Abstract
Purpose
This study investigated whether the reductions in finger blood flow induced by 125-Hz vibration applied to different locations on the hand depend on thresholds for perceiving vibration at these locations.
Methods
Subjects attended three sessions during which vibration was applied to the right index finger, the right thenar eminence, or the left thenar eminence. Absolute thresholds for perceiving vibration at these locations were determined. Finger blood flow in the middle finger of both hands was then measured at 30-s intervals during five successive 5-min periods: (i) pre-exposure, (ii) pre-exposure with 2-N force, (iii) 2-N force with vibration, (iv) post-exposure with 2-N force, (v) recovery. During period (iii), vibration was applied at 15 dB above the absolute threshold for perceiving vibration at the right thenar eminence.
Results
Vibration at all three locations reduced finger blood flow on the exposed and unexposed hand, with greater reductions when vibrating the finger. Vibration-induced vasoconstriction was greatest for individuals with low thresholds and locations of excitation with low thresholds.
Conclusions
Differences in vasoconstriction between subjects and between locations are consistent with the Pacinian channel mediating both absolute thresholds and vibration-induced vasoconstriction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vascular disorder associated with occupational exposures to hand-transmitted vibration is commonly known as vibration-induced white finger (VWF), which is characterised by attacks of finger blanching provoked by exposure to cold (Griffin 1990; Griffin and Bovenzi 2002). The development of this disorder in the fingers of workers operating vibratory tools is thought to depend on the frequency, the magnitude, and the duration of the vibration (International Organization for Standardization 2001).
In experimental studies, vibration-induced acute reductions in finger blood flow (FBF) have been found to depend on the magnitude of vibration (e.g. increased vasoconstriction during and after exposure to vibration with greater magnitudes: Bovenzi et al. 1999; Griffin et al. 2006; Thompson and Griffin 2009), the frequency vibration (increased vasoconstriction during and after exposure to vibration acceleration at some frequencies: Welsh 1980; Bovenzi et al. 2000; Thompson and Griffin 2009), and the duration of exposure to vibration (increased vasoconstriction after exposure to vibration for greater durations: Egan et al. 1996; Bovenzi et al. 1998; Luo et al. 2000). The variations in blood flow associated with these three vibration characteristics have been found on both the fingers of the hand exposed to vibration and fingers on the contralateral hand not exposed to vibration.
The mechanisms responsible for the vasoconstriction arising from acute and chronic exposures to hand-transmitted vibration are not understood. Vibration may cause changes in finger blood flow through a central sympathetic reflex (Gemne 1994; Bovenzi et al. 2006; Ye and Griffin 2011, 2013, 2014). Any such a reflex requires a stimulus, such as the excitation by vibration of mechanoreceptors in the hand (Ye and Griffin 2011, 2013, 2014).
Vibrotactile thresholds in the glabrous skin of the hand are mediated by one or more of four psychophysical channels (Bolanowski et al. 1988; Gescheider et al. 2002). The Pacinian (P) channel is mediated by Pacinian corpuscles and generally has greatest sensitivity at high frequencies (e.g. >40 Hz) and exhibits both spatial summation and temporal summation: Thresholds reduce as either the area of excitation increases or the duration of vibration increases (Verrillo 1962, 1963). Of the three non-Pacinian channels, the response of the NPI channel may determine thresholds at frequencies between about 4 and 40 Hz and is associated with Meissner corpuscles (FA I). The NPII and NPIII channels are associated with slow adapting fibres and have been identified from psychophysical studies (Capraro et al. 1979; Bolanowski et al. 1988; Verrillo et al. 2002). The NPII channel is excited by vibration in the same frequency range as the Pacinian channel, but is sensitive to stretching of the skin and in most conditions has a higher threshold than the Pacinian channel (Bolanowski et al. 1988). The NPIII channel may have a lower threshold than other channels in the frequency range 0.4–4 Hz (Greenspan and Bolanowski 1996). By selecting the frequency and the magnitude of vibration and the contact conditions between the source of vibration and the skin, different mechanoreceptors can be activated (Morioka and Griffin 2005; Ye and Griffin 2011, 2013, 2014).
Standards for evaluating the severity of exposures to hand-transmitted vibration recognise that factors other than the magnitude of vibration, the frequency of vibration, and the duration of exposure to vibration may influence the consequences of occupational exposures to hand-transmitted vibration. International Organization for Standardization (2001) says ‘the area and location of the parts of the hands which are exposed to vibration’ may be one of the factors influencing the effects of exposure to hand-transmitted vibration in working conditions. When a greater area of the finger is exposed to 125-Hz vibration, there are greater reductions in finger blood flow, both during and after exposure and on both the hand exposed to vibration and on the unexposed hand (Ye and Griffin 2013). This is consistent with the Pacinian channel being involved in the activation of a central sympathetic reflex.
The extent to which finger blood flow is reduced by 125-Hz vibration in an individual is dependent on the individual’s thresholds for the perception of 125-Hz vibration (Ye and Griffin 2011). This is also consistent with the Pacinian channel being involved in changes in finger blood flow during exposure to vibration. Over the frequency range from 8 to 250 Hz, reductions in finger blood flow at higher frequencies (63,125 and 250 Hz) are associated with individual thresholds for perceiving these frequencies, but at the lower frequencies (8, 16 and 31.5 Hz), there was no relation between thresholds and vasoconstriction (Ye and Griffin 2014). The difference in vascular response to the higher and lower frequencies may arise from the different mechanoreceptors activated when determining thresholds at high and low frequencies. The findings are consistent with only the P channel being involved in mediating the vascular response to vibration.
This study was designed to determine whether reductions in finger blood flow induced by 125-Hz vibration at different locations on the hand are associated with differences in thresholds for perceiving the same vibration at the same locations. With a vibration magnitude 15 dB greater than the threshold for perceiving 125-Hz vibration applied to the right thenar eminence, it was expected that the Pacinian channel would be activated at each of the three locations: the right index finger, the right thenar eminence, and the left thenar eminence. It was hypothesised differences in vasoconstriction during exposure to vibration at the different locations would be correlated with differences in thresholds for perceiving vibration at the three locations.
Method
Apparatus
Thresholds for the perception of 125-Hz vibration were obtained with the HVLab Vibrotactile Perception Meter (VPM, University of Southampton) (using a 6-mm-diameter probe with 2-mm gap between the probe and a fixed circular surround) and the von Békésy algorithm. The magnitude of vibration decreased at 3 dB/s until the subject no longer perceived vibration and then increased at 3 dB/s until the subject perceived vibration. The subject responded by pressing a button when perceiving the vibration stimulus and releasing the button when it was not perceived. The test proceeded for a minimum of 30 s and a minimum of six reversals (i.e. button presses or button releases). If necessary, the measurements continued beyond 30 s until six reversals were obtained. Thresholds were calculated as the arithmetic mean of the mean peak (button pressed) and the mean trough (button released) expressed in ms−2 r.m.s., with the first two responses ignored.
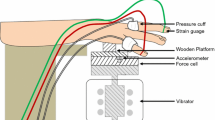
Finger blood flow (FBF) was measured in the middle finger of both hands using an HVLab multi-channel plethysmograph (University of Southampton). The strain gauges were placed at the base of the finger nails, and plastic pressure cuffs for air inflation were fixed around the proximal phalanges. The pressure cuffs and strain gauges were connected to the plethysmograph. The FBF was measured with a venous occlusion method. The pressure cuffs were inflated to a pressure of 60 mm Hg (8.0 kPa), and the rise in fingertip volume was detected by means of the strain gauge according to the criteria given by Greenfield et al. (1963). The FBF measurements were expressed as millilitres per 100 millilitres a second (ml/100 ml/s). The finger blood flow was expressed as a percentage of the median pre-exposure finger blood flow measured in period (i) (i.e. FBF%).
Finger skin temperature (FST) was measured using k-type thermocouples attached by micro pore tape to the distal phalanges of the right and left middle fingers. The room temperature was measured by a mercury-in-glass thermometer to an accuracy of ±0.5 °C. The thermometer was located close to the heads of the subjects.
Vertical sinusoidal 125-Hz vibration at a magnitude 15 dB greater than the threshold measured at the right thenar eminence of each individual was produced by the applicator of the VPM and monitored using the accelerometer inside the VPM. The waveform of the signal was displayed on a monitor. Visual feedback for the control of the downward force was supplied to the subject by the control unit of the VPM.
Subjects
Twelve healthy male volunteers with a mean age of 25.6 years (SD: 3.4; range: 20–30) participated in the study. All subjects were university students, non-smokers, right handed, and had no history of regular use of hand-held vibratory tools in occupational or leisure activities. The subjects completed a health questionnaire, read a list of medical contraindications, and gave their written informed consent to the study. The mean stature of the subjects was 175.8 (SD: 6.5, range: 165–196) cm, their mean weight was 77.9 (SD: 10.5, range: 62–105) kg, and their mean body mass index (BMI) was 24.5 (SD: 3.2, range: 19.6–27.3). The finger length and the width and depth of each phalanx of the right and left middle fingers were measured with vernier callipers to an accuracy of 0.5 mm, and then the volumes of the fingers calculated. The mean finger volumes were 17.6 (SD: 6.0) cm3 and 17.3 (SD: 6.2) cm3 for the middle fingers of the right and left hand.
The subjects were requested to avoid consuming caffeine for 2 h and alcohol for 12 h prior to the testing. The experiment was approved by the Human Experimentation Safety and Ethics Committee of the Institute of Sound and Vibration Research.
Sessions
Each subject participated in three sessions conducted on three different days. In each session, the vascular response was measured with vibration applied to one of three locations: (i) fingertip of right index finger (R In), (ii) thenar eminence of the right hand (R TE), or (iii) thenar eminence of the left hand (L TE). The order of presentation of conditions was randomised. Absolute thresholds for vibration perception at each of the three locations were determined at the beginning of the first session.
In each session, finger blood flow was measured in the left and right middle finger at 30-s intervals throughout five successive experimental periods, with no break between the five periods: (i) pre-exposure (5 min): no force and no vibration; (ii) pre-exposure application with force (5 min): 2-N force and no vibration; (iii) vibration (5 min): 2-N force and 125-Hz vibration 15 dB above the threshold measured at the right thenar eminence; (iv) post-exposure with force (5 min): 2-N force and no vibration; and (v) recovery (5 min): no force and no vibration.
Procedure
The experiment was performed in a laboratory with a mean air temperature of 25.2 (SD 0.5) °C.
Initially, subjects sat on a seat next to a table supporting the vibrotactile perception meter. The height of the seat was adjusted so that subjects were comfortable and able to maintain a force of 2-N between their hands and the surround on the VPM applicator. Their vibrotactile perception thresholds at 125-Hz were measured at three locations: the fingertip of the right index finger and the thenar eminences of the right and left hands. The skin temperatures at these three locations were measured and the experiment proceeded only if the skin temperature was >30 °C.
The subjects lay supine throughout the measurement of finger blood flow, with both arms and both hands supported at heart level. The vascular response to vibration was determined using vibration applied by the HVLab VPM with the same contact conditions used when measuring thresholds (i.e. 6-mm-diameter probe with 2-mm gap between the probe and a fixed circular surround). After a period of acclimatisation (around 20 min), finger blood flow and finger skin temperature were measured simultaneously in the left and right hand. For the first 5 min of measurement period (i), the baseline values of FBF were obtained with the hand supported at heart height alongside the subject. Then, with the help of experimenter, the subjects were asked to apply downward force of 2-N on the applicator of the VPM during period (ii). The hand was in a comfortable posture with all fingers suspended without contact. During period (iii), vibration was produced for 5 min at 125-Hz, followed by a 5-min period with force but no vibration in period (iv). The exposed hand was then moved by the experimenter and supported at heart height alongside the subject for another 5 min during period (v). The unexposed hand was supported at heart level and kept motionless with no force and no vibration throughout all five periods.
Statistical methods
Data analysis was performed using the software package SPSS (version 19.0). The data were summarised with the median as a measure of central tendency and the inter-quartile range (IQR) as a measure of dispersion. Nonparametric tests (Friedman test for k-related samples, Wilcoxon matched-pairs signed ranks test for two-related samples, and Spearman test for correlation coefficient) were employed in the statistical analysis.
The Friedman test was used to test for differences between the ten measurements of finger blood flow during the 5-min pre-exposure period, the 5-min pre-exposure application of force, the 5-min vibration period, the 5-min post-exposure application of force, and the 5-min recovery period. A Wilcoxon matched-pairs signed ranks test was then used to investigate any changes in finger blood flow between the five periods (with force and vibration applied) and any differences between the three body locations. A Spearman test was used to investigate the relation between individual perception thresholds and finger blood flow.
The criterion for statistical significance was p < 0.05. The p values were adjusted for multiple comparisons.
Results
Vibration thresholds
The medians and inter-quartile ranges (IQR) of the perception thresholds at the right index finger, the right thenar eminence, and the left thenar eminence were 0.29 (0.13), 0.54 (0.27) and 0.61 (0.31) ms−2 r.m.s., respectively. The range of thresholds at the right thenar eminence (0.39–1.19 ms−2 r.m.s.) resulted in vibration magnitudes 15 dB greater when investigating finger blood flow (i.e. 2.21–6.74 ms−2 r.m.s., unweighted; 0.28–0.84 ms−2 r.m.s., weighted). The acceleration thresholds determined at the fingertip of the right index finger, the thenar eminence of the right hand, and the thenar eminence of the left hand are shown for each subject in Fig. 1.
The thresholds at the three locations would be expected to be correlated since they are all expected to be mediated by the Pacinian channel. Thresholds at right index finger, the right thenar eminence, and the left thenar eminence were positively correlated (p < 0.05, Spearman), except for the right thenar eminence and the left thenar eminence (p = 0.079, Spearman). This shows that subjects with a lower threshold on the right index finger tended to have lower thresholds at the thenar eminence of both hands.
Room temperature and finger skin temperature
The temperature in the laboratory did not vary during the 25-min duration of the measurement of finger blood flow in any of the three experimental conditions (p = 0.46–0.83, Friedman). The median room temperature did not differ between the three conditions (p = 0.41, Friedman).
On neither hand was there a systematic change in finger skin temperature during the 25-min measurement of finger blood flow within any of the three experimental conditions (p = 0.27–0.78; Friedman). There was no differences in finger skin temperature across the three experimental conditions during the pre-exposure period, pre-exposure application of force, vibration exposure, post-exposure application of force, or recovery period for either the left or right hand (p = 0.19–0.53).
Finger blood flow
Variations in finger blood flow (medians and quartiles) on both the right and left middle fingers over the five experimental periods in each of the three conditions (i.e. vibration at the right index finger or the left or right thenar eminence during the third of the five 5-min periods) are shown in Fig. 2.
Medians and quartiles of finger blood flow in the middle fingers of the right and left hands over the five periods (vibration at the right index finger or the left or right thenar eminence during the third of the five 5-min periods). Significant reductions in FBF compared with pre-exposure period (i) are indicated: *p < 0.01; **p < 0.001
Finger blood flow was initially expressed as a percentage of the median finger blood flow during period (i), the pre-exposure period (i.e. FBF%). The FBF% (median and quartiles) varied over the five experimental periods of all three conditions on both the right and left middle fingers (Fig. 3).
Medians and quartiles of percentage change in finger blood flow (% of median pre-exposure blood flow) in the middle fingers of the right and left hands over the five periods (vibration at the right index finger or the left or right thenar eminence during the third of the five 5-min periods). Significant reductions in FBF% compared with the pre-exposure period (i) are indicated: *p < 0.01; **p < 0.001
Finger blood flow during period (i): pre-exposure period
There were no significant changes in FBF on either the exposed hand or the unexposed hand over the 10 measurements during the 5-min pre-exposure period in any condition (p = 0.31–0.44; Friedman). The median individual FBF over the 10 measurements did not differ between the three conditions on either hand (p = 0.24–0.33; Friedman). There was no significant difference in FBF between the exposed and the unexposed hand in any of the three conditions (p = 0.11–0.38; Wilcoxon).
Finger blood flow during period (ii): pre-exposure application of force
Finger blood flow on the exposed hand and the unexposed hand did not change over the ten measurements during the pre-exposure application of force in any of the three conditions (p = 0.53–0.76; Friedman), and the median FBF% did not differ across the three conditions on either hand (p = 0.27–0.44; Friedman).
There was no significant difference in the median FBF% during period (ii) (pre-exposure application of force) compared to period (i) (the pre-exposure period) (p = 0.23–0.56 on both hands for all three conditions) on either the exposed hand or the unexposed hand, except for the middle finger of the exposed hand in the condition with vibration of the right index finger (p = 0.033, Wilcoxon). However, after the Bonferroni correction, there was no significant effect of the force in any of the three conditions. This shows that when the 2-N force was applied to the right index finger, the right thenar eminence, or the left thenar eminence there was no change in finger blood flow on either hand.
Finger blood flow during period (iii): vibration exposure period
In all three conditions, there were no significant differences in FBF% over the ten measurements during period (iii) (force with vibration) on either the exposed hand or the unexposed hand (p = 0.13–0.27; Friedman), except on the middle finger of the exposed hand during vibration of the right index finger (p = 0.027, Friedman). On both hands, there were significant reductions in the individual median FBF% during period (iii) (force with vibration) compared to period (i) (pre-exposure period) (p < 0.001; Wilcoxon) and compared to period (ii) (pre-exposure force period) (p < 0.001; Wilcoxon) with vibration applied to all three locations. This shows that vibration of the right index finger, vibration of the thenar eminence of the right hand, and vibration of the thenar eminence of the left hand reduced finger blood flow in the middle finger of both hands. With all three locations of vibration excitation, there was a greater reduction in FBF% in the middle finger of the exposed hand than in the middle finger of the unexposed hand (p < 0.05, Wilcoxon).
The medians and inter-quartile ranges of the FBF% during exposure to 125-Hz vibration in period (iii) are listed in Table 1. This shows reductions in FBF for all three locations of vibration excitation (right index finger, left thenar eminence, and right thenar eminence) caused by the same vibration magnitude (15 dB above the threshold at the right thenar eminence). The FBF% on both the right and left middle fingers differed between the three locations of vibration excitation (p < 0.001, Friedman). The reduction in FBF was greater on both hands when vibration was applied to the right index finger (p < 0.001, Wilcoxon). This shows that vibration of the same frequency and magnitude applied to different body locations provoked different reductions in finger blood flow.
Finger blood flow during period (iv): post-exposure application of force
In all three conditions, there was no significant change in FBF% over the ten measurements during period (iv) (post-exposure application of force) (p = 0.11–0.23, Friedman), except with vibration applied to the right index finger where the FBF% in the unexposed left hand progressively increased during period (iv) (p = 0.041, Freidman).
In all three conditions, the median FBF% on both the exposed hand and the unexposed hand were less during period (iv) (post-exposure application of force) than during period (ii) (pre-exposure application of force) (p < 0.01, Wilcoxon), but slightly greater than during period (iii) (force and vibration) (p < 0.05, Wilcoxon).
Finger blood flow during period (v): recovery period
Over the ten measurements of FBF during period (v) (recovery period), there were significant changes in FBF on both the exposed hand and the unexposed hand (p < 0.001, Friedman) in all three conditions, except on the unexposed hand where the FBF did not change during vibration of the right thenar eminence (p = 0.12, Friedman).
In all three conditions, on the exposed hand the individual median finger blood flow during period (v) (recovery) was significantly less than during period (i) (pre-exposure) and less than during period (ii) (pre-exposure application of force) (p < 0.05; Wilcoxon) but significantly greater than during period (iii) (vibration exposure) and greater than period (iv) (post-exposure application of force) (p < 0.01; Wilcoxon). On the unexposed hand, the FBF during period (v) was significant less than during period (i) and during period (ii) with vibration of the right index finger (p < 0.05; Wilcoxon), but not with the vibration of the right or left thenar eminence (p > 0.05; Wilcoxon).
Relationship between finger blood flow and vibrotactile perception threshold
During exposure to 125-Hz vibration with the same acceleration magnitude at each of the three locations, exposure at the right index finger or the left thenar eminence provoked reductions in finger blood flow on both the exposed right hand and the unexposed left hand that were positively correlated with subject vibrotactile perception thresholds at the location of excitation (excitation of the right index finger: right finger, p = 0.011; left finger, p = 0.037; excitation of the left thenar eminence: right finger, p = 0.033; left finger, p = 0.051; Spearman). The results indicate that the vascular responses to vibration depend on individual thresholds at the location of application of vibration. As expected, with vibration applied to the right thenar eminence there was no correlation between thresholds at the right thenar eminence and FBF% on either hand (p > 0.1, Spearman). This is explained by the vibration magnitude being determined by the threshold for perceiving vibration at this location.
To investigate whether the vasoconstriction caused by vibration applied to the three different locations on the hand was associated with individual perception thresholds at these locations, differences in both FBF% and vibrotactile thresholds were calculated between pairs of conditions. For example, for each individual, the ratio of the difference in FBF% between the right index finger and the right thenar eminence was given by FBF%R–In/FBF%R–TE. There were positive correlations between the three ratios for FBF% on the exposed hand and the corresponding ratios for thresholds on the exposed hand (p < 0.01, Spearman; Fig. 4). This is consistent with the vasoconstriction induced by 125-Hz vibration depending on the thresholds for perceiving the vibration at the point of application of the vibration: the greater the ratio in thresholds between locations of excitation, the greater the ratio in the vasoconstriction caused by excitation at these locations.
Associations between ratios of differences between pairs of conditions for finger blood flow on the exposed hand during vibration exposure (period (iii)) and corresponding ratios of differences in perception thresholds. Associations shown for three locations: right index finger (R In), left thenar eminence (L TE), right thenar eminence (R TE)
Discussion
Effect of vibration on finger blood flow
The finding that 125-Hz vibration of a small area of one hand produces bilateral reductions in finger blood flow is consistent with previous studies. The present study employed magnitudes of 125-Hz vibration in the range 2.21–6.74 ms−2 r.m.s. (i.e. 0.28–0.84 ms−2 r.m.s. frequency-weighted), whereas previous studies have found similar reductions bilaterally with 0.5 and 1.5 ms−2 r.m.s. (Ye and Griffin 2011) and 0.88–9.17 ms−2 r.m.s. (Ye and Griffin 2013). Using the same experimental set up, 63-, 125-, and 250-Hz vibration 10 dB above threshold also reduced finger blood flow on both hands (Ye and Griffin 2014). With 250- and 315-Hz vibration, magnitudes as low as 7.8 and 9.8 ms−2 r.m.s., respectively, have produced vasoconstriction (Thompson and Griffin 2009). It is clear that vibration applied to various parts of the hand (palm, thenar eminence, and fingertip) at magnitudes much lower than occur on very many vibratory tools results in immediate reduction in finger blood flow on both the exposed hand and the unexposed hand.
With 125-Hz vibration 15 dB above the threshold at the right thenar eminence, there was greater vasoconstriction when the vibration was applied to the right index finger than when it was applied to the right or left thenar eminence. The same contactor (6-mm-diameter probe with 2-mm gap), the same vibration magnitude, and the same vibration frequency were used in all three vibration conditions, only the exposure location changed from the fingertip to the thenar eminence. There is no known previous study of the effect of the location of vibration excitation on finger blood flow, but two studies have used the same experimental set up with vibration applied at different locations. With 125-Hz vibration at 64 ms−2 r.m.s. with a 5-N push force, FBF% was reduced to 50 % when the vibration was applied to the middle finger (Bovenzi et al. 2006) but only 75 % when applied to the palm of the hand (Griffin et al. 2006). This is consistent with the reduction in finger blood flow depending on the location of application of vibration to the hand, with greater reduction when the vibration is applied to the fingers than to the palm.
After removing the force and the vibration, the finger blood flow on both hands gradually returned to the baseline value, consistent with previous studies using similar vibration provocation (Ye and Griffin 2011, 2013). Some studies have reported reductions in finger blood flow after cessation of vibration, with the extent of the reduction dependent on the magnitude and the duration of the vibration exposure (Bovenzi et al. 1998, 1999, 2000). In one study, there were reductions in finger blood flow on a vibrated finger after exposure to 22–62 ms−2 r.m.s., but not after exposure to 5.5 ms−2 r.m.s. (Bovenzi et al. 1999). In another study, during a 45-min recovery period, finger blood flow returned to pre-exposure levels after a 7.5-min exposure to 125-Hz vibration at 87 ms−2 r.m.s. but not after 15- and 30-min exposures to the same vibration (Bovenzi et al. 1998). With greater magnitudes of vibration and longer durations of exposure to vibration, the vasoconstriction after cessation of exposure is stronger and lasts longer. The absence of reductions in finger blood flow following exposure to vibration in the present study may reflect the brevity of the 5-min exposure to the vibration and the low magnitude of the vibration.
Vibratory tools are often used in a standing posture and rarely in the recumbent posture used to minimise variations in blood flow during the present study. There is an increase in diastolic blood pressure and a decrease in systolic blood pressure when sitting upright compared to standing (e.g. Terent and Breig-Asberg 1994; Netea et al. 2003). The diastolic pressure reflects the peripheral resistance of the vessels, and so finger blood flow may tend to be less when sitting than when standing. Finger blood flow reduces when the hand is elevated above the heart, but the percentage change in finger blood flow induced by vibration is independent of hand elevation (Ye et al. 2015). It therefore seems reasonable to expect that there may be similar percentage changes in finger blood flow in standing persons to those found in this study with supine subjects.
Effect of location of vibration excitation on vibrotactile perception thresholds
Vibrotactile perception thresholds differed between the three locations of vibration excitation used in the present study, with the finger having greater sensitivity than the thenar eminence. Thresholds at eight points on the glabrous skin of the hand measured with a 6-mm-diameter probe and a fixed surround showed greatest sensitivity at distal locations on the fingers and less sensitivity at proximal locations on the palm (Morioka and Griffin 2005), consistent with the suggestion of Löfvenberg and Johansson (1984) that greater densities of Pacinian corpuscles give lower psychophysical thresholds. The average density of Pacinian corpuscles in areas of glabrous skin on the human hand is reported to be about 25 units/cm2 at the fingertip compared with 10 units/cm2 at the palm (Johansson and Vallbo 1979). The higher thresholds obtained on the right and left thenar eminence compared with the right index finger indicates that the Pacinian channel has a greater sensitivity on the finger.
There is no known study suggesting the perception of vibration at the hand differs between the two body postures: sitting upright and supine. The variation in the angle between the upper-arm and the fore-arm may affect the transmission of hand-transmitted vibration, but in the experiment the transmission of vibration from the excitation area was controlled by the use of a surround and the vibration magnitude was too low to be felt at locations other than very close to the point of contact.
Effect of location of vibration excitation on finger blood flow: involvement of the Pacinian channel
The greater reductions in FBF with vibration applied at the right index finger may be primarily due to the greater sensitivity of the P channel at the fingertip. The similar reduction in FBF with vibration applied at the thenar eminences of the right and left hands is consistent with similar sensitivity in the Pacinian channel in the palms of the right and left hands. These results indicate that ‘contact location’ influences the sensitivity of the Pacinian channel and the extent to which the Pacinian channel mediates finger blood flow. This is consistent with the hypothesis that variations in the sensitivity of Pacinian channel vary the vasoconstriction in different subjects and also vary the vasoconstriction caused by vibration applied at different locations.
The associations between reductions in finger blood flow and vibrotactile thresholds at three body locations are consistent with the Pacinian channel mediating vasoconstriction (Ye and Griffin 2011, 2013, 2014). The involvement of the Pacinian system in mediating changes in finger blood flow induced by 125-Hz vibration was previously studied by investigating the correlation between reductions in individual FBF and individual thresholds (Ye and Griffin 2011). Further study found that increasing the excitation area reduced thresholds and increased vasoconstriction (Ye and Griffin 2013), with both effects explained by ‘spatial summation’ in the Pacinian channel. Over a range of frequencies of vibration (from 8 to 250 Hz), it was found that with the higher frequencies (63, 125, and 250 Hz) vasoconstriction was associated with individual thresholds for perceiving the vibration, but at the lower frequencies (8, 16, and 31.5 Hz) there was no correlation between thresholds and vasoconstriction (Ye and Griffin 2014). This difference between higher and lower frequencies corresponds to the Pacinian channel being the dominant channel involved in vibration perception at frequencies greater than about 40 Hz.
Other explanations might be offered for the stronger vasoconstriction arising from vibration of the fingers than vibration at the thenar eminence. As mechanical systems, the dynamic responses of the fingers and the hand are highly dependent on the posture and location of contact with vibration, but can contain resonances at various frequencies <500 Hz (Concettoni and Griffin 2009). The contact condition used in this study was designed to restrict the vibration to within a 10-mm-diameter area, so studies of the response of the whole finger or whole hand do not provide useful estimates of any resonances applicable to this study. Variations in contact area and contract force affect vibrotactile thresholds (e.g. Morioka and Griffin 2005; Ye and Griffin 2013) and can affect the dynamic responses of tissues close to the point of contact with vibration. Changes in thresholds caused by changes in contact area and force seem to be more easily explained by the characteristics of the psychophysical channels (e.g. spatial summation in the Pacinian channel) than changes in a local mechanical resonance of the tissues (Whitehouse and Griffin 2002). Nevertheless, local tissue dynamic responses may have some effect on both vibrotactile thresholds and reductions in blood flow associated with vibration at the fingers and the thenar eminence.
The present results complement previous findings of an association between perception thresholds and vibration-induced vasoconstriction, which explain differences in vasoconstriction between females and males, between large and small contact areas, and between vibration frequencies in the range 8–250 Hz (Ye and Griffin 2011, 2013, 2014). All findings suggest that the Pacinian channel is involved in the regulation of digital circulation through a central sympathetic reflex, with the extent of the vasoconstriction induced by vibration dependent on the activation of the Pacinian channel: people with lower Pacinian thresholds, or exposed to vibration over a larger area of skin on the hand, or at a location with greater Pacinian sensitivity, will have a greater reduction in finger blood flow on both the exposed hand and the unexposed hand.
Conclusions
The application of 125-Hz vibration to an index finger provokes greater reductions in finger blood flow on both the exposed hand and the unexposed hand than the same vibration applied to the thenar eminence of either hand. The greater vasoconstriction is explained by lower vibrotactile perception thresholds at the fingertip, which reflect the greater sensitivity of the Pacinian channel at the fingertip. The findings are consistent with the Pacinian channel being involved in the control of finger circulation via a central sympathetic reflex.
References
Bolanowski SJ, Gescheider GA, Verrillo RT, Checkosky CM (1988) Four channels mediate the mechanical aspects of touch. J Acoust Soc Am 84:1680–1694
Bovenzi M, Lindsell CJ, Griffin MJ (1998) Duration of acute exposure to vibration and finger circulation. Scand J Work Environ Health 24(2):130–137
Bovenzi M, Lindsell CJ, Griffin MJ (1999) Magnitude of acute exposure to vibration and finger circulation. Scand J Work Environ Health 25(3):278–284
Bovenzi M, Lindsell CJ, Griffin MJ (2000) Acute vascular responses to the frequency of vibration transmitted to the hand. Occup Environ Med 57:422–430
Bovenzi M, Welsh AJL, Della Vedova A, Griffin MJ (2006) Acute effects of force and vibration on FBF. Occup Environ Med 63:84–91
Capraro AJ, Verrillo RT, Zwislocki JJ (1979) Psychophysical evidence for a triplex system of mechanoreception. Sens Process 3:334–352
Concettoni E, Griffin MJ (2009) The apparent mass and mechanical impedance of the hand and the transmission of vibration to the fingers, hand and arm. J Sound Vib 325(3):664–678
Egan CE, Espie BH, McGrann S, McKenna KM, Allen JA (1996) Acute effects of vibration on peripheral blood flow in healthy subjects. Occup Environ Med 53:663–669
Gemne G (1994) Diagnostics of hand-arm system disorders in workers who use vibrating tools. Occup Environ Med 54:90–95
Gescheider GA, Bolanowski SJ, Pope JV, Verrillo RT (2002) A four-channel analysis of the tactile sensitivity of the fingertip: frequency selectivity, spatial summation, and temporal summation. Somatosens Motor Res 19:114–124
Greenfield ADM, Whitney RJ, Mowbray JF (1963) Methods for the investigation of peripheral blood flow. Br Med Bull 19:101–109
Greenspan JD, Bolanowski SJ (1996) The psychophysics of tactile perception and its peripheral physiological basis. In: Kruger L (ed) Handbook of perception and cognition 7: pain and touch. Academic Press, San Diego, pp 25–103
Griffin MJ (1990) Handbook of human vibration. Academic Press, London
Griffin MJ, Bovenzi M (2002) The diagnosis of disorders caused by hand-transmitted vibration: Southampton Workshop 2000. Int Arch Occup Environ Health 75(1–2):1–5
Griffin MJ, Welsh AJL, Bovenzi M (2006) Acute response of finger circulation to force and vibration applied at the palm of the hand. Scand J Work Environ Health 32(5):383–391
International Organization for Standardization (2001) Mechanical vibration—measurement and evaluation of human exposure to hand-transmitted vibration—part 1: general requirements. International Standard, ISO 5349-1
Johansson RS, Vallbo ÅB (1979) Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J Physiol 286:283–300
Löfvenberg J, Johansson RS (1984) Regional differences and inter-individual variability in sensitivity to vibration in the glabrous skin on the human hand. Brain Res 301:65–72
Luo J, Sakakibara H, Zhu S-H, Kondo T, Toyoshima H (2000) Effect of vibration magnitude and repetitive exposure on finger blood flow in healthy subjects. Int Arch Occup Environ Health 73:281–284
Morioka M, Griffin MJ (2005) Thresholds for the perception of hand-transmitted vibration: dependence on contact area and contact location. Somatosens Mot Res 22:281–297
Netea RT, Lenders JW, Smits P, Thien T (2003) Influence of body and arm position on blood pressure readings: an overview. J Hypertens 21:237–241
Terent A, Breig-Asberg E (1994) Epidemiological perspective of body position and arm level in blood pressure measurement. Blood Press 3:156–163
Thompson AJL, Griffin MJ (2009) Effect of the magnitude and frequency of hand-transmitted vibration on finger blood flow during and after exposure to vibration. Int Arch Occup Environ Health 82:1151–1162
Verrillo RT (1962) Investigation of some parameters of the cutaneous threshold for vibration. J Acoust Soc Am 34:1768–1773
Verrillo RT (1963) Effect of contact area on the vibrotactile threshold. J Acoust Soc Am 35:1962–1971
Verrillo RT, Bolanowski SJ, Gescheider GA (2002) Effect of aging on the subjective magnitude of vibration. Somatosens Mot Res 19:238–244
Welsh CL (1980) The effect of vibration on digital blood flow. Br J Surg 67(10):708–710
Whitehouse DJ, Griffin MJ (2002) A comparison of vibrotactile thresholds obtained using different diagnostic equipment: the effect of contact conditions. Int Arch Occup Environ Health 75:85–89
Ye Y, Griffin MJ (2011) Reductions in finger blood flow in men and women induced by 125-Hz vibration: association with vibration perception thresholds. J Appl Physol 111:1606–1613
Ye Y, Griffin MJ (2013) Reductions in finger blood flow induced by 125-Hz vibration: effect of area of contact with vibration. Eur J Appl Physiol 113:1017–1026
Ye Y, Griffin MJ (2014) Relation between vibrotactile perception thresholds and reductions in finger blood flow induced by vibration of the hand at frequencies in the range 8–250 Hz. Eur J Appl Physiol 114(8):1591–1603
Ye Y, Mauro M, Bovenzi M, Griffin MJ (2015) Reduction in finger blood flow induced by hand-transmitted vibration: effect of hand elevation. Int Arch Occup Environ Health. doi:10.1007/s00420-015-1027-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ye, Y., Griffin, M.J. Reductions in finger blood flow induced by 125-Hz vibration: effect of location of contact with vibration. Int Arch Occup Environ Health 89, 425–433 (2016). https://doi.org/10.1007/s00420-015-1081-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-015-1081-7