottery. All participants underwent a familiarization session to allow them to become accustomed to the training modes, experimental measurements (e.g., evaluation of maximal muscle strength to avoid a potential practice effect during the training period), and procedures. To eliminate the effects of diurnal variations and food intake (Hakkinen et al. 1988), all participants visited the laboratory between 9:00 and 12:00 AM, at least 2 h after eating a light breakfast. Additionally, participants were asked to avoid strenuous physical activity and alcohol for 24 h prior to experimentation. Experimental procedures were conducted in a quiet, environmentally controlled room, at a constant room temperature between 23 and 25 °C. The following variables were measured prior to the training period, at 2 weeks, and after 4 weeks of training: body weight, thigh circumference, heart-ankle pulse wave velocity (haPWV), resting heart rate (HR), four-limb blood pressure (BP), microvascular function, and maximal strength for knee extension and leg press. Specifically, these outcomes were assessed approximately 48 hours after 2 and 4 weeks of training. Thigh circumference was measured with a non-elastic tape scale for both thigh muscles at the height of the middle portion between the patella and iliac bone. After 30 min of supine rest, BP cuffs were applied bilaterally to the upper arms and ankles, and the legs were placed in commercial vacuum packs to prevent lower limb movement (Horiuchi and Stoner 2021, 2022). The cuffs were attached to a commercial vascular screening system (Vasera-1000; Fukuda-Denshi, Co. Ltd., Japan). To assess microvascular function, near-infrared spectroscopy (NIRS) probes (BOM-L1TRW; OmegaWave, Tokyo, Japan) were attached to the calf muscle, and a pneumatic cuff was also wrapped around the thigh muscle. Macrovascular, microvascular, BP, and HR measures were collected in the supine position following an additional 10 min of rest.
Training protocol
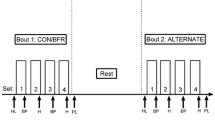
Resistance exercise occurred after the assessment of vascular function, consisting of bilateral knee extensions and leg presses. In the HLRT group, each subject performed knee extensions and leg presses at 75% of one repetition maximum (1-RM), for 3 sets of 10 reps, with 2 min rest intervals per one day. In the BFR group, each subject performed these exercises at 30% of 1-RM with BFR, for 4 sets of 20 reps, with 30 s rest intervals per one day. This training protocol was modified based on previous studies (Fahs et al. 2012; Ozaki et al. 2013) and a preliminary study at our lab. The intent was to match the training load between conditions was matched to attempt to isolate the effect of BFR versus non-BFR on macrovascular and microvascular outcomes.
For the 1-RM measurements, each participant was seated in the adjustable chair of the knee extension or leg press apparatus and was stabilized with straps across the waist to prevent additional body movement. Participants were instructed to lift the load through the range of motion, preventing assistance from any other body part (e.g., the arm holding the apparatus, the back). The 1-RM was determined as a successful contraction on each apparatus, while following these criteria. The 1-RM trials were designed using increments of 10 kg until 60–80% of the perceived maximum was reached (Takada et al. 2012). Next, the load was gradually increased by adding 0.5-5 kg weights until lift failure, which was defined as the participant’s failure to maintain proper form or to completely lift the weight. The last acceptable lift with the highest possible load was determined as the 1-RM (Takada et al. 2012). These trainings were carried out 4 days per week over the period of 4 weeks. In the BFR group, circulatory occlusion was applied using a custom-built tourniquet reformed by a commercial device (Oscar 2 Ambulatory Blood Pressure Monitor; SunTech Medical, Inc., NC, USA) (Horiuchi and Stoner 2022) around the both thigh muscles with a pressure that was 1.3 times the individual’s ankle systolic blood pressure (Takada et al. 2012). The cuff was inflated for the entirety of each training session (i.e., 4 sets including the resting period for knee extension or leg press) with a commercial cuff (width × length; 11 × 85 cm, SC10™, Hokanson, Inc., WA, USA). All exercise training was performed under supervision.
We also confirmed that none of our participants experienced any excess discomfort and pain throughout the training period, which could have led to training interruption or drop out.
Cardiovascular outcomes
Macrovascular function measurement
As an indicator of arterial stiffness, haPWV was measured using a commercial vascular screening system (Vasera-1000; Fukuda-Denshi, Co. Ltd., Japan) (Spronck et al. 2022). The VaSera device calculates haPWV using the equation: distance/pulse transit time. Distance was measured between the left sternal border (in the second intercostal space) and the center of the cuff applied to the ankle. To calculate pulse transit time as the sum of the time interval between the S2 heart sound on phonocardiogram and the dicrotic notch of the brachial pulse wave was recorded, and time interval between the foot of the brachial pressure wave to the foot of the ankle pressure wave. The difference between these time intervals yields the time required for pulse waves to travel from the heart (aortic orifice) to the ankle. During pulse wave measurements, a low cuff pressure from 30 to 50 mm Hg was used to minimize the effect of cuff pressure on hemodynamics. Thereafter and using the same device, upper arm and ankle BP (oscillometric method), and HR (electrocardiography) were measured.
Microvascular function measurement
Microvascular function was quantified as the reperfusion rate of the medial gastrocnemius after occlusion. Following haPWV and BP measurements, local tissue oxygenation profiles at the calf muscles were measured using NIRS (BOM-L1TRW; OmegaWave, Tokyo, Japan). This instrument uses three laser-diodes (780, 810, and 830 nm) and calculates the relative tissue levels of oxygenated and deoxygenated hemoglobin according to the modified Beer-Lambert law (Horiuchi and Stoner 2021, 2022). Given that large vessels (> 1 mm diameter) contain sufficient hemoglobin to maximally absorb near-infrared light, the NIRS signals reflect changes in light absorption caused by hemoglobin in the small arterioles, capillaries, and venules (Mancini et al. 1994). To guide accurate placement, the NIRS optodes were placed on the calf muscles after carefully examining the target muscle using Doppler ultrasound (Logic-e; GE Healthcare, Tokyo, Japan). The NIRS probe was placed unilaterally along the vertical plane of the central belly of the medial gastrocnemius at the position of maximum circumference. The location was marked, and between visits participants were asked to re-mark themselves each day to ensure identical site placement. To eliminate the effects of adipose tissue thickness on NIRS signals, adipose tissue and muscle thickness of calf muscle were also measured using Doppler ultrasound (Logic-e; GE Healthcare, Tokyo, Japan) for all participants. It is known that the measurement depth of the near-infrared signal is about half of the distance between the two fiber optic bundles that are placed over the skin, one comprising the light source and the other the detector (Patterson et al. 1989). With this in mind, a distance of 4 cm between probes was used, which would provide a NIRS signal traversing approximately 20 mm. Therefore, when NIRS probes were placed over the skin of each muscle, the near-infrared light was transmitted to the desired muscle bed (Patterson et al. 1989).
Microvascular function was quantified as area under the curve (AUC; units: %∙s) of the medial gastrocnemius tissue oxygen saturation (StO2) (Horiuchi and Okita 2020; Horiuchi and Stoner 2022; Soares and Murias 2018; Soares et al. 2017). Reactive hyperemia was induced by inflating a custom-built tourniquet reformed by a commercial device (Oscar 2 Ambulatory Blood Pressure Monitor; SunTech Medical, Inc., NC, USA) wrapped around the thigh to 220 mmHg for 5 min (Horiuchi and Stoner 2022). The same cuff (SC10™, Hokanson, Inc., WA, USA) used for exercise training was adopted for this evaluation. Measurements were made in duplicate, separated by 10 min, and the mean value was calculated. We confirmed that the resting baseline (pre-occlusion) value was apparent prior to initiation of the subsequent trial (Horiuchi and Okita 2020; Horiuchi and Stoner 2022). Total hemoglobin was calculated as the sum of oxygenated and deoxygenated hemoglobin. Next, StO2 was calculated by dividing the oxygenated hemoglobin by the total hemoglobin (represented as a percentage) and used for further analysis (Horiuchi and Okita 2020; Horiuchi and Stoner 2022).
Sample size
As there is no consensus regarding how BFR training impacts vascular function, sample size calculation was based on muscle strength, for which we could obtain comparable effects of BFR training for effect size estimation. A previous study reported that 10 weeks of low-intensity resistance training with BFR (twice per week) increased muscle strength by ~ 20% (Madarame et al. 2008). To detect a beneficial (or detrimental) effect at 90% statistical power with the maximum chances of a type-I error controlled at 5%, approximately 7 participants were required for each group. We recruited 12 participants for each group to mitigate the possible risk of drop-out during the exercise training period.
Statistical analyses
Statistical analyses were performed using the R programming language (RKWard, version 0.7.2). Only participants who had complete data for the primary outcomes were included in the analyses. Raw data are presented as mean [standard deviation, SD], and mixed model data are presented as mean (95% confidential interval: [95%CI]) and standard error. The corresponding author had full access to the data in the study and was responsible for the integrity of the data set and the data analyses. The α level was set a priori for main effects at 0.05, and for interaction effects at 0.10. Model assumptions were tested using Q–Q plots to diagnose normal distribution, and by plotting residual against fitted values to inspect bias.
An unpaired t test was used to compare the total exercise training workload between HLRT and BFR. The effects of time (pre vs post) and condition (HLRT vs. BRF) on haPWV and StO2 AUC were analyzed using linear mixed models, with subject (intercept) specified as a random effect and time (slope), and condition specified as fixed effects. To limit within-subject variance, the models were adjusted for baseline values as specified by Kenward and Roger (Kenward and Roger 2010). Baseline differences between HLRT and BFR were compared using Student’s unpaired t test. Effect size (ES) was calculated according to Cohen’s d levels, defined as a trivial (< 0.2), small (0.2), moderate (0.5), and large (0.8) (Hopkins et al. 2009). For the mixed models, Cohen’s d was calculated as the effect β divided by SD.
Results
Participants
We recruited 24 young men for the study, of which 12 were randomized to BFR (age: 22 [SD: 2] years, body mass index: 21.7 [1.1] kg・(m2)−1) and 12 to HLRT (22 [SD: 2] years, and 21.6 [1.1] kg・(m2)−1. All participants successfully completed both experimental trials, with no missing data.
Exercise training workload
Total training volumes of knee extension were 1396 ± 143 kg for HLRT and 1451 ± 158 kg for BFR with no difference between the two groups (P = 0.38). Similarly, those of leg press were 2892 ± 316 kg for HLRT and 2979 ± 401 kg for BFR with no difference between the two groups (P = 0.56).
Physical characteristics
Baseline calf adipose tissue (HLRT: 4.8 [0.6] mm vs. BFR: 5.0 [0.8] mm, P > 0.05) and muscle thickness (HLRT: 18.2 [1.3] mm vs. BFR: 17.7 [1.6] mm, P > 0.05) were not significantly different between groups. Changes in body composition and muscle strength at each timepoint are reported in Table 1. There were non-significant interaction and condition effects for all variables, but there were moderate-large time effects for all variables, including a 14% increase (Δ8.6 kg, 95% CI 7.6–9.6) in knee extension 1-RM, a 13% (Δ16.3 kg, 95% CI 14.0–18.5) increase in leg press strength 1-RM, and a 1.3% (Δ0.7 cm, 95% CI 0.5–0.8) in thigh circumference.
Cardiovascular outcomes
Cardiovascular outcomes are reported in Table 2. The interaction effect for mean arterial pressure (MAP, P = 0.763) and HR (P = 0.884) were both non-significant. MAP did not significantly differ by condition (Δ −0.3 mmHg, 95%%CI − 1.2 to 0.7) but there was a negligible, significant decrease of 1% with time (Δ –0.4 mmHg, 95%%CI − 1.1 to 0.4, ES = 0.2). For HR, both the condition and time effects were non-significant. The haPWV and StO2 AUC data are reported in Figs. 2 and 3. There was a marginal interaction effect (P = 0.087) for haPWV. The haPWV slightly decreased by 5% (Δ −0.32 m s−1, 95%CI − 0.51 to − 11.8, ES = 0.50) for BFR and increased by 1% (Δ−0.03 mm s−1, 95%CI − 0.17 to 0.23 ES = − 0.05) for HLRT (Fig. 2). There was a significant interaction effect for StO2 AUC (P = 0.013), which increased by 16.6% (Δ159%・s, 95%CI 108–209, ES = 0.93) and 4.9% (Δ47.5%・s, 95%CI − 3.07 to 98.1, ES = 0.23) for BFR and HLRT, respectively (Fig. 3).
Heart-ankle pulse wave velocity (haPWV) response to 4 weeks of exercise training: (i) high-load resistance training (HLRT) or (ii) low-intensity resistance training with blood flow restriction (BFR). (n = 12 for each group, data points = 72 = 12 participants × 2 groups × 3 measurement points). In the line graph, data are mean ± standard error of the mean (SE). ES effect size. Circles indicate individual data
Area under the curve (AUC) of tissue oxygen saturation (StO2) to 4 weeks of exercise training: (i) high-load resistance training (HLRT) or (ii) low-intensity resistance training with blood flow restriction (BFR). (n = 12 for each group, data points = 72 = 12 participants × 2 groups × 3 measurement points). In the line graph, data are mean ± standard error of the mean (SE). ES effect size. Circles indicate individual data
Discussion
Macrovascular function (haPWV) improved by 5% following BFR, but did not significantly change following HLRT. Additionally, microvascular function StO2 AUC improved for both BFR and HLRT, but significantly more so for BFR (17%) compared to HLRT (5%). Collectively, these findings suggest that, at least in young, healthy men, BFR may improve macro- and microvascular function compared to HLRT.
Macrovascular function
In the present study, we found that BFR training led to an improvement in macrovascular function, as quantified by a decrease in haPWV (Δ −0.32 m s−1, ES = 0.50) for BFR, whereas no such change resulted from HLRT (Δ−0.03 −1, ES = − 0.05). While the stimuli for macrovascular adaptations are multi-factorial, it is thought that the major stimulus for macrovascular improvements is shear stress (Cunningham and Gotlieb 2005; Zhou et al. 2014). Hypothetically, restricting blood flow to the lower limbs induces metabolite accumulation, thereby stimulating local vasodilation and a sustained post-exercise increases in blood flow-induced shear stress (Kooijman et al. 2008), which is shown as a hypothetical illustration (Fig. 4). In support, low-intensity exercise training with BFR decrease leg (femoral-tibial)- but not arm (carotid-radial) or central (carotid-femoral)- PWV (Karabulut et al. 2020). Nonetheless, our choice to evaluate macrovascular function with only haPWV should be further discussed. Different segments used in PWV measurements represent varying degrees of contributions from central elastic arteries and peripheral muscular arteries (Kim et al. 2018). Ideally, macrovascular function should be evaluated at central arteries such as carotid–femoral, heart-carotid, or heart-femoral PWV. The device used in the present study evaluates haPWV and brachial-ankle (ba)PWV, and both indices include the central aorta and peripheral muscular arteries. However, anatomically, haPWV is a direct combination of these arteries, while baPWV is not a simple unidirectional pathway for arterial pulse waves, resulting in the potential loss of some segments of central and peripheral arteries may be lost (Kim et al. 2018). Moreover, previous studies found less variability in haPWV than baPWV measurements when measured simultaneously (Liao et al. 2016; Lin et al. 2012). Although future studies measuring various segments of PWV may be required, especially, central artery only, the current findings clearly demonstrated that BFR may have a positive effect on macrovascular function. With respect to HLRT, it is possible that while this condition likely induced a greater blood-flow-induced shear stress response during the exercise bout, there would have been a lesser post-exercise hyperemic response, i.e., less of a sustained increase in shear stress.
Microvascular function
In the present study, improvements in macrovascular function, as quantified by StO2 AUC, occurred within both the BFR (Δ159%・s, ES = 0.93) and HLRT (Δ47.5%・s, ES = 0.23) groups, but greater improvements resulted from BFR training. This improvement in microvascular function post-BFR training is intuitive, as the stimuli for microvascular adaptations include hypoxia, increased myocellular metabolism, shear stress, and/or dynamic stretching of the vascular wall (Egginton 2009), all of which can be expected after ischemic exercise. The current literature appears to support the hypothesis that BFR leads to greater hypoxia relative to exercise without occlusion, and therefore a more robust stimulus for angiogenesis; one study has shown an acute angiogenic signaling response (i.e., increases in endothelial nitric oxide synthase, vascular endothelial growth factor, and hypoxia-inducible factor 1α mRNA expression) to low-load BFR training (Ferguson et al. 2018). Another study showed that short-term BFR training of the calves enhanced calf filtration capacity, which could be due to increased capillarization (Evans et al. 2010; Hunt et al. 2013). Therefore, we theorize that hypoxia resulted in angiogenesis post-BFR, leading to a greater StO2 AUC.
Clinical implications
The aim of this study was to progress the current literature pertaining to the impacts of BFR training on vascular function, as there is currently no clear consensus on the safety and efficacy of this modality. We aimed to assess vascular function more comprehensively by including measurements of both macro- and microvascular function. Contrary to a previous study (Credeur et al. 2010), we found that BFR training yielded improvements in both measurements. While we cannot fully ascertain the physiological mechanisms explaining these results, these preliminary findings do suggest that BFR is more effective than HLRT (and furthermore, safe) in the short-term.
It remains unknown whether BFR training is safe in the long-term, or if it is the optimal long-term modality for gaining strength. Therefore, BFR cannot currently be recommended as the sole optimal training modality for all populations. However, it may be efficacious in the rehabilitation process, or for previously untrained individuals, and those with orthopedic complications. Future studies should investigate the long-term impact of this training in such populations, to ensure these benefits remain consistent.
Limitations and strengths
First, we recruited only male participants, and thus, our results cannot be currently applied to women. Although future studies may be needed, a previous study demonstrated that post exercise-induced hyperemia with blood flow restriction does not differ between the sexes (Dankel et al. 2017). Second, we measured adipose tissue thickness (ATT) of calf muscle at only pre-training, and did not assess at 2- and 4- weeks of the training period. Moreover, we did not perform a correction that was previously used as a phantom model (Lin et al. 2000). Thus, we cannot completely rule out the effects of ATT, particularly resistance training-induced changes in ATT. However, the aim of this measurement was simply to confirm any differences in ATT between the two groups. Also, the exercise training protocol consisted of knee extension and leg press which mainly recruit thigh muscles. Additionally, while static StO2 values may be influenced by ATT, dynamic changes in StO2 during the hypermic phase after cuff deflation should not be affected by ATT, as changes in StO2 are independent of absolute values (Bopp et al. 2011, 2014). Thus, our results may not be strongly affected by ATT. Third, we did not change training load throughout the 4-weeks training period. Thus, it may be possible that relative exercise load was different across the participants (i.e., during the latter 2 weeks period). However, improvements in knee extension and leg press strength from baseline to the 2-week time point were not markedly different between the groups (approximately 7% for each group, Table 1). Finally, while the applied occlusive pressure was 130% that of systolic blood pressure, we did not confirm that blood flow was ablated. Similarly, as we did not measure heart rate and blood pressure during exercise, we are unable to ascertain whether the BFR induced a pressor reflex.
Conclusion
Prior to the current study, the effect of BFR training on macro- and microvascular function was unknown, with mixed meta-analytic findings. The present study fills this gap in the knowledge by demonstrating that low-intensity resistance training with BFR may improve macrovascular (haPWV) and microvascular function (StO2 responses). In contrast, high-intensity resistance training without BFR did not affect the vascular function of healthy young men. Both training modalities significantly increased muscle strength, with no differences between the groups. These data support the notion that short-term local resistance training with BFR may improve macro- and microvascular function, at least, in the downstream vasculature of the healthy male population in this study.
Availability of data and material
All relevant data are within the paper. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability (software application or custom code)
Not applicable.
Abbreviations
- ATT:
-
Adipose tissue thickness
- AUC:
-
Area under the curve
- BFR:
-
Blood flow restriction
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- bpm:
-
Beats per minute
- CI:
-
Confidential interval
- ES:
-
Effect size
- haPWV:
-
Heart-ankle pulse wave velocity
- HLRT:
-
High-load resistance training
- HR:
-
Heart rate
- MAP:
-
Mean arterial pressure
- NIRS:
-
Near-infrared spectroscopy
- RM:
-
Repetition maximum
- SD:
-
Standard deviation
- StO2 :
-
Tissue oxygen saturation
References
Bopp CM, Townsend DK, Barstow TJ (2011) Characterizing near-infrared spectroscopy responses to forearm post-occlusive reactive hyperemia in healthy subjects. Eur J Appl Physiol 111(11):2753–2761. https://doi.org/10.1007/s00421-011-1898-z
Bopp CM, Townsend DK, Warren S, Barstow TJ (2014) Relationship between brachial artery blood flow and total [hemoglobin+myoglobin] during post-occlusive reactive hyperemia. Microvasc Res 91:37–43. https://doi.org/10.1016/j.mvr.2013.10.004
Credeur DP, Hollis BC, Welsch MA (2010) Effects of handgrip training with venous restriction on brachial artery vasodilation. Med Sci Sports Exerc 42(7):1296–1302. https://doi.org/10.1249/MSS.0b013e3181ca7b06
Cunningham KS, Gotlieb AI (2005) The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest 85(1):9–23. https://doi.org/10.1038/labinvest.3700215
Dankel SJ, Mouser JG, Jessee MB, Mattocks KT, Buckner SL, Loenneke JP (2017) Post-exercise blood flow restriction attenuates hyperemia similarly in males and females. Eur J Appl Physiol 117(8):1707–1712. https://doi.org/10.1007/s00421-017-3663-4
Egginton S (2009) Invited review: activity-induced angiogenesis. Pflugers Arch 457(5):963–977. https://doi.org/10.1007/s00424-008-0563-9
Evans C, Vance S, Brown M (2010) Short-term resistance training with blood flow restriction enhances microvascular filtration capacity of human calf muscles. J Sports Sci 28(9):999–1007. https://doi.org/10.1080/02640414.2010.485647
Fahs CA, Rossow LM, Loenneke JP, Thiebaud RS, Kim D, Bemben DA, Bemben MG (2012) Effect of different types of lower body resistance training on arterial compliance and calf blood flow. Clin Physiol Funct Imaging 32(1):45–51. https://doi.org/10.1111/j.1475-097X.2011.01053.x
Ferguson RA, Hunt JEA, Lewis MP, Martin NRW, Player DJ, Stangier C, Taylor CW, Turner MC (2018) The acute angiogenic signalling response to low-load resistance exercise with blood flow restriction. Eur J Sport Sci 18(3):397–406. https://doi.org/10.1080/17461391.2017.1422281
Hakkinen K, Pakarinen A, Alen M, Kauhanen H, Komi PV (1988) Neuromuscular and hormonal responses in elite athletes to two successive strength training sessions in one day. Eur J Appl Physiol 57(2):133–139. https://doi.org/10.1007/BF00640652
Hopkins WG, Marshall SW, Batterham AM, Hanin J (2009) Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc 41(1):3–13. https://doi.org/10.1249/MSS.0b013e31818cb278
Horiuchi M, Okita K (2020) Microvascular responses during reactive hyperemia assessed by near-infrared spectroscopy and arterial stiffness in young, middle-aged, and older women. Microvasc Res 129:103972. https://doi.org/10.1016/j.mvr.2019.103972
Horiuchi M, Stoner L (2021) Effects of compression stockings on lower-limb venous and arterial system responses to prolonged sitting: a randomized cross-over trial. Vasc Med 26(4):386–393. https://doi.org/10.1177/1358863X20988899
Horiuchi M, Stoner L (2022) Macrovascular and microvascular responses to prolonged sitting with and without bodyweight exercise interruptions: a randomized cross-over trial. Vasc Med 27(2):127–135. https://doi.org/10.1177/1358863X211053381
Houben A, Martens RJH, Stehouwer CDA (2017) Assessing microvascular function in humans from a chronic disease perspective. J Am Soc Nephrol 28(12):3461–3472. https://doi.org/10.1681/ASN.2017020157
Hunt JE, Galea D, Tufft G, Bunce D, Ferguson RA (2013) Time course of regional vascular adaptations to low load resistance training with blood flow restriction. J Appl Physiol 115(3):403–411. https://doi.org/10.1152/japplphysiol.00040.2013
Karabulut M, Esparza B, Dowllah IM, Karabulut U (2020) The impact of low-intensity blood flow restriction endurance training on aerobic capacity, hemodynamics, and arterial stiffness. J Sports Med Phys Fitness. https://doi.org/10.23736/S0022-4707.20.11526-3
Kenward MG, Roger JH (2010) The use of baseline covariates in crossover studies. Biostatistics 11(1):1–17. https://doi.org/10.1093/biostatistics/kxp046
Kim ED, Tanaka H, Ballew SH, Sang Y, Heiss G, Coresh J, Matsushita K (2018) Associations between kidney disease measures and regional pulse wave velocity in a large community-based cohort: the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis 72(5):682–690. https://doi.org/10.1053/j.ajkd.2018.04.018
Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT (2008) Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol 586(4):1137–1145. https://doi.org/10.1113/jphysiol.2007.145722
Krentz AJ, Clough G, Byrne CD (2009) Vascular disease in the metabolic syndrome: do we need to target the microcirculation to treat large vessel disease? J Vasc Res 46(6):515–526. https://doi.org/10.1159/000226220
Liao CW, Lin LY, Hung CS, Lin YT, Chang YY, Wang SM, Wu VC, Wu KD, Ho YL, Satoh F, Lin YH (2016) Time course and factors predicting arterial stiffness reversal in patients with aldosterone-producing adenoma after adrenalectomy: prospective study of 102 patients. Sci Rep 6:20862. https://doi.org/10.1038/srep20862
Lin L, Niwayama M, Shiga T, Kudo N, Takahashi M, Yamamoto K (2000) Influence of a fat on muscle oxygenation measurement using near-IR spectroscopy: quantitative analysis based on two-layered phantom experiments and Monte Carlo simulation. Front Med Biol Eng 10(1):43–58. https://doi.org/10.1163/15685570052061531
Lin YH, Lin LY, Chen A, Wu XM, Lee JK, Su TC, Wu VC, Chueh SC, Lin WC, Lo MT, Wang PC, Ho YL, Wu KD, Group TS (2012) Adrenalectomy improves increased carotid intima-media thickness and arterial stiffness in patients with aldosterone producing adenoma. Atherosclerosis 221(1):154–159. https://doi.org/10.1016/j.atherosclerosis.2011.12.003
Liu Y, Jiang N, Pang F, Chen T (2021) Resistance training with blood flow restriction on vascular function: a meta-analysis. Int J Sports Med 42(7):577–587. https://doi.org/10.1055/a-1386-4846
Loenneke JP, Wilson JM, Wilson GJ, Pujol TJ, Bemben MG (2011) Potential safety issues with blood flow restriction training. Scand J Med Sci Sports 21(4):510–518. https://doi.org/10.1111/j.1600-0838.2010.01290.x
Madarame H, Neya M, Ochi E, Nakazato K, Sato Y, Ishii N (2008) Cross-transfer effects of resistance training with blood flow restriction. Med Sci Sports Exerc 40(2):258–263. https://doi.org/10.1249/mss.0b013e31815c6d7e
Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR (1994) Validation of near-infrared spectroscopy in humans. J Appl Physiol 77(6):2740–2747. https://doi.org/10.1152/jappl.1994.77.6.2740
Ozaki H, Yasuda T, Ogasawara R, Sakamaki-Sunaga M, Naito H, Abe T (2013) Effects of high-intensity and blood flow-restricted low-intensity resistance training on carotid arterial compliance: role of blood pressure during training sessions. Eur J Appl Physiol 113(1):167–174. https://doi.org/10.1007/s00421-012-2422-9
Paiva FM, Vianna LC, Fernandes IA, Nobrega AC, Lima RM (2016) Effects of disturbed blood flow during exercise on endothelial function: a time course analysis. Braz J Med Biol Res 49(4):e5100. https://doi.org/10.1590/1414-431X20155100
Patterson MS, Chance B, Wilson BC (1989) Time resolved reflectance and transmittance for the non-invasive measurement of tissue optical properties. Appl Opt 28(12):2331–2336. https://doi.org/10.1364/AO.28.002331
Pereira-Neto EA, Lewthwaite H, Boyle T, Johnston K, Bennett H, Williams MT (2021) Effects of exercise training with blood flow restriction on vascular function in adults: a systematic review and meta-analysis. PeerJ 9:e11554. https://doi.org/10.7717/peerj.11554
Roth SM, Martel GF, Ivey FM, Lemmer JT, Metter EJ, Hurley BF, Rogers MA (2000) High-volume, heavy-resistance strength training and muscle damage in young and older women. J Appl Physiol 88(3):1112–1118. https://doi.org/10.1152/jappl.2000.88.3.1112
Schulz KF, Altman DG, Moher D, Group C (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 152(11):726–732. https://doi.org/10.7326/0003-4819-152-11-201006010-00232
Soares RN, Murias JM (2018) Near-infrared spectroscopy assessment of microvasculature detects difference in lower limb vascular responsiveness in obese compared to lean individuals. Microvasc Res 118:31–35. https://doi.org/10.1016/j.mvr.2018.01.008
Soares RN, Reimer RA, Murias JM (2017) Changes in vascular responsiveness during a hyperglycemia challenge measured by near-infrared spectroscopy vascular occlusion test. Microvasc Res 111:67–71. https://doi.org/10.1016/j.mvr.2017.01.003
Spronck B, Obeid MJ, Paravathaneni M, Gadela NV, Singh G, Magro CA, Kulkarni V, Kondaveety S, Gade KC, Bhuva R, Kulick-Soper CM, Sanchez N, Akers S, Chirinos JA (2022) Predictive ability of pressure-corrected arterial stiffness indices: comparison of pulse wave velocity, cardio-ankle vascular index (CAVI), and CAVI0. Am J Hypertens 35(3):272–280. https://doi.org/10.1093/ajh/hpab168
Takada S, Okita K, Suga T, Omokawa M, Kadoguchi T, Sato T, Takahashi M, Yokota T, Hirabayashi K, Morita N, Horiuchi M, Kinugawa S, Tsutsui H (2012) Low-intensity exercise can increase muscle mass and strength proportionally to enhanced metabolic stress under ischemic conditions. J Appl Physiol 113(2):199–205. https://doi.org/10.1152/japplphysiol.00149.2012
Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N (2000) Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 88(6):2097–2106
Zhou J, Li YS, Chien S (2014) Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol 34(10):2191–2198. https://doi.org/10.1161/ATVBAHA.114.303422
Acknowledgements
We appreciate the time and effort spent by our volunteers. We also thank Ms. Junko Endo for her technical assistance.
Funding
This work was supported in part by Japan Society for the Promotion of Science (JSPS, KAKENHI, Number; JP18K11012 to M.H.).
Author information
Authors and Affiliations
Contributions
MH, LS, and JP conceived and designed the study. MH performed the experiments. MH, LS, and JP analyzed and interpreted results. MH drafted the first manuscript. LS, and JP critically revised the manuscript, and all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest, financial or otherwise, are declared by the authors.
Ethics approval and consent to participate
All study procedures were approved by the ethical committee of the Yamanashi Institute of Environmental Science University of Yamanashi (Mount Fuji Research Institute after renaming) in Japan and performed in accordance with the Declaration of Helsinki 2013 (ECHE-03-2012), with written informed consent obtained from all study participants.
Additional information
Communicated by I. Mark Olfert.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Horiuchi, M., Stoner, L. & Poles, J. The effect of four weeks blood flow restricted resistance training on macro- and micro-vascular function in healthy, young men. Eur J Appl Physiol 123, 2179–2189 (2023). https://doi.org/10.1007/s00421-023-05230-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05230-3