Abstract
Aim
Blood flow restriction (BFR) exercise is a common alternative to traditional high-load resistance exercise used to increase muscle size and strength. Some populations utilizing BFR at a low load may wish to limit their cardiovascular response to exercise. Different contraction patterns may attenuate the cardiovascular response, but this has not been compared using BFR.
Purpose
To compare the cardiovascular response to unilateral (UNI), bilateral (BIL), and alternating (ALT) BFR exercise contraction patterns.
Methods
Twenty healthy participants performed four sets (30 s rest) of knee extensions to failure, using 30% one-repetition maximum, 40% arterial occlusion pressure, and each of the three contraction patterns (on different days, at the same time of day, separated by 2–10 days, randomized). Cardiovascular responses, presented as pre- to post-exercise mean changes (SD), were measured using pulse wave analysis and analyzed with Bayesian RMANOVA.
Results
ALT caused greater changes in: aortic systolic [ΔmmHg: ALT = 21(8); UNI = 13(11); BIL = 15(8); BF10 = 29.599], diastolic [ΔmmHg: ALT = 13(8); UNI = 7(11); BIL = 8(8); BF10 = 5.175], and mean arterial [ΔmmHg: ALT = 19(8); UNI = 11(11); BIL = 13(7); BF10 = 48.637] blood pressures. Aortic [ΔmmHg bpm: ALT = 4945(2340); UNI = 3294(1408); BIL = 3428 (1461); BF10 = 113.659] and brachial [ΔmmHg bpm: ALT = 6134(2761); UNI = 4300(1709); BIL = 4487(1701); BF10 = 31.845] rate pressure products, as well as heart rate [Δbpm: ALT = 26(14); UNI = 19(8); BIL = 19(11); BF10 = 5.829] were greatest with ALT. Augmentation index [Δ%: UNI = -6(13); BIL = − 7(11); ALT = − 5(16); BF10 = 0.155] and wave reflection magnitude [Δ%: UNI = − 5(9); BIL = − 4(7); ALT = − 4(7); BF10 = 0.150] were not different.
Conclusion
Those at risk of a cardiovascular event may choose unilateral or bilateral BFR exercise over alternating until further work determines the degree to which it can be tolerated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditionally, recommendations to increase muscle size and strength include resistance training two or more times a week with a high-load of ~ 60 to 80% of an individual’s one-repetition maximum (1RM) (American College of Sports Medicine, 2009). However, high-load resistance exercise places mechanical stress on the joints which can make this modality uncomfortable and/or unsafe for elderly, clinical, or injured populations. Low-load resistance exercise in combination with blood flow restriction (BFR) may be an alternative modality to traditional resistance training for those populations (Loenneke et al. 2012). BFR is accomplished by applying a pneumatic cuff to the upper portion of a limb, and then, increasing cuff inflation pressure until arterial blood inflow is reduced and venous outflow is occluded (Patterson et al. 2019). Compared to high-load resistance exercise, low-load BFR exercise elicits similar increases in muscle size and some measurements of strength (Jessee et al. 2018) at a reduced overall workload even when compared to low load without BFR (Fahs et al. 2015; Farup et al. 2015; Jessee et al. 2017). In addition, using BFR alone can reduce muscular weakness after a period of disuse (Kubota et al. 2008), in combination with passive movement can reduce muscle wasting (Barbalho et al. 2019), and with aerobic exercise can increase maximal oxygen uptake and muscle size (Abe et al. 2010a). In contrast to the benefits of BFR, there is a theoretical concern that BFR may increase the risk of developing a blood clot, but low-load BFR exercise does not seem to activate the coagulation system in healthy subjects (Madarame et al. 2010). Additionally, there is a concern that BFR resistance exercise may augment the cardiovascular response compared to the traditional resistance exercise and could pose a risk for an adverse cardiovascular event (Spranger et al. 2015).
The cardiovascular concern for BFR exercise may be due to early BFR methodology when researchers would apply one absolute pressure (i.e., 100 mmHg) to all participants regardless of individual differences (Farup et al. 2015). In a review assessing the cardiovascular concern for BFR exercise, Spranger et al. (2015) included many studies that would make it difficult to determine what individual level of pressure participants were exercising with, some of which could have been near ischemic conditions (Jessee et al. 2016). When participants exercise with very little blood flow or with complete arterial occlusion their metaboreflex activation is heightened as metabolites pool distal to the cuff, thus, increasing the cardiovascular response (Spranger et al. 2015). As mentioned previously, the goal of BFR exercise is not to completely occlude blood flow to the exercising limb, but rather occlude venous outflow and reduce arterial inflow (Patterson et al. 2019). To reduce the risk of exercising under ischemic conditions, BFR pressures can be made relative by determining the participant’s arterial occlusion pressure (AOP) and using a percentage of that AOP to apply the restrictive stimulus (Jessee et al. 2016). Using this method of relative BFR application, one could reduce the cardiovascular response to BFR by applying lower relative pressures, as seen with changes in AOP from before to after exercise (Jessee et al. 2017). When a greater relative pressure is applied, there is an increase in the cardiovascular response (Jessee et al. 2017). Additionally, adjusting the load lifted can modify the cardiovascular response. For example, when using a lower load with BFR applied at the same pressure and number of repetitions, the cardiovascular response is attenuated (Jessee et al. 2017). Recently, a study also suggested that using a unilateral (UNI) contraction pattern for BFR exercise may be another beneficial method for limiting the central and peripheral cardiovascular response when compared to the traditional non-BFR resistance exercise (Credeur et al. 2019).
With the traditional non-BFR resistance exercise, manipulating exercise contraction patterns, i.e., exercising with UNI, alternating (ALT), or bilateral (BIL) contraction patterns, may attenuate the cardiovascular response (Matos-Santos et al. 2017; Moreira et al. 2017). One study found that BIL and ALT contraction patterns in the lower body, when compared to UNI, had a greater peripheral cardiovascular response following the last set of exercise (Moreira et al. 2017). Another study found that BIL exercise had a greater heart rate (HR), systolic blood pressure (bSBP), diastolic blood pressure (bDBP), cardiac output, and brachial rate pressure product (bRPP) in the last set when compared to UNI exercise (Matos-Santos et al. 2017). Based on these findings, it would seem that UNI exercise may have a more favorable cardiovascular response when compared to BIL and ALT. However, different contraction patterns have yet to be investigated in combination with low-load BFR and may be beneficial to further attenuate the cardiovascular response, especially in clinical or at-risk populations. In addition, as the central and peripheral components of the cardiovascular system may respond differently, they should be examined with BFR and different contraction patterns.
Currently, when using BFR with resistance exercise, the cardiovascular response can be limited by reducing the relative pressure and load lifted, but evidence suggests that we may be able to further attenuate the cardiovascular response by utilizing UNI contraction patterns. Thus, the purpose of the current study was to quantify and compare the central and peripheral cardiovascular response to different lower body BFR exercise contraction patterns (UNI, BIL, ALT) using a low load and relative pressure. The results may help to identify potential modifications or provide guidelines to attenuate the risk of cardiovascular events when using BFR protocols with at-risk populations.
Methods
Participants
Twenty-two volunteers (18–35 years old) were recruited, of which 20 completed the study. Two participants dropped out due to reasons unrelated to the study. Participants were excluded if they met the following criteria: taking medications that influenced HR and/or blood pressure, orthopedic issues inhibiting lower body resistance exercise, or exhibiting two or more risk factors for thromboembolism (Motykie et al. 2000). Participants were informed of the benefits and risks of the investigation prior to signing an informed consent document to participate in the study. Participants were asked to avoid alcohol and exercise 24 h prior and avoid caffeine 8 h prior to each visit. This study was approved by the Institutional Review Board of The University of Southern Mississippi (IRB-19-211) and was conducted following the ethical standards set forth in 1964 by the Declaration of Helsinki and its current amendments or comparable ethical standards.
Experimental design
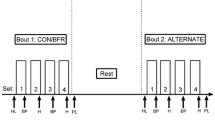
Using a within-subjects, repeated-measures design, participants completed UNI, BIL, and ALT conditions over four separate visits with 2–10 days between each visit. On visit one, participants completed screening criteria for inclusion, informed consent, and a Physical Activity Readiness Questionnaire for Everyone 2019. If participants were eligible and willing to participate, their height (stadiometer) and body mass (digital scale) were measured, followed by 1RM testing, and familiarization with scales to measure ratings of perceived effort (RPE-E) and perceived discomfort (RPE-D). Then, participants were familiarized with each exercise contraction pattern by performing five unloaded repetitions of each condition, to a metronome, with a deflated cuff on their left upper leg.
For visits two through four, participants performed four sets of knee extension BFR exercise to momentary failure using either UNI, BIL, or ALT contraction patterns (randomized). Participants completed all testing visits at approximately the same time of day (within 2 h). Exercise was performed at ~ 30% of the participant’s averaged bilateral 1RM, with 30 s of rest between sets, and BFR was applied at 40% AOP. Pulse wave analysis (PWA) was measured twice before and once immediately after each exercise condition. RPE-E and RPE-D were taken before, between sets, and immediately after exercise.
Arterial occlusion pressure
A 10 cm-wide, pneumatic nylon cuff (SC10D; Hokanson, Bellevue, WA) was placed on the proximal portion of each upper leg. The participant was asked to sit in a knee extension machine and rest for 5 min. Then, an Ultrasonic Pocket Doppler probe (SD3 Vascular; Edan, Pingshan, Shenzhen) was used to detect a pulse at the posterior tibial artery. The cuff was inflated immediately to 50 mmHg and then slowly increased (E20-Rapid Cuff Inflator; Bellevue, WA) until a pulse was no longer audible; this pressure was recorded to the nearest mmHg and determined to be AOP. AOP was measured on the first leg, and following cuff deflation, immediately measured on the next leg prior to each condition.
One-repetition maximum
Participants were seated with a lap belt secured around their waist in an iso-lateral knee extension machine (IL-LE; Hammer Strength, Rosemont, Illinois). Following a warm-up of ten unloaded knee extensions for each leg, a lighter weight that the participant was confident in lifting was added to the bar and used to complete one practice/warm-up repetition. With arms crossed over the chest, participants were asked to perform one repetition by extending the knee from ~ 90 degrees of flexion until reaching full extension (gauged objectively by reaching a pre-set safety bar on the machine). A repetition was deemed successful if the safety bar was reached. Upon completion of successful attempts, the load was increased. If an attempt was unsuccessful, the load was lowered. This process continued until the greatest load the participant could successfully complete was found and determined to be the 1RM. Attempts were alternated between legs (starting leg was randomized), with 60 s between each leg. Each 1RM was determined within approximately five attempts per leg. If the 1RM was different between legs, they were averaged together for the participant’s bilateral 1RM.
Pulse wave analysis
Central and peripheral cardiovascular hemodynamics were assessed using PWA (SphygmoCor XCEL, AtCor Medical, Itasca, Illinois). A pneumatic pressure cuff (size in accordance with manufacturer recommendations) was placed on the upper arm and inflated to find bSBP and bDBP. Then, inflated to a subsystolic pressure, to determine the brachial pressure waveform and central cardiovascular response (Davies and Struthers 2003; Butlin and Qasem 2016). The pulse pressure (PP) waveform is dependent on the viscoelastic properties of the artery, wave reflection, and wave dispersion (Davies and Struthers 2003). From these inflations, measures of HR, PP, augmentation pressure (AP), augmentation index (AIx), augmentation index corrected for HR (AIx75), wave reflection magnitude (WR), forward wave height, and reflected wave height are produced. Measures related to stiffness of large arteries include WR and AIx (Butlin and Qasem 2016). With PWA, AIx is calculated as a percentage of AP divided by PP (Butlin and Qasem 2016) and WR is calculated as a percentage of reflected wave height relative to forward wave height. bRPP and aortic rate pressure product (aRPP) were calculated as the product of HR and bSBP or aortic blood pressure (aSBP), respectively (baseline values calculated from the average of two premeasurements; post-exercise value calculated based on the single post-measure). Two PWA measurements were performed prior to exercise, separated by approximately 1 min. For each variable, the two pre-exercise values were averaged, and the average then used as the baseline pre-exercise value (Table 1). To evaluate the response of each variable to each condition, the difference in the baseline value and the value derived from the post-exercise measurement (i.e., the change) was calculated and used for subsequent statistical analyses.
Ratings of perceived effort and perceived discomfort
RPE-E and RPE-D were measured with two different scales, ranging from 0 to 10, which have been validated previously (Steele et al. 2016). The participant was familiarized with each scale on the first visit by reading a standardized statement in accordance with Steele et al. Prior to the exercise protocol and immediately after, RPE-E and RPE-D were measured. During the exercise protocol, RPE-E was measured immediately after each set, and RPE-D was measured 20 s after each set.
Exercise protocols
For all exercise protocols, participants had a 10 cm pneumatic nylon cuff on the proximal portion of each leg. For BIL, cuffs were inflated on each leg, while both legs exercised synchronously. For UNI, a cuff was placed on both legs, but only the cuff on the exercising leg was fastened and inflated. Once all sets were completed on the first leg, the cuff was deflated and unfastened, then the cuff on the opposite leg was fastened and inflated to undergo the exercise protocol on the second leg. UNI left and right leg results were added together for the volume of load lifted, RPE-E and RPE-D were averaged. For ALT, both cuffs were inflated for the duration of the condition, and following one leg completing a repetition and relaxing, the opposing leg completed a repetition and relaxed in an alternating pattern. Regardless of condition, repetitions were counted if participants reached the pre-set safety bar, which was used as an objective measure of completing a full knee extension range of motion. Participants continued exercise until momentary failure. If participants missed the safety bar or were unable to maintain a 2-s cadence (1 s concentric and 1 s eccentric) for more than two consecutive attempts per leg, then the set was ended.
Volume of load lifted
Volume was calculated by multiplying the number of total repetitions completed for each condition by the exercise load.
Statistical analysis
A one-way (condition) Bayesian repeated-measures ANOVA was performed to compare the calculated changes (pre- to post-exercise) in each cardiovascular variable obtained from PWA and exercise volume across conditions. Two-way (condition and time) Bayesian repeated-measures ANOVA were conducted for RPE-E and RPE-D to determine the effect of condition, the effect of time, and the interaction of the two. We utilized Bayesian analyses as it is a growing method in our field (Bernards et al. 2017; Mattocks et al. 2018; Spitz et al. 2019; Wong et al. 2019) and allows one to identify the level of evidence for either the null or alternative hypothesis (Wagenmakers et al. 2018b), rather than simply making a binary decision on whether to reject or fail to reject the null hypothesis. A Bayes Factor (BF10) of 1 would indicate the null hypothesis and the alternative hypothesis are equally likely. A BF10 greater than 1 would support the alternative, and less than 1 would support the null. Strength of evidence can also be considered, as the Bayes Factor gets further from one, the greater the evidence is for a hypothesis. For example, a BF10 greater than 3 indicates moderate, greater than 10 is strong, greater than 30 is very strong, and greater than 100 is extreme evidence for the alternative hypothesis (Wagenmakers et al. 2018a), that there is a difference across UNI, BIL, and ALT or across time. A BF10 less than 0.333 indicates moderate, less than 0.1 indicates strong, less than 0.03 is very strong, and less than 0.001 is extreme evidence for the null hypothesis that there is not a difference across UNI, BIL, and ALT or across time. BF10 values between 0.333 and 3 are considered anecdotal evidence, and more data points would be necessary before determining if the evidence strongly supports one hypothesis over another. Participants were excluded from analysis for a particular variable if they did not have a value for all conditions. During ALT, one participant was unable to complete the exercise protocol and their data were excluded from analysis. Analyses were performed using JASP 0.11.1 and SPSS 26. All results presented as mean (SD) unless otherwise noted. For test–retest reliability of cardiovascular variables intraclass correlation coefficients (ICC) were calculated using a two-way mixed-effects model with absolute agreement. ICCs were calculated for each day using the two measurements of PWA taken prior to exercise. The reported values are the lowest ICC coefficients (average measurement since the average of the measures was used for statistical analyses) across the 3 days, and thus, reliability is assumed to be greater than or equal to the value reported.
Results
Participants
Thirteen males and seven females [age: 26.8 (3.6) years, height: 173.7 (7.6) cm, weight: 77.7 (10.5) kg, body mass index: 25.8 (3.4) kg/cm2, 1RM right leg: 32.9 (8.4) kg, 1RM left leg: 31.8 (7.8) kg] completed the study.
Systolic and diastolic blood pressure
All cardiovascular measures and comparisons (n = 19 due to missing data) are presented in Table 1 with the exception of WR, forward wave height, and reflected wave height found in Supplementary Table 1. Comparisons of bSBP showed changes in ALT were greater than UNI (BF10 = 14.547), but not different from BIL (BF10 = 0.646), nor was UNI different from BIL (BF10 = 0.348). For bDBP, changes in ALT were greater than UNI (BF10 = 2.140) and BIL (BF10 = 2.016), while UNI and BIL were not different (BF10 = 0.248). Changes in aSBP were greater with ALT compared to UNI (BF10 = 244.444) and BIL (BF10 = 3.286), which were not different (UNI versus BIL, BF10 = 0.314). Similarly, changes in aortic diastolic blood pressure (aDBP) were greater with ALT versus UNI (BF10 = 7.181) and BIL (BF10 = 8.252), which were not different (UNI versus BIL, BF10 = 0.259).
Aortic mean arterial pressure, heart rate, and rate pressure product
Changes in aortic mean arterial pressure (aMAP) were greater with ALT compared to UNI (BF10 = 72.329) and BIL (BF10 = 16.895), whereas UNI and BIL were not different (BF10 = 0.292). Changes in HR were greater in ALT versus UNI (BF10 = 1.940) and BIL (BF10 = 8.361), while UNI and BIL were not different (BF10 = 0.240). The changes in aRPP with ALT were greater than UNI (BF10 = 14.981) and BIL (BF10 = 35.218) with no difference between UNI and BIL (BF10 = 0.257). ALT elicited greater changes in bRPP compared to UNI (BF10 = 7.299) and BIL (BF10 = 13.654), but the changes were not different between UNI and BIL (BF10 = 0.262).
AIx75 was analyzed with 15 participants, because the instrument was unable to provide data for 5 participants following ALT. The changes in AIx75 were greater with ALT than UNI (BF10 = 1.524), but not BIL (BF10 = 0.875). UNI changes were not different from BIL (BF10 = 0.262). Changes in AP, AIx, and PP were not different across conditions. Wave component changes (n = 18), such as WR, forward wave height, and reflected wave height, were not different across conditions.
Ratings of perceived effort and discomfort
There was no interaction for RPE-E (BF10 = 0.013; Table 2), nor was there a main effect of condition (BF10 = 0.089). However, there was a main effect of time (BF10 = 3.991e+144). RPE-E increased from baseline to set 1 (BF10 = 3.019e+31), set 1 to set 2 (BF10 = 1086.684), set 2 to set 3 (BF10 = 1.754), and set 3 to set 4 (BF10 = 1640.738).
While there was no interaction (BF10 = 0.082) for RPE-D, there was evidence for a main effect of time (BF10 = 2.014e+73) and condition (BF10 = 5552.067) (Table 2). Collapsed across time, ALT had a greater discomfort than UNI (BF10 = 20,058.268) and BIL (BF10 = 4.741e+6), while the UNI and BIL conditions were not different (BF10 = 0.114). Collapsed across condition, discomfort increased from before exercise to set 1 (BF10 = 1.999e+22), set 1 to set 2 (BF10 = 1625.964), set 2 to set 3 (BF10 = 5.169), and set 3 to set 4 (BF10 = 5.925).
Volume of load lifted
The volume of load lifted differed between conditions (BF10 = 40.434, Fig. 1). Post hoc comparisons show that ALT was greater than UNI [2234 (2028) kg vs. 1093 (344) kg; BF10 = 3.905] and BIL [2234 (2028) kg vs. 1052 (351) kg; BF10 = 5.500]. UNI and BIL were not different [1093 (344) kg vs. 1052 (351) kg; BF10 = 0.195]. To determine if the greater volume was explaining the cardiovascular responses (rather than the contraction patterns being used), we checked for any correlations between volume and cardiovascular variables. Results revealed evidence for a positive correlation between volume and changes in AIx (Pearson’s r = 0.517; BF10 = 3.109), AIx75 (Pearson’s r = 0.650; BF10 = 7.339), WR (Pearson’s r = 0.558; BF10 = 4.254), and AP (Pearson’s r = 0.455; BF10 = 1.693) for ALT. BIL had a negative correlation with volume and AP (Pearson’s r = − 0.382; BF10 = 1.007). There was a negative correlation for volume and AIx (Pearson’s r = − 0.413; BF10 = 1.278), AIx75 (Pearson’s r = − 0.387; BF10 = 1.043), AP (Pearson’s r = − 0.436; BF10 = 1.561), and WR (Pearson’s r = − 0.442; BF10 = 1.644), and positive correlations for bSBP (Pearson’s r = 0.480; BF10 = 2.361) and bRPP (Pearson’s r = 0.395; BF10 = 1.115) in the UNI condition. However, the variables that were different between conditions were not correlated with volume for ALT, leading us to believe that the differences in cardiovascular responses seen between conditions are due to something other than just the work performed.
Volume of load lifted. The average total volume of load lifted for each condition is represented with lower and upper 95% credible intervals for unilateral (926.837 kg, 1258.874 kg), bilateral (882.724 kg, 1221.184 kg), and alternating (1256.336 kg, 3211.221 kg) conditions. If two conditions do not share the same letter, they are different from each other (BF10 ≥ 3)
Test–retest reliability
The lowest intraclass correlation coefficients (95%CI) across the testing visits were: bSBP = 0.753 (0.395, 0.901); bDBP = 0.876 (0.677, 0.951); aSBP = 0.807 (0.474, 0.926); aDBP = 0.876 (0.685, 0.951); PP = 0.519 (− 0.183, 0.808); aMAP = 0.923 (0.711, 0.973); HR = 0.758 (0.400, 0.904); AP = 0.740 (0.344, 0.897); AIx = 0.830 (0.559, 0.933); AIx75 = 0.865 (0.649, 0.947); WR = 0.340 (− 0.729, 0.742); forward wave height = 0.623 (0.058, 0.850); reflected wave height = 0.160 (− 1.061, 0.664).
Discussion
The purpose of this study was to compare the central and peripheral cardiovascular response to three different contraction patterns: UNI, BIL, and ALT. Our PWA data suggest that although there is an increase in the central cardiovascular response, there is no difference in arterial stiffness across conditions. In contrast to other studies without BFR, we found that the cardiovascular response for UNI and BIL conditions are not different from each other. These results suggest that when exercising to momentary failure, with BFR, a UNI or BIL contraction pattern may attenuate the cardiovascular response when compared to ALT.
Previous studies investigating the peripheral cardiovascular response to knee extension exercise without BFR indicate that UNI exercise has a lower response than that of BIL (Matos-Santos et al. 2017) and ALT (Moreira et al. 2017). Moreira et al. and Matos-Santos et al. matched total time of exercise for UNI to BIL by having participants only exercise one leg, then the cardiovascular response was measured. However, Costa et al. found that UNI and BIL exercise were not different when exercising both legs until momentary failure, rather than matching protocols for exercise duration (Costa et al. 2015). In line with Costa et al., we measured the UNI cardiovascular response after 8 sets of exercise (4 sets in one leg and then 4 sets in the opposing leg), and since both legs exercised until momentary failure, there was no statistical difference in volume of load lifted between UNI and BIL (Fig. 1). When matching conditions for exercise time, as is the case in Moreira et al. and Matos-Santos et al., UNI may have a more favorable cardiovascular response, because the system needs only meet an increased demand for blood flow in one muscle group, whereas BIL and ALT would have a higher demand for oxygenated blood in both legs. Taking into account the limitations of only exercising one leg and comparing it to conditions that required exercise with both legs, we chose to have participants exercise both legs for the UNI condition. In the current study, the mechanism leading to an increased cardiovascular response using an ALT pattern of BFR exercise is unclear. However, we speculate that the duration of cuff inflation (i.e., time under restriction) may explain the greater cardiovascular response. Since participants were asked to exercise until momentary failure, we did not control for volume in the current study. As can be seen in Fig. 1, ALT resulted in a greater average exercise volume compared to the other patterns. To determine if the difference in volume was driving the differential cardiovascular responses, we ran correlations to investigate the relationship between volume and every variable in each condition. We found that volume for the ALT contraction pattern only correlated with variables that did not differ across conditions. Thus, we assume that something besides volume may account for the greater cardiovascular response in ALT. Since the load lifted was set at 30% 1RM and exercise was performed to a metronome, the greater volume was due to a greater number of repetitions completed. Due to the exercise protocol, the ALT contraction pattern had rest between each leg as one leg lifted and lowered within 2 s, while the opposing leg was resting for those two seconds. This resting period may have allowed a slight recovery in the ALT condition, but with UNI and BIL contraction patterns, there was no rest between repetitions within a set. Thus, the ALT condition would require the cuffs to be inflated for a longer period of time, possibly trapping more blood in the exercising limbs. In response, the amount of preload back to the heart would decrease stroke volume and require a subsequent increase in HR to maintain cardiac output (Table 1). The continual decrease in stroke volume could place greater and greater strain on the heart causing a heightened cardiovascular response. While stroke volume was not assessed in this study, one could see a greater change in heart rate did occur in response to ALT BFR exercise. As BFR exercise also leads to a build-up of metabolites distal to the cuff (Yasuda et al. 2010), the greater time with BFR in ALT may have led to a greater increase in metabolites, stimulating the metaboreflex and leading to a heightened cardiovascular response (Spranger et al. 2015).
During typical resistance exercise, without BFR, there is localized swelling in the exercising muscles and an accumulation of metabolites (Costa et al. 2015). Muscle metaboreceptors sense the accumulation of metabolites, send a signal via afferent nerves to the brainstem, and the cardiovascular center responds by increasing blood pressure, HR, local vasculature dilation, and peripheral vasculature constriction (Spranger et al. 2015). When comparing different contraction patterns of BFR exercise in the lower body, we found that most cardiovascular measurements were higher with ALT compared to BIL or UNI exercise. The greater cardiovascular response with ALT BFR exercise seems to be driven by changes in central cardiovascular variables (i.e., aortic blood pressures and HR), rather than changes in indices of arterial stiffness (i.e., PP, AP, AIx, AIx75, or WR). It should be noted that the reliability of WR and PP was poor, which could be one reason why no differences were observed (data reported in Supplementary Table 1). Similar findings (no differences in PP, AP, AIx, or WR) were observed when investigating the response to unilateral handgrip exercise at moderate and low loads, with and without BFR (Credeur et al. 2019). Interestingly, bilateral BFR exercise in the lower body has been shown to reduce arterial stiffness, based on AIx and time to wave reflection, immediately following exercise (Rossow et al. 2012). While our results are more similar to Credeur et al., given that we saw no changes in variables indicative of arterial stiffness, our protocol was more like Rossow et al., in that we used lower body BFR exercise in both limbs. Both Credeur et al. and Rossow et al. measured PWA, while the limbs were still under BFR. Rossow et al. postulated that reductions in AIx could be from a reduced preload due to the restriction of blood flow. With the present study, the cuffs were immediately deflated and removed following cessation of exercise; thus, pooled venous blood may have returned to the heart and possibly negated any effect on arterial stiffness by the time PWA measurements were taken.
The cardiovascular response to BFR has been assessed using changes in AOP (Barnett et al. 2016; Jessee et al. 2017; Bell et al. 2018; Mattocks et al. 2018; Dankel et al. 2019), peripheral blood pressures (Neto et al. 2014, 2016; Brandner et al. 2015; Moriggi et al. 2015), and PWA (Rossow et al. 2012; Credeur et al. 2019), each method with advantages and disadvantages. An advantage of using changes in AOP to quantify cardiovascular responses to BFR is that you can immediately assess the cardiovascular response without having to remove and replace cuffs. Using brachial blood pressures to quantify the cardiovascular response may be advantageous, because blood pressure measurements are inexpensive and have clinically relevant values. An advantage of using PWA is that one can estimate changes in peripheral and central hemodynamics (Avolio et al. 2010; Rossow et al. 2012; Credeur et al. 2019). When examining changes in AOP with BFR exercise, the responses are augmented by applying greater relative pressures and/or using higher loads (Jessee et al. 2017). Similarly, the bSBP response to low-load BFR exercise is increased in magnitude when applying a higher BFR pressure or exercising traditionally (i.e., no BFR) using high loads (Brandner et al. 2015). Central cardiovascular responses, assessed via PWA, have also been experimentally observed to be driven by variables such as exercise load (Credeur et al. 2019) and pressure (Rossow et al. 2012) where an increase in either seems to elicit a greater increase in the cardiovascular response. Therefore, regardless of measurement tool, it seems as though common variables, such as exercise load and restriction pressure, affect the magnitude of the cardiovascular response to BFR exercise. In healthy populations, it seems that when using a low load with a relative BFR pressure, there is no significant increase in the cardiovascular response compared to traditional exercise (Neto et al. 2014). However, since BFR may be a viable exercise option for clinical populations, it is beneficial to explore ways to limit the cardiovascular response to exercise. In the current study, using moderate relative pressures (40% AOP) and a relatively low load (~ 30% 1RM), we found that different exercise patterns (UNI, BIL, and ALT) in the lower body elicit different cardiovascular responses. Thus, based on previous findings and our results, changing the contraction pattern during exercise will extend the number of possible variables that can be manipulated for reducing the cardiovascular response to BFR exercise for those with heightened cardiovascular risk.
We observed RPE-E and RPE-D increase with successive sets of exercise; however, only RPE-D differed between contraction patterns (greater with ALT). When very low-load (15% 1RM) BFR exercise is taken to momentary failure, ratings of perceived exertion seem to be greater when applying a higher BFR pressure (80% AOP versus 40% and 0% AOP) in the upper body (Bell et al. 2018). However, in the lower body, perceived exertion seems unaffected by pressure (0%, 40%, and 80% AOP), but a greater load (70% 1RM versus 15% 1RM) results in lower perception of exertion (Dankel et al. 2019). In the current study, load and pressure were constant, only contraction pattern and volume differed across conditions, and thus, it seems as though neither has a large effect on RPE-E. In contrast, ALT elicited a greater RPE-D. Bell et al. (2018) and Dankel et al. (2019) both found that discomfort is increased when applying greater levels of BFR pressure (80% > 40% > 0% AOP) to exercise with a very low load (15% 1RM), and, when no BFR is applied, a traditional high-load (70%1RM) causes less discomfort than 15%1RM take to momentary failure. Their results suggest positive relationships between applied pressure and discomfort as well as exercise volume and discomfort. While we did not compare differing pressures or loads, our contraction patterns did result in varying amounts of exercise volume, with the ALT condition being greater than UNI and BIL. Having also seen a greater RPE-D in the ALT condition, our results support the conclusions of Bell et al. and Dankel et al. in stating that exercise volume influences the degree of discomfort associated with varying exercise protocols. Given that our experiment was acute in nature, the degree to which each of these protocols can be tolerated over time was not investigated. However, research has shown that the degree of discomfort associated with BFR exercise may decrease over time during a chronic training program (Mattocks et al. 2017).
Limitations
Each leg had 2 s of rest between successive repetitions for ALT, while the UNI and BIL conditions had no rest, and since exercise was taken to momentary failure, this may have led to a greater volume of load lifted. However, volume did not seem to be correlated with the differences in the cardiovascular response between conditions, which suggests that other factors may be responsible. Second, the device used to assess cardiovascular responses took up to 3 min to capture participants’ waveform in some cases. Even 1–2 min after exercise, HR has been shown to significantly decrease (Javorka et al. 2002). This could be a potential reason some variables (i.e., arterial stiffness) were not different across conditions, or could have resulted in an underestimated magnitude of change in others. Additionally, the population studied was young and healthy. However, the implications of the study are more pertinent to elderly and/or clinical populations, as they are more likely to have complications related to elevated blood pressure, arterial stiffness, etc. Therefore, future studies should investigate the effects of the current contraction patterns in healthy older populations, then with clinical populations at risk for a cardiovascular event. Since momentary failure protocols may not be typical in clinical settings, further research would also benefit by controlling for volume, or comparing standard protocols to determine if ALT still produces a greater cardiovascular response when compared to UNI and BIL BFR contraction patterns. Although the focus of this manuscript is to limit the cardiovascular response during low-load BFR exercise with a moderate pressure, there likely exists a minimal threshold where some cardiovascular response may be necessary for stimulating improvements in cardiovascular or vascular function. While BFR exercise has been shown to improve both cardiorespiratory fitness (Abe et al. 2010b) and peripheral vascular function (Mouser et al. 2019), the minimal stimulus necessary to do so remains uninvestigated (to our knowledge). Additionally, further studies should investigate the acute and chronic muscular response to contraction patterns, as it is not known whether avoiding an ALT contraction pattern may result in different muscular adaptations.
Conclusion
The results of this study indicate that when exercising with a commonly used blood flow restriction pressure (40% arterial occlusion pressure) and load (30% one-repetition maximum) to momentary failure, an alternating contraction pattern generally produces a greater cardiovascular response than unilateral or bilateral. Populations who are at an increased risk of a cardiovascular event could utilize the unilateral or bilateral condition to avoid greater discomfort and reduce the risk of a possible cardiovascular event. More research should be conducted to determine if an alternating condition when controlled for volume of load lifted leads to a heightened cardiovascular response when compared to unilateral and bilateral.
Abbreviations
- 1RM:
-
One-repetition maximum
- aDBP:
-
Aortic diastolic blood pressure
- AIx:
-
Augmentation index
- AIx75:
-
Augmentation index corrected for heart rate
- ALT:
-
Alternating
- aMAP:
-
Aortic mean arterial pressure
- AOP:
-
Arterial occlusion pressure
- AP:
-
Augmentation pressure
- aRPP:
-
Aortic rate pressure product
- aSBP:
-
Aortic systolic blood pressure
- bDBP:
-
Brachial diastolic blood pressure
- BF10 :
-
Bayes factor
- BFR:
-
Blood flow restriction
- BIL:
-
Bilateral
- bRPP:
-
Brachial rate pressure product
- bSBP:
-
Brachial systolic blood pressure
- HR:
-
Heart rate
- PP:
-
Pulse pressure
- PWA:
-
Pulse wave analysis
- RPE-D:
-
Ratings of perceived discomfort
- RPE-E:
-
Ratings of perceived effort
- UNI:
-
Unilateral
- WR:
-
Wave reflection magnitude
References
Abe T, Fujita S, Nakajima T et al (2010a) Effects of low-intensity cycle training with restricted leg blood flow on thigh muscle volume and VO2MAX in young men. J Sports Sci Med 9:452–458
Abe T, Sakamaki M, Fujita S et al (2010b) Effects of low-intensity walk training with restricted leg blood flow. J Geriatr Phys Ther 33:34–40. https://doi.org/10.1097/JPT.0b013e3181d07a73
American College of Sports Medicine (2009) Progression models in resistance training for healthy adults. Med Sci Sports Exerc 41:687–708
Avolio AP, Butlin M, Walsh A (2010) Arterial blood pressure measurement and pulse wave analysis-their role in enhancing cardiovascular assessment. Physiol Meas 31:R1–47. https://doi.org/10.1088/0967-3334/31/1/R01
Barbalho M, Rocha AC, Seus TL et al (2019) Addition of blood flow restriction to passive mobilization reduces the rate of muscle wasting in elderly patients in the intensive care unit: a within-patient randomized trial. Clin Rehabil 33:233–240. https://doi.org/10.1177/0269215518801440
Barnett BE, Dankel SJ, Counts BR et al (2016) Blood flow occlusion pressure at rest and immediately after a bout of low load exercise. Clin Physiol Funct Imaging 36:436–440. https://doi.org/10.1111/cpf.12246
Bell ZW, Buckner SL, Jessee MB et al (2018) Moderately heavy exercise produces lower cardiovascular, RPE, and discomfort compared to lower load exercise with and without blood flow restriction. Eur J Appl Physiol 118:1473–1480. https://doi.org/10.1007/s00421-018-3877-0
Bernards J, Sato K, Haff G, Bazyler C (2017) Current research and statistical practices in sport science and a need for change. Sports 5:87. https://doi.org/10.3390/sports5040087
Brandner CR, Kidgell DJ, Warmington SA (2015) Unilateral bicep curl hemodynamics: low-pressure continuous vs high-pressure intermittent blood flow restriction. Scand J Med Sci Sport 25:770–777. https://doi.org/10.1111/sms.12297
Butlin M, Qasem A (2016) Large artery stiffness assessment using SphygmoCor technology. Pulse 4:180–192. https://doi.org/10.1159/000452448
Costa EC, Moreira A, Cavalcanti B et al (2015) Effect of unilateral and bilateral resistance exercise on maximal voluntary strength, total volume of load lifted, and perceptual and metabolic responses. Biol Sport 32:35–40. https://doi.org/10.5604/20831862.1126326
Credeur DP, Jones R, Stanford D et al (2019) Central cardiovascular hemodynamic response to unilateral handgrip exercise with blood flow restriction. Eur J Appl Physiol. https://doi.org/10.1007/s00421-019-04209-3
Dankel SJ, Jessee MB, Mattocks KT et al (2019) Perceptual and arterial occlusion responses to very low load blood flow restricted exercise performed to volitional failure. Clin Physiol Funct Imaging 39:29–34. https://doi.org/10.1111/cpf.12535
Davies JI, Struthers AD (2003) Pulse wave analysis and pulse wave velocity: a critical review of their strengths and weaknesses. J Hypertens 21:463–472. https://doi.org/10.1097/00004872-200303000-00004
Fahs CA, Loenneke JP, Thiebaud RS et al (2015) Muscular adaptations to fatiguing exercise with and without blood flow restriction. Clin Physiol Funct Imaging 35:167–176. https://doi.org/10.1111/cpf.12141
Farup J, de Paoli F, Bjerg K et al (2015) Blood flow restricted and traditional resistance training performed to fatigue produce equal muscle hypertrophy. Scand J Med Sci Sport 25:754–763. https://doi.org/10.1111/sms.12396
Javorka M, Žila I, Balhárek T, Javorka K (2002) Heart rate recovery after exercise: Relations to heart rate variability and coplexity. Brazilian J Med Biol Res 35:991–1000. https://doi.org/10.1590/S0100-879X2002000800018
Jessee MB, Buckner SL, Grant Mouser J et al (2018) Muscle adaptations to high-load training and very low-load training with and without blood flow restriction. Front Physiol. https://doi.org/10.3389/fphys.2018.01448
Jessee MB, Buckner SL, Mouser JG et al (2016) Letter to the editor: Applying the blood flow restriction pressure: the elephant in the room. Am J Physiol Hear Circ Physiol 310:H132–H133. https://doi.org/10.1152/ajpheart.00820.2015
Jessee MB, Dankel SJ, Buckner SL et al (2017) The cardiovascular and perceptual response to very low load blood flow restricted exercise. Physiol Biochem 38:597–603. https://doi.org/10.1055/s-0043-109555
Kubota A, Sakuraba K, Sawaki K et al (2008) Prevention of disuse muscular weakness by restriction of blood flow. Med Sci Sports Exerc 40:529–534. https://doi.org/10.1249/MSS.0b013e31815ddac6
Loenneke J, Abe T, Wilson J et al (2012) Blood flow restriction: an evidence based progressive model (Review). Acta Physiol Hung 99:235–250. https://doi.org/10.1556/APhysiol.99.2012.3.1
Madarame H, Sasaki K, Ishii N (2010) Endocrine responses to upper- and lower-limb resistance exercises with blood flow restriction. Acta Physiol Hung 97:192–200. https://doi.org/10.1556/APhysiol.97.2010.2.5
Matos-Santos L, Farinatti P, Borges PJ et al (2017) Cardiovascular responses to resistance exercise performed with large and small muscle mass. Int J Sports Med 38:883–889. https://doi.org/10.1055/s-0043-116671
Mattocks KT, Jessee MB, Counts BR et al (2017) The effects of upper body exercise across different levels of blood flow restriction on arterial occlusion pressure and perceptual responses. Physiol Behav 171:181–186. https://doi.org/10.1016/j.physbeh.2017.01.015
Mattocks KT, Mouser JG, Jessee MB et al (2018) Acute hemodynamic changes following high load and very low load lower body resistance exercise with and without the restriction of blood flow. Physiol Meas 39:125007. https://doi.org/10.1088/1361-6579/aaefc9
Moreira OC, Farci LL, De Matos DG et al (2017) Cardiovascular responses to unilateral, bilateral, and alternating limb resistance exercise performed using different body segments. J Strength Cond Res 31:644–652. https://doi.org/10.1519/JSC.0000000000001160
Moriggi R, Di Mauro HS, Dias SC et al (2015) Similar hypotensive responses to resistance exercise with and without blood flow restriction. Biol Sport 32:289–294. https://doi.org/10.5604/20831862.1163691
Motykie GD, Zebala LP, Caprini JA et al (2000) A guide to venous thromboembolism risk factor assessment. J Thromb Thrombol 9:253–262. https://doi.org/10.1023/A:1018770712660
Mouser JG, Mattocks KT, Buckner SL et al (2019) High-pressure blood flow restriction with very low load resistance training results in peripheral vascular adaptations similar to heavy resistance training. Physiol Meas. https://doi.org/10.1088/1361-6579/ab0d2a
Neto GR, Novaes JS, Dias I et al (2016) Effects of resistance training with blood flow restriction on haemodynamics: a systematic review. Clin Physiol Funct Imaging 37:567–574. https://doi.org/10.1111/cpf.12368
Neto GR, Sousa MSC, Costa e Silva GV et al (2014) Acute resistance exercise with blood flow restriction effects on heart rate, double product, oxygen saturation and perceived exertion. Clin Physiol Funct Imaging 36:53–59. https://doi.org/10.1111/cpf.12193
Patterson SD, Hughes L, Warmington S et al (2019) Blood flow restriction exercise position stand: Considerations of methodology, application, and safety. Front Physiol 10:1–15
Rossow LM, Fahs CA, Loenneke JP et al (2012) Cardiovascular and perceptual responses to blood-flow-restricted resistance exercise with differing restrictive cuffs. Clin Physiol Funct Imaging 32:331–337. https://doi.org/10.1111/j.1475-097X.2012.01131.x
Spitz RW, Chatakondi RN, Bell ZW et al (2019) The impact of cuff width and biological sex on cuff preference and the perceived discomfort to blood-flow-restricted arm exercise. Physiol Meas. https://doi.org/10.1088/1361-6579/ab1787
Spranger MD, Krishnan AC, Levy PD et al (2015) Blood flow restriction training and the exercise pressor reflex: a call for concern. Am J Physiol Hear Circ Physiol 309:H1440–H1452. https://doi.org/10.1152/ajpheart.00208.2015
Steele J, Fisher J, McKinnon S, McKinnon P (2016) Differentiation between perceived effort and discomfort during resistance training in older adults: reliability of trainee ratings of effort and discomfort, and reliability and validity of trainer ratings of trainee effort. J Trainol 6:1–8. https://doi.org/10.17338/trainology.6.1_1
Wagenmakers EJ, Love J, Marsman M et al (2018a) Bayesian inference for psychology. Part II: example applications with JASP. Psychon Bull Rev 25:58–76. https://doi.org/10.3758/s13423-017-1323-7
Wagenmakers EJ, Marsman M, Jamil T et al (2018b) Bayesian inference for psychology. Part I: Theoretical advantages and practical ramifications. Psychon Bull Rev 25:35–57. https://doi.org/10.3758/s13423-017-1343-3
Wong V, Abe T, Chatakondi RN et al (2019) The influence of biological sex and cuff width on muscle swelling, echo intensity, and the fatigue response to blood flow restricted exercise. J Sports Sci 37:1865–1873. https://doi.org/10.1080/02640414.2019.1599316
Yasuda T, Abe T, Brechue WF et al (2010) Venous blood gas and metabolite response to low-intensity muscle contractions with external limb compression. Metabolism 59:1510–1519. https://doi.org/10.1016/j.metabol.2010.01.016
Acknowledgements
The authors would like to thank the participants for contributing their time toward the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Massimo Pagani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stanford, D.M., Park, J., Jones, R. et al. Acute cardiovascular response to unilateral, bilateral, and alternating resistance exercise with blood flow restriction. Eur J Appl Physiol 120, 1921–1930 (2020). https://doi.org/10.1007/s00421-020-04401-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-020-04401-w