Abstract
Objectives
This study investigated the effect of hand elevation on reductions in finger blood flow (FBF) induced by hand-transmitted vibration.
Methods
Fourteen males attended six sessions on six separate days, with a control sessions and a vibration session (125-Hz vibration at 44 ms−2 rms) with the right hand supported at each of three elevations: 20 cm below heart level (HL), at HL, and 20 cm above HL. Finger blood flow on the left and right hand was measured every 30 s during each 25-min session comprised of five periods: (1) no force and no vibration (5 min), (2) 2-N force and no vibration (5 min), (3) 2-N force and vibration (5 min), (4) 2-N force and no vibration (5 min), and (5) no force and no vibration (5 min).
Results
Without vibration, FBF decreased with increasing elevation of the hand. During vibration of the right hand, FBF reduced on both hands. With elevation of the right hand, the percentage reduction in FBF due to vibration (relative to FBF on the same finger at the same elevation before exposure to vibration) was similar on the middle and little fingers of both hands. After cessation of vibration, there was delayed return of FBF with all three hand heights.
Conclusions
Vibration of one hand reduces FBF on both exposed and unexposed hands, with the reduction dependent on the elevation of the hand. The mechanisms responsible for vibration-induced reductions in FBF seem to reduce blood flow as a percentage of the blood flow without vibration. Tasks requiring the elevation of the hands will be associated with lower FBF, and the FBF will be reduced further if there is exposure to hand-transmitted vibration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Workers who use hand-held vibrating tools are at risk of developing vascular, neurological, and musculoskeletal disorders of the upper limbs, known collectively as the hand-arm vibration syndrome. The principal vascular disorder associated with hand-transmitted vibration, vibration-induced white finger (VWF), is characterised by episodic blanching of the fingers due to reduced finger blood flow (FBF). The whiteness of the digits, often provoked by exposure to cold, is a visible sign of an abnormality in the regulation of FBF (Griffin and Bovenzi 2002). The blanching may occur on the distal, middle, or proximal phalanges of the fingers. Although various tests can assist diagnosis (e.g. the measurement of finger systolic blood pressure following cold provocation; ISO 14835-2:2005), the mechanisms involved in the causation of this vascular disorder are unclear.
Symptoms of VWF mostly arise after many years of regular occupational exposure to hand-transmitted vibration. Experimental studies in healthy people show that vibration of one hand provokes digital vasoconstriction, not only in the exposed hand but also in fingers of the non-vibrated hand (e.g. Bovenzi et al. 1998, 1999, 2000; Griffin et al. 2006; Thompson and Griffin 2009). It has been hypothesised that the vasoconstriction in fingers on a non-exposed hand is evidence of a central sympathetic vasomotor reflex (Bovenzi et al. 2006; Ye and Griffin 2011a, 2013, 2014).
The current International Standard for evaluating the severity of occupational exposures to hand-transmitted vibration says that in addition to the physical characteristics of the vibration (the magnitude, the frequency, and the duration of exposure to vibration), there are other factors to take into account when considering the risks from occupational exposures to hand-transmitted vibration (ISO 5349-1, 2001). These include “The position of the hand and arm, and body posture during exposure (angles of wrist, elbow and shoulder joints)”. The vertical position of the arm has a major influence on brachial blood pressure: there is increased pressure when the forearm is below heart level (HL) and reduced blood pressure when the forearm is above HL. The differences can be attributed to the effects of hydrostatic pressure and may be 10 mm Hg or more (Mitchell et al. 1964; Netea et al. 1999; Pickering et al. 2005). To control the effects of body posture, there is a standard posture for measuring brachial blood pressure (Pickering et al. 2005). There are no known studies of how posture affects finger circulation when operating of vibratory tools. It seems reasonable to anticipate that elevation of the hand will reduce FBF and may increase the risks arising from occupational exposures to hand-transmitted vibration.
The effect of elevating the hand on finger circulation may be similar to the effect of reducing the environmental temperature on finger circulation. When room temperature was reduced from 28 to 20 °C, FBF was reduced but vibration provoked less reduction in absolute FBF at the lower temperature. However, the percentage reduction in FBF was similar at both temperatures (Ye and Griffin 2011b). Elevating the hand will reduce FBF but if vibration causes the same percentage reduction in FBF, the absolute reduction in blood flow will be less at higher elevations.
This study was designed to increase understanding of the effect of hand position on finger circulation before and during exposure to hand-transmitted vibration. Three elevations of the hand were investigated: 20 cm below HL, HL, and 20 cm above HL. It was hypothesised that increasing the height of the hand relative to the heart would decrease FBF. It was also hypothesised that with the three elevations of the hand the reductions in FBF induced by the vibration would either have a similar percentage or a similar absolute amount.
Methods
Apparatus
Finger blood flow in the middle and little fingers of both hands was measured with a venous occlusion method using an HVLab multichannel plethysmograph (University of Southampton). On both fingers, a strain gauge was placed at the base of the finger nail and a pressure cuff for air inflation was fixed around the proximal phalanx. The pressure cuffs were inflated to a pressure of 60 mm Hg (8.0 kPa), and the rise in fingertip volume detected by means of the strain gauge according to the criteria given by Greenfield et al. (1963). The FBF measurements were expressed as millilitres per 100 millilitres per second (ml/100 ml/s).
Brachial systolic and diastolic blood pressures were measured in the upper left arm by an auscultatory technique while the participants were supine.
Finger skin temperature (FST) was measured using k-type thermocouples attached by micropore tape to the centres of the palmar surfaces of the distal phalanges of the right and left ring fingers. The room temperature was measured by a mercury-in-glass thermometer to an accuracy of ±0.5 °C. The thermometer was located close to the heads of the subjects.
Vertical sinusoidal vibration at 125 Hz with an unweighted acceleration magnitude of 44 ms−2 rms (corresponding to a frequency-weighted acceleration of 5.5 ms−2 rms according to International Standard 5349-1, 2001) was generated by an electrodynamic vibrator (VP4, Derritron). The vibration was the same as used previously to investigate the effects of environmental temperature on vibration-induced reductions in FBF (Ye and Griffin 2011b). The perception of 125-Hz vibration is mediated via the Pacinian channel which is involved in vibration-induced vasoconstriction (Ye and Griffin 2011a, 2013, 2014).
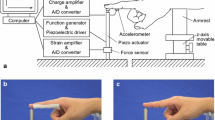
The vibration was applied to the right hand of each subject through a spherical wooden surface supported by a force cell (Huntleigh). The force cell was connected to a metre that provided visual feedback to the subject of the downward force applied by the hand. Subjects applied a 2-N downward force, sufficient to maintain contact between the palm and vibrator without affecting FBF. The vibration was monitored by an accelerometer (Entran 233E) attached to the metal plate supporting the force cell. The arrangement for controlling contact force and for generating and monitoring the vibration is shown in Fig. 1, and was the same as in previous studies (Griffin et al. 2006; Ye et al. 2012, 2014).
Subjects
Fourteen male volunteers participated in the study (the results from two subjects were excluded from data analysis due to very low baseline FBFs). All subjects were university students, non-smokers, and right-handed, and had no history of regular use of hand-held vibratory tools in occupational or leisure activities. The subjects completed a health questionnaire, read a list of medical contraindications, and gave their written informed consent to the study. No subject reported cardiovascular or neurological disorders, connective-tissue diseases, injuries to the upper extremities, or a history of cold hands. Subjects had a mean age of 26.1 (SD 3.3; range 20–32) years, a mean stature of 176 (SD 9.0; range 165–196) cm, a mean weight of 70.3 (SD 14.9; range 48–105) kg, and mean body mass index (BMI) of 22.4 (SD 2.7; range 17.6–27.3). From measurements of the length, width, and depth of each phalanx using vernier callipers, mean finger volumes were calculated as 19.1 (SD 5.3) and 19.1 (SD 5.5) cm3 for the middle fingers of the right and left hands, respectively, and 10.4 (SD 2.4) and 10.2 (SD 2.4) cm3 for the little fingers of the right and left hands. The subjects were requested to avoid consuming caffeine for 2 h and alcohol for 12 h prior to the testing. The experiment was approved by the Human Experimentation Safety and Ethics Committee of the Institute of Sound and Vibration Research.
Experimental sessions
Each subject participated in six sessions conducted on six different days, consisting of three control sessions (without vibration) and three sessions with vibration (Table 1). The vascular response to vibration was measured with the hand in one of three positions: (1) 20 cm below HL, (2) HL, or (3) 20 cm above HL. The order of presentation of conditions was randomised.
In all sessions, FBF was measured in the middle and little fingers of both the left and right hand at 30-s intervals throughout five successive experimental periods, with no breaks between the periods: (1) pre-exposure (5 min): no force and no vibration; (2) pre-exposure application with force (5 min): 2-N force and no vibration; (3) vibration (5 min): 2-N force and vibration at 125 Hz with vibration magnitudes at 44 ms−2 rms (unweighted); (4) post-exposure with force (5 min): 2-N force and no vibration; (5) recovery (5 min): no force and no vibration. In the three control conditions, the 2-N force was applied for 5 min during period (3) without vibration.
Procedure
The skin temperatures were measured, and the experiment proceeded only if the FST was greater than 30 °C.
The subjects lay supine throughout the measurement of FBF, with the right hand and arm supported at one of three positions. After a period of acclimatisation (around 20 min), FBF and FST were measured simultaneously in the left and right hand. For the first 5 min of measurement, the baseline values of FBF were obtained for both hands during period (1). Then, with the help of experimenter, the right hand was moved gently to place the centre of the palm on the spherical wooden piece connected to the vibrator, with all fingers suspended in air. The 2-N push force was then applied during period (2). During period (3), vibration was produced for 5 min at 125 Hz, followed by a 5-min period with 2-N force but no vibration in period (4). The exposed right hand was then moved by the experimenter and supported alongside the subject [at the same level as during period (1)] for another 5 min during period (5). The unexposed hand was supported at HL and kept motionless with no force and no vibration throughout all five periods.
Statistical methods
Statistical analysis was performed using the software package Stata (version 13.1 SE, Stata Corporation, College Station, TX, USA). The data were summarised with the mean as a measure of central tendency and the standard deviation (SD) or the 95 % confidence intervals (95 % CI) as measures of dispersion. Pairwise correlations between variables were tested by means of the Pearson coefficient.
Maximum-likelihood (ML) random-effects linear models for repeated-measures data set were used to test the hypothesis of no difference in the vascular responses in different exposure conditions taking into account the within-subject correlation over time. A p value of 0.05 was set as the limit of the statistical significance for the regression coefficients estimated by the fitted ML random-effects models. The p values were adjusted by the Bonferroni method for multiple comparisons.
Results
There were no statistically significant correlations between the FBF in any finger and the age, height, weight, BMI, or finger volume during any experimental session (p = 0.14–0.99). The FBF was not correlated with FST during any period of any of the six conditions (p = 0.19–0.96).
Systolic/diastolic brachial arterial pressures measured before the first period ranged from 130/60 to 90/50 mmHg, with no significant differences within subjects across conditions. No differences were observed between brachial arterial blood pressures measured at the beginning and at the end of the six experimental conditions (data not shown).
The air temperature in the laboratory did not show significant differences across the six experimental conditions (p = 0.16–0.24), ranging between 24.0 and 26.0 (mean 24.9) °C. There was no significant correlation between FBF and room temperature for any finger during the pre-exposure period (p = 0.83) or over the whole experiment (p = 0.62–0.84).
Finger circulation before exposure [period (1)]
The mean and standard deviation of the FBFs on the middle and little fingers of the right hand and the middle and little fingers of the left hand during period (1), with the right hand resting without force at one of three heights, are shown in Table 2. On the right hand, there were significant differences between the FBFs across the three different heights in both the middle finger (p < 0.001) and the little finger (p = 0.034). On the left hand, there were no significant differences in the three measures of FBF at HL (middle finger: p = 0.18; little finger: p = 0.44). Prior to vibration exposure, the mean FBF on the right middle finger decreased from 1.40 to 0.98 ml/100 ml/s and that on the right little finger decreased from 1.08 to 0.87 ml/100 ml/s as the hand was raised from 20 cm below HL to 20 cm above HL. This shows that raising a hand reduces blood flow to the fingers, but has little effect on blood flow in a contralateral hand maintained at heart height.
The FST during the first period averaged 35.1 (SD 0.9) °C in the right ring finger and 35.1 (0.8) °C in the left ring finger, with no significant differences across the six experimental conditions (p = 0.64). This indicates that raising the right hand did not provoke a change in FST on either hand.
Finger circulation during force application pre-exposure period (2)
Changes in %FBF (mean FBF expressed as a percentage of the mean FBF measured during the first period) in the exposed and unexposed fingers during the five experimental periods within each of the six conditions are shown in Fig. 2. The corresponding absolute changes in FBF in the exposed and unexposed fingers during the five experimental periods within each of the six conditions are shown in Fig. 3.
Percentage change in finger blood flow (% of pre-exposure) in the middle and little right fingers (exposed hand) and the middle and little left fingers (unexposed hand) during the six conditions and the five exposure periods (see Table 1). The plotted symbols are mean values. See Table 1 for the codes of conditions and periods
Absolute change in finger blood flow in the middle and little right fingers (exposed hand) and the middle and little left fingers (unexposed hand) during the six conditions and the five exposure periods (see Table 1). The plotted symbols are mean values. See Table 1 for the codes of conditions and periods
Changes in FST (Δ °C of pre-exposure period) in the exposed and unexposed finger during five experimental periods with each of six conditions are shown in Fig. 4.
Changes in finger skin temperature (Δ °C of pre-exposure) in the right and left ring finger during the six conditions and the five exposure periods: (1) pre-exposure, (2) force, (3) vibration, (4) force, and (5) recovery (see Table 1). The plotted symbols are mean values. See Table 1 for the codes of conditions and periods
During period (2), there were no significant changes in FBF compared with the pre-exposure period (1) in any of the four fingers in any of the six conditions (p = 0.33–0.96) (Fig. 2).
In none of the six conditions was there a significant change in FST during period (2) compared with period (1) in either the left or right ring finger (p = 0.24–1.0; Fig. 4).
This indicates that the application of 2-N downward push force by the right hand at each of the three positions (20 cm above HL, HL, and 20 cm below HL) did not provoke changes in finger circulation on either the exposed right hand or the unexposed left hand.
Finger circulation during vibration period (3)
Table 3 shows the effect of elevating the right hand on the absolute FBF and the percentage change in FBF (% of pre-exposure) during period (3).
Vibration provoked vasoconstriction in both fingers of both hands when the exposed hand was 20 cm above HL compared with no vibration with the same hand position (i.e. E+20 compared with C+20; p < 0.001). There were similar patterns with the hand at HL (E0 compared with C0; p < 0.001) and 20 cm below HL (E−20 compared with C−20; p < 0.001).
There were no significant differences in the reduction of %FBF in any finger between conditions E+20, E0, and E−20 on either the exposed hand or the unexposed hand (p = 0.10–0.17), except for the little finger of the exposed hand between E0 and E−20 (p = 0.034).
Relative to the corresponding control condition without vibration, on the middle finger of the exposed right hand, the mean absolute reductions in FBF were 0.49, 0.54, and 0.57 ml/100 ml/s in conditions E+20, E0, and E−20, respectively. On the little finger of the exposed right hand, the absolute reductions were 0.24, 0.48, and 0.58 ml/100 ml/s, respectively. With increasing height of the exposed right hand, there was a trend for less absolute reduction in FBF on both fingers of the exposed right hand. However, after adjustment of the p value for multiple comparisons, a statistically significant reduction in the absolute FBF was only found for the right middle finger in condition E+20 compared with conditions E0 and E−20 (p < 0.001), with no significant reductions in absolute FBF for the little finger of the right hand (p > 0.05). On the unexposed left hand, the mean absolute reductions in FBF did not differ across the three elevations of the right hand (p > 0.05). The results therefore show that elevation of the right hand decreased the absolute reduction in FBF in the right hand caused by the vibration of the right hand, but had no effect on the reduction in absolute FBF in the unexposed left hand maintained at HL.
Relative to the corresponding control condition without vibration, on the middle finger of the exposed right hand, the mean percentage reductions in FBF were similar at all three elevations. In the un-vibrated left hand resting at HL, the vibration-induced reduction in %FBF was independent of the height of the vibrated right hand.
Table 4 reports the FST in the exposed and unexposed fingers during exposure to 2-N push force and vibration [i.e. during period (3)] with the hand placed at each of the three heights. There were reductions in finger skin temperature in the three vibration conditions with the hand placed 20 cm above HL, at HL, and 20 cm below HL (conditions E+20, E0, and E−20) compared with the three corresponding control conditions without vibration but with the hand placed at the same positions (conditions C+20, C0, and C−20) (p < 0.001).
Finger circulation during force application post-exposure period (4)
In conditions C+20, C0, and C−20, there were no significant differences in FBF across fingers during period (4) compared with period (1) (i.e. pre-exposure) (p > 0.33).
Table 5 reports the effect of exposure to push force alone (2 N) on the percentage change in FBF (% of pre-exposure) over the period (4).
In conditions E+20, E0, and E−20, the FBFs in all four fingers were lower compared with the corresponding control conditions (i.e. C+20, C0, and C−20) (0.001 < p < 0.05), except for the middle finger of the exposed hand between E+20 and C+20 (p = 0.074) and E+0 and C+0 (p = 0.13), and for little finger of the exposed hand between E+0 and C+0 (p = 0.07).
The pattern of changes in finger skin temperature was similar to the changes in FBF, with applied force after vibration inducing lower finger skin temperature in both the exposed and unexposed fingers with all three hand positions (p < 0.01). Furthermore, the FST was lower in the exposed hand than the unexposed hand with all three vibration conditions (E+20, E0, and E−20) (p < 0.001).
Finger circulation during recovery period (5)
Table 6 reports the percentage change in FBF (% of pre-exposure) over the recovery period (5).
After the removal of vibration and force, the FBF gradually returned to the baseline. During period (5), there was no significant difference in %FBF on any of the four fingers in conditions E+20, E0, and E−20 compared with the corresponding control conditions C+20, C0, and C−20 (p = 0.15–0.90), except for the little finger of the exposed hand between E+20 and C+20 (p = 0.046) and little finger of unexposed hand between E−20 and C−20 (p = 0.026).
In both fingers, the FST was lower during the recovery period for condition E+20 than condition C+20 (p < 0.01). In the right (exposed) ring finger, the FST was lower for condition E0 than condition C0 (p < 0.05).
Discussion
Changes in finger blood flow induced by vibration
Acute exposure of one hand to vibration at a frequency of 125 Hz and a magnitude of 44 ms−2 rms (a frequency-weighted acceleration magnitude of 5.5 ms−2 rms) reduced FBF in fingers of both the exposed and the unexposed hand. This is consistent with previous studies: FBF in exposed and unexposed fingers has been reduced by 125-Hz vibration at magnitudes in the range of 0.69–7.75 ms−2 rms (weighted) (Bovenzi et al. 1999), and with the 5.5 ms−2 rms vibration used here (Bovenzi et al. 2004, Ye and Griffin 2011b). Although the same frequency and magnitude were applied, the % FBF observed in those studies differed, probably due to differences in the location of contact with vibration (Bovenzi et al. 2006, Griffin et al. 2006), the area of contact (Ye and Griffin 2013), and the applied force (Bovenzi et al. 2006). Although these factors do not influence the daily exposure A(8) value, they do influence the vasoconstriction caused by exposure to hand-transmitted vibration, so they may need to be controlled.
Reflex control of digital blood flow is considered to be mediated through sympathetic vasoconstriction and vasodilation (Roddie 1983; Bovenzi et al. 2006; Griffin et al. 2006). The finding in this study of reduced FBF and reduced FST contralateral to the location of vibration stimulation is consistent with previous findings (Bovenzi et al. 2004, 2006; Thompson and Griffin 2009; Ye and Griffin 2011a, 2013, 2014). It has been suggested that vibration causes a central sympathetic reflex that results in vasomotor responses in areas of the human body distant from the site of application of the vibration (Furuta et al. 1991; Mckenna et al. 1994; Bovenzi et al. 1995). Any such a reflex requires a stimulus, such as vibration excitation of one or more mechanoreceptor channel, and it has been found that vasoconstriction is dependent on the sensitivity of the Pacinian channel (Ye and Griffin 2011a, 2013, 2014).
After removing the force and the vibration, the FBF on both hands gradually returned to the baseline value, consistent with previous study using similar vibration provocation (Ye and Griffin 2011b). Previous studies have reported an association between the reductions in FBF during and after vibration exposure: subjects with greater reduction during vibration exposure tend to have lower FBF and longer recovery periods after removal of vibration (Ye et al. 2014). The extent of the reduction in blood flow after vibration exposure is dependent on the magnitude and the duration of vibration during exposure (Bovenzi et al. 1998, 1999, 2000). In one study, reductions in FBF were found on a vibrated finger after exposure to 22–62 ms−2 rms (unweighted), but not after exposure to 5.5 ms−2 rms (unweighted) (Bovenzi et al. 1999). In another study, during a 45-min recovery period, FBF returned to pre-exposure levels after a 7.5-min exposure to 125-Hz vibration at 87 ms−2 rms (unweighted) but not after 15- and 30-min exposures to the same vibration (Bovenzi et al. 1998). With greater magnitudes of vibration and longer durations of exposure to vibration, the vasoconstriction after cessation of exposure is stronger and lasts longer. The absence of reductions in FBF following exposure to vibration in the present study may reflect the brevity of the 5-min exposure to the vibration.
Effect of hand and arm position on finger blood flow
As expected, without vibration or force, the vertical position of the hand affected finger circulation: lifting the hand by 40 cm (from 20 cm below HL to 20 cm above HL) reduced FBF by about 0.42 and 0.23 ml/100 ml/s in the right middle and little fingers, respectively (about 30 and 21 % of the FBF measured with the hand 20 cm below HL). The blood pressure has been reported to drop 2 mm Hg for each inch (2.54 cm) increase in height (Netea et al. 1999). For the 40-cm (about 15.7 inches) increase in height in the present study, this would correspond to a 30-mm Hg drop in blood pressure. Such changes may be attributed to the effects of hydrostatic pressure (Mitchell et al. 1964). Various mechanisms mediate blood pressure and blood flow, so a linear relationship between FBF and finger blood pressure cannot be assumed, although the current results seem consistent with reductions in blood flow and blood pressure due to the lifting of the hand.
The absolute reduction in FBF provoked by vibration decreased with the hand elevated to 20 cm above the HL, but the percentage reduction in FBF was similar at all three heights. This is similar to the pattern of changes in FBF associated with the effects of room temperature: subjects with greater FBF at higher room temperature showed greater absolute reductions in blood flow in response to vibration, but the percentage reduction was similar (Ye and Griffin 2011b). Without vibration, a 20-N push force applied by the palm provoked a greater reduction in FBF than a 5-N push force, but when vibration was applied there was a similar percentage reduction in FBF on fingers of the exposed hand, but not on fingers of the unexposed hand (Griffin et al. 2006). The height of the hand, the temperature, and the applied force alter finger circulation irrespective of any hand-transmitted vibration, but it seems that in each case the vibration may cause an approximately similar percentage reduction in FBF.
Another physiological factor that may influence circulation is the muscle tension (Perez Gonzalez 1981). Although the arm was well supported during the measurements, subjects needed to maintain the 2-N downward force to the applicator. Holding the hand and arm 20 cm above HL, required extra effort and this isometric exercise may have reduced blood flow (Pickering et al. 2005; Takano et al. 2005).
Control the risk of vibration exposure
The effects of posture (and factors such as grip force and environmental temperature) are usually ignored when assessing the risks associated with occupational exposures to hand-transmitted vibration. They are not considered in most epidemiological studies and not taken into account in the exposure–response relationships for VWF proposed in International Standard 5349-1 (2001). Epidemiology studies have generally found only weak agreement between the occurrence of VWF and predictions based on the ISO 5349-1:2001 model (e.g. Griffin et al. 2003; Bovenzi 2010, 2012; Brammer and Pitts 2012). Both overestimation and underestimation of the occurrence of VWF have been reported (Futatsuka et al. 1984; Gemne et al. 1993; Griffin 1994), leading to doubts as to whether the frequency weighting for hand-transmitted vibration is appropriate for the assessment of vibration-induced vascular effects (Griffin 2012). Although the frequency weighting has a large influence on the assessment of risk from measures of vibration, even the perfect weighting will not predict risk if other factors, including posture, have a large influence.
Because FBF is highly variable and affected by factors other than vibration and posture, this study controlled the conditions and subjects rested in a supine posture. Users of vibratory tools are mostly standing and exerting muscular forces to undertake work. There are no known studies of the effects of body orientation on FBF. Measures of finger blood pressure suggest the diastolic pressure is greater when sitting than when supine (Netea et al. 2003) and the systolic pressure is lower while sitting upright than while supine (Terent and Breig-Asberg 1994). Bending of the elbows and the back can also increase diastolic blood pressure (Cushman et al. 1990). The diastolic pressure reflects the peripheral resistance of the vessels, and it may be assumed that body postures that increase the diastolic pressure will tend to be associated with decreased blood flow.
The working environment (e.g. hand and arm posture, grip force, and environmental temperature) will influence finger circulation in workers exposed to hand-transmitted vibration. This study shows that lifting the hand reduces blood flow in the fingers so that there is a lower baseline blood flow while working, which is unlikely to be beneficial to maintain active and healthy finger circulation. Considering many vibrating tools are heavy to lift, holding them at high positions requires extra force and muscle tension, which also causes the vasoconstriction. This also implies that to minimise the adverse effects of vibration, the height of the hands holding vibratory tools should be as low as practicable. Furthermore, the force needed to operate the tools should be as low as practicable. Further study is needed to understand the effects of vibration on FBF with a wider range of elevations than studied here, and with a range of grip, push, and pull forces, and a range of temperatures.
Previous studies of the effects of temperature and push force revealed a similar pattern as the effect of hand height: finger circulation varied with each of these factors before the application of vibration, and the application of vibration provoked a similar percentage reduction in FBF so that greater absolute reductions in FBF occurred when the FBF was greater. It may therefore be concluded that the maximisation of FBF when using vibratory tools requires consideration of factors influencing baseline blood flow as well as the vasoconstriction effects of vibration.
Conclusions
FBF reduces when raising the hand above the HL. The application of 125-Hz vibration to the palm of the right hand provokes an immediate reduction in blood flow in fingers on the exposed right hand and the unexposed left hand. There was lower absolute reduction in FBF when the hand was supported 20 cm above HL, but the percentage reduction in FBF caused by vibration was similar with all three elevations of the hand. The hand height should be controlled during studies of the vascular response to vibration. Tasks requiring the use of vibrating tools overhead are likely to involve low FBF.
References
Bovenzi M (2010) A prospective cohort study of exposure-response relationship for vibration induced white finger. Occup Environ Med 67(1):38–46
Bovenzi M (2012) Epidemiological evidence for new frequency weightings of hand-transmitted vibration. Ind Health 50(5):377–387
Bovenzi M, Griffin MJ, Ruffell CM (1995) Acute effects of vibration on digital circulatory function in healthy men. Occup Environ Med 52:834–841
Bovenzi M, Lindsell CJ, Griffin MJ (1998) Duration of acute exposure to vibration and finger circulation. Scand J Work Environ Health 24(2):130–137
Bovenzi M, Lindsell CJ, Griffin MJ (1999) Magnitude of acute exposure to vibration and finger circulation. Scand J Work Environ Health 25(3):278–284
Bovenzi M, Lindsell CJ, Griffin MJ (2000) Acute vascular responses to the frequency of vibration transmitted to the hand. Occup Environ Med 57(6):422–430
Bovenzi M, Welsh AJL, Griffin MJ (2004) Acute effects of continuous and intermittent vibration on finger circulation. Int Arch Occup Environ Health 77(4):255–263
Bovenzi M, Welsh AJL, Della Vedova A, Griffin MJ (2006) Acute effects of force and vibration on finger blood flow. Occup Environ Med 63:84–91
Brammer AJ, Pitts PM (2012) Frequency weighting for vibration-induced white finger compatible with exposure-response models. Ind Health 50:397–411
Cushman WC, Cooper KM, Horne RA, Meydrech EF (1990) Effect of back support and stethoscope head on seated blood pressure determinations. Am J Hypertens 3:240–241
Furuta M, Sakakibara H, Miyao M, Kondo T, Yamada S (1991) Effect of vibration frequency on finger blood flow. Int Arch Occup Environ Health 63:221–224
Futatsuka M, Sakurai T, Ariizumi M (1984) Preliminary evaluation of dose effect relationship for vibration-induced white finger in Japan. Int Arch Occup Environ Health 54:201–221
Gemne G, Lundström R, Hansson JE (1993) Disorders induced by work with hand-held vibrating tools. Arb Halsa 6:1–83
Greenfield ADM, Whitney RJ, Mowbray JF (1963) Methods for the investigation of peripheral blood flow. Br Med Bull 19:101–109
Griffin MJ (1994) Foundations of hand-transmitted vibration standards. Nagoya J Med Sci 57(Suppl):147–164
Griffin MJ (2012) Frequency-dependence of psychophysical and physiological responses to hand-transmitted vibration. Ind Health 50(5):354–369
Griffin MJ, Bovenzi M (2002) The diagnosis of disorders caused by hand-transmitted vibration: Southampton Workshop 2000. Int Arch Occup Environ Health 75:1–5
Griffin MJ, Bovenzi M, Nelson CM (2003) Dose-response patterns for vibration-induced white finger. Occup Environ Med 60:16–26
Griffin MJ, Welsh AJL, Bovenzi M (2006) Acute response of finger circulation to force and vibration applied at the palm of the hand. Scand J Work Environ Health 32(5):383–391
International Organization for Standardization (2001) Mechanical vibration: guidelines for the measurement and the assessment of human response to hand-transmitted vibration. International Standard, ISO 5349–1
International Organization for Standardization (2005) Mechanical vibration and shock: cold provocation tests for the assessment of peripheral vascular function—Part 2: measurement and evaluation of finger systolic blood pressure. International standard, ISO 14835–2
McKenna KM, Blann AD, Allen JA (1994) Vascular responses in chain saw operators. Occup Environ Med 51:366–370
Mitchell PL, Parlin RW, Blackburn H (1964) Effect of vertical displacement of the arm on indirect blood-pressure measurement. N Engl J Med 271:72–74
Netea RT, Lenders JW, Smits P, Thien T (1999) Arm position is important for blood pressure measurement. J Hum Hypertens 13:105–109
Netea RT, Lenders JW, Smits P, Thien T (2003) Influence of body and arm position on blood pressure readings: an overview. J Hypertens 21:237–241
Perez Gonzalez JF (1981) Factors determining the blood pressure responses to isometric exercise. Circ Res 48:176–186
Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ (2005) Recommendations for blood pressure measurement in humans and experimental animals–Part 1: blood pressure measurement in humans—a statement for professionals from the subcommittee of professional and public education of the American heart association council on high blood pressure research. Circulation 111:697–716
Roddie IC (1983) Circulation to skin and adipose tissue. In: Handbook of Physiology. The cardiovascular system. Peripheral circulation and organ blood flow. Am Physiol Soc, Bethesda, sec 2, vol 3, chapter 10, 285–317
Takano H, Morita T, Iida H, Asada K, Kato M, Uno K, Hirose K, Matsumoto A, Takenaka K, Hirata Y, Eto F, Nagai R, Sato Y, Nakajima T (2005) Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol 96:65–73
Terent A, Breig-Asberg E (1994) Epidemiological perspective of body position and arm level in blood pressure measurement. Blood Press 3:156–163
Thompson AJL, Griffin MJ (2009) Effect of the magnitude and frequency of hand-transmitted vibration on finger blood flow during and after exposure to vibration. Int Arch Occup Environ Health 82:1151–1162
Ye Y, Griffin MJ (2011a) Reductions in finger blood flow in men and women induced by 125-Hz vibration: association with vibration perception thresholds. J Appl Physiol 111:1606–1613
Ye Y, Griffin MJ (2011b) Effects of temperature on reductions in finger blood flow induced by vibration. Int Arch Occup Environ Health 84:315–323
Ye Y, Griffin MJ (2013) Reduction in finger blood flow induced by 125-Hz vibration: effect of area of contact with vibration. Eur J Appl Physiol 113:1017–1026
Ye Y, Griffin MJ (2014) Relation between vibrotactile perception thresholds and reductions in finger blood flow induced by vibration of the hand at frequencies in the range 8 to 250 Hz. Eur J Appl Physiol 114:1591–1603
Ye Y, Mauro M, Bovenzi M, Griffin MJ (2012) Acute effects of mechanical shocks on finger blood flow: influence of shock repetition rate and shock magnitude. Int Arch Occup Environ Health 85:605–614
Ye Y, Mauro M, Bovenzi M, Griffin MJ (2014) Association between vasoconstriction during and following exposure to hand-transmitted vibration. Int Arch Occup Environ Health 87:41–49
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, Y., Mauro, M., Bovenzi, M. et al. Reduction in finger blood flow induced by hand-transmitted vibration: effect of hand elevation. Int Arch Occup Environ Health 88, 981–992 (2015). https://doi.org/10.1007/s00420-015-1027-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-015-1027-0