Abstract
Objectives
To compare the acute response of finger circulation to continuous and intermittent vibration having the same total duration of vibration exposure and the same energy-equivalent acceleration magnitude.
Methods
Finger blood flow (FBF) was measured in the middle and little fingers of both hands of ten healthy men. Finger skin temperature (FST) was measured in the middle right finger. With a static load of 10 N, the middle finger of the right hand was exposed to 125 Hz at 44 m s−2 root mean square (r.m.s.) in five conditions: (1) 30 min continuous exposure, (2) two periods of 15 min, separated by a 15 min period with no vibration, (3) four periods of 7.5 min, separated by 7.5 min periods with no vibration, (4) eight periods of 3.75 min, separated by 3.75 min periods with no vibration, (5) 16 periods of 1.88 min, separated by 1.88 min periods with no vibration. All five exposures correspond to an 8 h energy-equivalent frequency-weighted acceleration magnitude of 1.4 m s−2 r.m.s. according to International Standard ISO 5349–1 (2001). Finger circulation was measured in all four digits before the application of vibration and at fixed intervals during vibration exposure and during a 45 min recovery period.
Results
The FST did not change during vibration exposure, whereas all vibration conditions produced significant reductions in FBF of the vibrated finger when compared with the pre-exposure FBF. During vibration exposure, the vibration caused a similar degree of vasoconstriction in the vibrated finger without evidence of cumulative effects during intermittent exposure. After the end of exposure to 30 min of continuous vibration there was a progressive decrease in the FBF, whereas there was no statistically significant reduction following exposure to intermittent vibration.
Conclusions
For the vibration stimuli investigated (exposure durations varying from 1.88 min to 30 min, with rest periods varying from 1.88 min to 15 min), the reduction of FBF during exposure was the same for continuous and intermittent vibration. The after effect of vibration was greater following the continuous vibration exposure. Although some evidence from this study is consistent with the notion that intermittent vibration has a less severe effect than continuous vibration, this evidence is not yet conclusive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Occupational exposure to hand-transmitted vibration from powered tools or vibrating work pieces may give rise to vascular, neurological and musculoskeletal disorders in the upper limbs [11]. The complex of symptoms and signs of these disorders is called the hand–arm vibration syndrome, which is recognised as an occupational disease in many industrialised countries [5].
The approximate vibration conditions associated with the occurrence of the vascular disorder caused by exposure to hand-transmitted vibration, called vibration-induced white finger, have been reported in some studies. However, although many jobs involve intermittent exposure to vibration, epidemiological studies do not clearly indicate whether intermittency is beneficial or detrimental: it is not known whether the total exposure is the determining factor or whether some allowance should be made for breaks in exposure. Notwithstanding that uncertainty, it is widely suggested that working conditions characterised by continuous exposure to hand-transmitted vibration are more hazardous to worker health than conditions in which vibration exposure is interrupted with regular rest periods (i.e. periods without exposure to vibration).
The assumed importance of exposure intermittency is not new. A 1967 Work Hygiene Regulation in Czechoslovakia defined limits that varied according to the intermittency in exposures [6]. This influenced Draft International Standard 5349 (1979), which contained a procedure to allow for the benefits of intermittency in exposures; for example, the severity of a cumulative 4 h total daily exposure to hand-transmitted vibration varied by a factor of 4 according to the durations of regular interruptions in exposures. According to the current International Standard, ISO 5349-1 [13], daily exposures are evaluated on the basis of the cumulative daily exposure, and there is no allowance for intermittency. However, at item E.3.c in an annex dedicated to preventive measures to be adopted by persons responsible for occupational health and safety, this standard says: “it is presumed that vibration hazards are reduced when continuous vibration exposure over long periods are avoided; therefore, work schedules should be arranged to include vibration-free periods”. In another annex it is suggested that “....working conditions, methods of use of the tool and exposure duration patterns (including intermittency) should be reported”. More recently, the Physical Agents Directive of the European Union states that if the exposure action value is exceeded, the introduction of “work schedules with adequate rest periods” should be considered [15].
The underlying assumption is that vibration-free rest periods could allow the human body tissues to recover from vibration-induced mechanical stress. At present, however, there is little experimental evidence and no epidemiological evidence that intermittency in exposures to hand-transmitted vibration has a beneficial effect, either to prevent the onset of vibration injuries or to reduce the occurrence of vibration-induced disorders [8, 14].
The aim of this study was to compare the response of finger circulation to acute exposures to continuous and intermittent vibration. All exposures had the same frequency of vibration and the same energy-equivalent acceleration magnitude: they would have equal risk according to current standards if they were to occur routinely in an occupation. A vibration frequency of 125 Hz was used because previous experimental studies have shown that this frequency induces greater changes in finger circulation than do some lower or higher frequencies [3, 12, 16]. Finger blood flow was measured simultaneously in four fingers of healthy men (two ipsilateral and two contralateral to vibration exposure), by use of a newly developed multi-channel, computer-controlled, strain-gauge plethysmograph.
Subjects and methods
Subjects
Ten healthy male volunteers gave their written informed consent to participate in the investigation. All subjects were students or office workers with no history of regular use of hand-held vibrating tools in occupational or leisure activities. Eight subjects were non-smokers. None of them reported cardiovascular or neurological disorders, connective tissue diseases, injuries to the upper extremities or a family history of Raynaud’s phenomenon. The mean age of the subjects was 29 (range 23–46; SD 7) years, their mean stature was 181 (range 170–195; SD 7) cm and their mean weight was 77 (range 62–107; SD 13) kg. Finger dimensions were measured with vernier callipers to a precision of 0.5 mm. Finger volume was calculated from that of a cylinder formed from an ellipse based on the dimensions of the proximal interphalangeal joint and the length of the finger. The mean (SD) volume of the middle right finger was 21.2 (3.7) cm3, the middle left finger was 19.7 (3.9) cm3, the little right finger was 11.4 (2.3) cm3 and the little left finger was 10.9 (2.1) cm3.
Measurements of finger circulation
Finger blood flow (FBF) was measured in the middle and little fingers of both the right and the left hand. Mercury-in-silastic strain gauges were placed around the distal phalanx at the base of the nails, and plastic pressure cuffs for air inflation (2.4 cm×9 cm) were fixed around the proximal phalanges and secured with a Velcro strip. The pressure cuffs and strain gauges were connected to a five-channel plethysmograph (HVLab, ISVR, University of Southampton, UK).
The FBF was measured by a venous occlusion technique: the pressure cuffs were inflated to a pressure of 60 mmHg and the increases in finger volumes were detected by means of strain gauges according to the criteria given by Greenfield et al. [9]. Three to five plethysmographic recordings of FBF were made for each digit during each measurement, and the mean values calculated. The FBF measurements were expressed as millilitres per hundred millilitres per minute.
Brachial systolic and diastolic blood pressures were measured in the upper right arm by an auscultatory technique with a standard rubber cuff (12 cm×23 cm).
Finger skin temperature (FST) was measured with a k-type thermocouple connected to an HVLab thermal aesthesiometer so as to measure temperature with an accuracy of ±0.2°C. The thermocouple was taped to the dorsal surface of the medial phalanx of the right middle finger with porous surgical tape.
The room temperature was measured by a mercury-in-glass thermometer.
Experimental procedure
The experiment was performed in a room with a mean (SD) temperature of 26.5 (0.9)°C. Subjects were requested to avoid caffeine consumption for 2 h and tobacco and alcohol for 12 h prior to being tested.
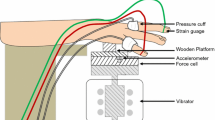
The subjects lay supine throughout the investigation with their hands resting on platforms alongside the body at about the level of the heart. After a period of acclimatisation of approximately 15 min, FBF was measured in the middle and little fingers of both hands; FST was measured in the middle right finger. After the pre-exposure measurements had been obtained, the subjects were asked to apply a downward force of 10 N with their right hands on a horizontal wooden platform that was mounted on an electrodynamic vibrator (VP4, Derritron). The signal from a Tedea Huntleigh force cell mounted between the platform and the shaker was used to provide visual feedback on a meter for the control of downward force. The index, middle and ring fingers of the right hand were in contact with the wooden platform and the little finger was independently supported at the same height. The arrangement for controlling contact force and for generating and monitoring the vibration has been described elsewhere [4].
Sinusoidal vibration was produced in the vertical direction at a frequency of 125 Hz at a root-mean-square (r.m.s.) acceleration magnitude of 44 m s−2 (unweighted), corresponding to a frequency-weighted acceleration of 5.6 m s−2 r.m.s. The total duration of vibration exposure on each occasion was 30 min, divided into the following intermittent vibration exposure periods and rest periods (Table 1):
- (1):
-
One period of 30 min continuous vibration; 45 minutes recovery.
- (2):
-
Two periods of 15 min, separated by a 15 min period with no vibration; 45 min recovery.
- (3):
-
Four periods of 7.5 min, separated by 7.5 min periods with no vibration; 45 min recovery.
- (4):
-
Eight periods of 3.75 min, separated by 3.75 min periods with no vibration; 45 min recovery.
- (5):
-
Sixteen periods of 1.88 min, separated by 1.88 min periods with no vibration; 45 min recovery.
All five exposures corresponded to an 8 h energy-equivalent frequency-weighted acceleration magnitude of 1.4 m s−2 r.m.s. according to International Standard ISO 5349-1 [13].
The measurements of FBF were made in the exposed (i.e. right) middle finger and in the unexposed right little finger, left middle finger and left little finger immediately before vibration exposure, throughout the vibration exposure period, and for 45 min following exposure.
During vibration exposures in conditions (1) to (4), measurements of finger circulation were obtained 0.5 min after the start of vibration and at 2 min intervals during vibration. Similarly, during rest periods, measurements of finger circulation were obtained 0.5 min following the cessation of vibration and at 2 min intervals during the rest period. In condition (5), with 16 periods of 1.88 min of exposure and rest, measurements were obtained at 1 min intervals, following the start of vibration.
During recovery, measurements were obtained 0.5 min after the cessation of vibration and at 2 min intervals from the cessation of vibration until the end of the recovery period.
Brachial blood pressures were measured at the beginning and at the end of each experimental session.
Some previous studies have found that measures of finger circulation (FST and FBF) do not change when a similar static force with a similar posture to that used in this experiment was applied [1, 3]. For this reason, the present study did not include a static condition.
Each of the ten subjects experienced all five experimental conditions on five separate days. Across the subject group, the five experimental conditions were presented in a balanced order. The experimental sessions lasted approximately 2 h. All sessions were completed within a 3 week period.
The study was approved by the Human Experimentation Safety and Ethics Committee of the Institute of Sound and Vibration Research at the University of Southampton (UK).
Statistical methods
Data analysis was performed with the software package Stata (version 7.0 SE). The data were summarised with the mean as a measure of central tendency and the SD or the standard error of mean as measures of dispersion.
The difference between paired means was tested by Student’s t-test.
Repeated measures analysis of variance (ANOVA) was used to test the hypothesis of no difference in the vascular responses in different exposure conditions (‘treatments’). To control for the effect of covariates on the response variables, we used repeated measures analysis of covariance (ANCOVA). When the compound symmetry assumption (that is, the measures have the same variance and the correlations between each pair of repeated measures are equal) was violated, we used a conservative test of the repeated measures factor by reducing the degrees of freedom of the F ratio (Greenhouse-Geisser method) [10]. The 95% Bonferroni confidence intervals for pairwise mean comparisons of the response by time were used when the probability value for the F test of repeated measures ANOVA was P<0.05 (two-sided).
We assessed the relationship between continuous variables with repeated measures by the generalised estimating equations (GEE) approach to repeated measures data sets in order to account for the within-subject correlation [7].
Results
Finger circulation before vibration exposures
The vascular measurements before the subjects’ exposure to vibration showed no changes in either FBF or FST in either the exposed or the unexposed fingers across the five experimental sessions. No differences in the pre-exposure measurements of digital circulation were found between the middle right and middle left finger or between the little right and little left finger within any session. Table 2 reports the baseline measurements of FBF before the volunteers’ exposures to continuous and intermittent vibration.
Brachial systolic and diastolic arterial pressures measured before exposure did not change significantly within subjects across sessions (range of values across subjects and sessions: 115/70 mmHg–130/80 mmHg). No difference was observed for the brachial arterial blood pressures measured at the beginning and the end of the five sessions.
In pre-exposure conditions, analysis of repeated measures by the GEE method showed that in the middle right finger FBF was positively related to FST (P<0.01). For all fingers, the FBF showed a positive, even though not statistically significant, relationship to room temperature (P=0.15–0.27)
Neither age nor the volume of the fingers was correlated with the baseline measurements of digital circulation.
Repeated measures ANOVA revealed no significant difference in the air temperature of the laboratory across the five experimental sessions, ranges of mean (SD) values being 26.6 (0.3)°C–26.8 (0.2)°C.
Finger circulation during exposure to continuous or intermittent vibration
Over the five experimental sessions, the GEE analysis of repeated measures showed no significant changes in the FST of the middle right (exposed) finger during either continuous or intermittent exposures to vibration (results not shown).
Acute exposure to vertical vibration with a frequency of 125 Hz and an unweighted acceleration magnitude of 44 m s−2 r.m.s. provoked an immediate reduction of FBF in the middle right (exposed) finger at the beginning of each exposure period for all experimental conditions (Figs. 1, 2, 3, 4 and 5).
Mean percentage changes in the finger blood flow of ten healthy men during and after exposure to 30 min of continuous vibration with a frequency of 125 Hz, an unweighted r.m.s. acceleration magnitude of 44 m s−2, and an 8 h energy-equivalent frequency-weighted acceleration magnitude of 1.4 m s−2 r.m.s., according to International Standard ISO 5349-1 (2001)
Mean percentage changes in the finger blood flow of ten healthy men during and after exposure to intermittent vibration [two vibration periods of 15 min (V), separated by a 15 min period with no vibration (R)] with a frequency of 125 Hz, an unweighted r.m.s. acceleration magnitude of 44 m s−2, and an 8 h energy-equivalent frequency-weighted acceleration magnitude of 1.4 m s−2 r.m.s., according to International Standard ISO 5349-1 (2001)
Mean percentage changes in the finger blood flow of ten healthy men during and after exposure to intermittent vibration [four vibration periods of 7.5 min (V), separated by 7.5 min periods with no vibration (R)] with a frequency of 125 Hz, an unweighted r.m.s. acceleration magnitude of 44 m s−2, and an 8 h energy-equivalent frequency-weighted acceleration magnitude of 1.4 m s−2 r.m.s., according to International Standard ISO 5349-1 (2001)
Mean percentage changes in the finger blood flow of ten healthy men during and after exposure to intermittent vibration [eight vibration periods of 3.75 min (V), separated by 3.75 min periods with no vibration (R)] with a frequency of 125 Hz, an unweighted r.m.s. acceleration magnitude of 44 m s−2, and an 8 h energy-equivalent frequency-weighted acceleration magnitude of 1.4 m s−2 r.m.s., according to International Standard ISO 5349-1 (2001)
Mean percentage changes in the finger blood flow of ten healthy men during and after exposure to intermittent vibration (16 vibration periods of 1.88 min (V), separated by 1.88 min periods with no vibration (R)] with a frequency of 125 Hz, an unweighted r.m.s. acceleration magnitude of 44 m s−2, and an 8 h energy-equivalent frequency-weighted acceleration magnitude of 1.4 m s−2 r.m.s., according to International Standard ISO 5349-1 (2001)
During the subjects’ 30 min exposure to continuous vibration (Fig. 1), the decrease of FBF in the exposed finger was persistent over the whole exposure period, and the percentage change in FBF at each measurement time was significant when compared with the pre-exposure FBF. After we had controlled for age, finger dimension and room temperature, repeated measures ANCOVA showed that the reduction of FBF in the exposed finger was significantly stronger than that measured in both the unexposed ipsilateral finger and the unexposed contralateral fingers (P<0.001). The percentage change in the FBF of the unexposed right little finger was greater than in the contralateral (unexposed) left fingers (P<0.001). Compared with the measurements before exposure, no significant change was observed for the FBF in the contralateral (unexposed) fingers throughout the 30 min vibration exposure period. There was no difference in the changes of FBF between the two contralateral fingers.
Finger circulation during intermittent vibration (44 m s−2 r.m.s. at 125 Hz) with durations of 15, 7.5, 3.75 and 1.88 min (spaced out by equal vibration-free rest periods), all with the same 8 h energy-equivalent acceleration magnitude of 1.4 m s−2 r.m.s., are shown in Figs. 2, 3, 4 and 5. Overall, the change in the FBF of the middle right (exposed) finger exhibited a similar pattern across all experimental sessions: a rapid decrease of FBF during vibration exposures followed by a prompt restoration of FBF during the vibration-free rest periods. As during the 30 min exposure to continuous vibration, the reduction of FBF in the ipsilateral (right) fingers was significantly stronger than in the contralateral (unexposed left) fingers (P<0.001) and the percentage changes of FBF in the middle right (exposed) finger was greater than in the little right (unexposed) finger (P<0.001). During each period of intermittent vibration exposure, FBF in the ipsilateral fingers decreased significantly when compared with both the pre-exposure FBF and the FBF during the vibration-free rest periods (0.001<P<0.05). Within both right (ipsilateral) fingers, there was no significant difference in the degree of the vasoconstrictor response within each vibration exposure period.
During intermittent exposures, immediately after the end of each vibration stimulus, there was an increase in the FBF of the right (ipsilateral) fingers. This vasodilation was observed more frequently in the middle right (exposed) finger. The FBF in both ipsilateral fingers during the vibration-free rest periods immediately after the end of the vibration stimuli was not significantly different from the pre-exposure values.
In the contralateral (unexposed left) fingers, there was no significant change in the FBF over the four sessions with intermittent vibration exposure when compared with the blood flow measured before exposure, neither during vibration nor during the rest periods.
Finger circulation after subjects’ exposure to continuous or intermittent vibration
During the recovery period following exposures to either continuous or intermittent vibration, repeated measures ANCOVA revealed no significant treatment-by-time interaction for either FST or FBF across the five experimental sessions. As a result, the overall patterns of the changes in FST and FBF could be compared over the whole recovery period across the various exposure conditions.
In the middle right (exposed) finger, FST during recovery did not change significantly across the experimental sessions (results not shown).
Within each finger, the patterns of the percentage change of FBF during recovery were not different across the five exposure conditions (P=0.49–0.97). Likewise, within each exposure condition there was no difference in the change of FBF during recovery across fingers, with the exception of the experimental condition with 30 min exposure to continuous vibration. With continuous vibration, a small, although significant, reduction in the FBF of both ipsilateral (right) fingers was observed when compared with that measured in the contralateral (left) fingers (P<0.05). Repeated measures ANCOVA showed that the difference between the ipsilateral and contralateral fingers was mainly due to a greater decrease in the FBF of the ipsilateral fingers during the second half of the recovery period.
The Bonferroni test revealed that there was a significant decrease in the FBF of the middle right (exposed) finger during the last 15 min of the recovery period after the end of 30 min exposure to continuous vibration, compared with its pre-exposure FBF (P<0.02). No significant differences from the baseline FBF were observed for the other fingers after exposure to any other condition (continuous or intermittent vibration exposure). Nevertheless, the mean values of FBF showed some evidence of vasoconstriction after the subjects’ exposure to vibration, as found in our previous investigations [1, 2].
Discussion
Comparison with previous studies
Our previous studies have consistently found that vibration of a finger produces vasoconstriction during exposure, followed by an immediate vasodilation and then the onset of a period of vasoconstriction lasting 30 min or more during a recovery period. The strength of vasoconstriction during exposure depends on the vibration frequency and the vibration magnitude [2, 3]. The strength and duration of the vasoconstriction following exposure depends on the duration of vibration exposure, in addition to the vibration frequency and vibration magnitude [1]. The previous studies have shown similar, although somewhat reduced, effects on an unexposed contralateral finger.
With the 30 min continuous vibration used in the current study, the patterns of the response of FBF in both the exposed right finger and the unexposed left fingers are similar to those obtained with the same stimulus (44 m s−2 r.m.s. at 125 Hz for 30 min) in a previous study [4]. However, a clear but weak reduction of FBF in the unexposed contralateral finger, seen in the previous study, is not observed in the results of the current study. In the previous studies there were no measurements on an unexposed ipsilateral finger.
In our series of experiments, we have not previously measured FBF response to intermittent vibration. Egan et al. [8] investigated the effects of three 2 min exposures to vibration from a pneumatic chisel, with each period of vibration separated by a 10 min rest without vibration. In exposed and unexposed fingers, vibration reduced the blood flow by an amount that did not appear to change greatly between the three periods of vibration: there was a downward trend in blood flow over the three periods, but a similar trend was observed during a control condition without vibration. Luo et al. [14] measured FBF over three 5 min periods of vibration separated by 5 min rests. They reported a downward trend in the FBF over the three periods of vibration in the unexposed hand but not the exposed hand. The present study found no evidence of a significant change in the degree of vascular response to vibration over the various intermittent exposures in either the exposed or the unexposed hand.
Finger circulation during exposure to continuous or intermittent vibration
In the exposed (right) finger, in all five conditions, there were significant reductions in FBF during vibration. There was no significant change in the reduction of FBF during the 30 min continuous vibration over the whole exposure period. Also, the overall mean change in the percentage FBF during exposure did not differ between conditions or between repeated exposures during the four intermittent conditions. This suggests that neither the accumulation of exposure (i.e. dose) nor periods of rest (varying from 1.88 min to 15 min) affected the FBF response to vibration exposure. This might indicate that, with the magnitude, frequency and durations of vibration studied here, the reduction of FBF was primarily a direct effect, with little cumulative influence. This does not exclude that a cumulative effect, or some compensatory mechanism, might come into play in some circumstances, as may be evident in a previous study where there was evidence of reduced vasoconstriction after approximately 15 min of vibration [1]. Any such changes seen during the present study were not significant, but the present study employed a lower magnitude of vibration (44 m s−2 r.m.s., compared with 87 m s–2 r.m.s. in the previous study).
In the ipsilateral unexposed right finger there was a significant reduction of FBF during vibration over the 30 min continuous exposure and during the four conditions of intermittent vibration. However, in this experiment there were no reductions in either of the contralateral (unexposed left) fingers. The absence of vasoconstriction in fingers on the contralateral hand differs from our previous studies, and this may be due, at least partially, to the lower vibration magnitude used in the present study [1].
Finger circulation after exposure to continuous or intermittent vibration
As in previous studies, there was evidence of a reduction in FBF following vibration exposure. However, this was only significant in the exposed (right) finger after exposure to the 30 min continuous vibration. In the present experiment, the apparently smaller after-effect in the unexposed contralateral fingers is consistent with the absence of significant vasoconstriction during exposure in these fingers. In previous experiments, where there was vasoconstriction in the unexposed fingers during exposure to vibration, there was also evidence of vasoconstriction following exposure [1, 2]. The absence of significant vasoconstriction in the contralateral fingers during or after intermittent vibration could indicate that the effects of intermittent vibration were less severe than those of continuous exposure.
The effects following the end of exposure to vibration in this experiment were fewer with intermittent exposures, but it should not be forgotten that there were also periods without vibration during the exposure period. The responses during these periods (a cumulative total of 15 min) need to be considered when one is assessing the overall impact of the five different cumulative 30 min exposures. During these ‘rest’ periods there was no reduction in the blood flow. So, if the duration and extent of vasoconstriction is an indicator of the cumulative risk of damage caused by an exposure to hand-transmitted vibration, the present results might indicate less risk with intermittent exposures.
Comparison with current standards
Current standards and guides for the evaluation and assessment of exposures to hand-transmitted vibration disregard the influence of periods without vibration and merely accumulate the total exposure duration over a day, regardless of whether it arises from one long exposure or many shorter exposures.
The reduction in FBF observed during the vibration exposure in the present study did not differ between the continuous and intermittent exposures, as implied by the evaluation method in current standards [13]. However, although these standards might be correct in not distinguishing between intermittent and continuous exposures, they are not good at predicting how the response of FBF to vibration exposure depends on the magnitude, frequency or duration of exposures [1, 2, 3].
The current results indicated that there was less after-effect of vibration exposure when the vibration included rest periods, suggesting that breaks in exposure might be beneficial. This is inconsistent with the evaluation methods in current standards. However, the findings of this study seem to be consistent with the general recommendation of current guides suggesting that rest periods may be beneficial.
Conclusion
The reduction of blood flow occurring in a finger exposed to vibration was similar during continuous and intermittent exposures. However, the decrease in FBF was less after subjects’ exposure to intermittent vibration. The results suggest that exposures to intermittent vibration might be less hazardous than exposure to the same vibration without breaks in exposure. Although some evidence from this study is consistent with the notion that intermittent vibration has a less severe effect than continuous vibration, this evidence is not yet conclusive.
The preliminary results obtained from this study require extension to more severe exposures: greater vibration magnitudes and longer exposures, similar to those associated with a known risk of vascular disorders (e.g. vibration-induced white finger) in many occupations.
References
Bovenzi M, Lindsell CJ, Griffin MJ (1998) Duration of acute exposures to vibration and finger circulation. Scand J Work Environ Health 24:130–137
Bovenzi M, Lindsell CJ, Griffin MJ (1999) Magnitude of acute exposures to vibration and finger circulation. Scand J Work Environ Health 25:278–284
Bovenzi M, Lindsell CJ, Griffin MJ (2000) Acute vascular response to the frequency of vibration transmitted to the hand. Occup Environ Med 57:422–430
Bovenzi M, Lindsell CJ, Griffin MJ (2001) Response of finger circulation to energy equivalent combinations of magnitude and duration of vibration. Occup Environ Med 58:185–193
Comité Européen de Normalisation (1996) Mechanical vibration—guide to the health effects of vibration on the human body. CEN Report 12349, Brussels
Czechoslovakian Ministry of Health (1967) Hygiene regulation no 33. Czechoslovakian Ministry of Health, Avicenum, Zdravotnicke Nakladatelstvi, Prague
Diggle PJ, Liang KY, Zeger SL (1994) Analysis of longitudinal data. Oxford Statistical Science Series No 13. Oxford University Press, Oxford
Egan CE, Espie BH, McGrann S, McKenna KM, Allen JA (1996) Acute effects of vibration on peripheral blood flow in healthy subjects. Occup Environ Med 53:663–669
Greenfield ADM, Whitney RJ, Mowbray JF (1963) Methods for the investigation of peripheral blood flow. Br Med Bull 19:101–109
Greenhouse SW, Geisser S (1959) On methods in the analysis of profile data. Psychometrika 24:95–111
Griffin MJ (1990) Handbook of human vibration. Academic Press, London
Hyvärinen J, Pyykkö I, Sundberg S (1973) Vibration frequencies and amplitudes in the etiology of traumatic vasospastic disease. Lancet i:791–794
International Organization for Standardization (2001) Mechanical vibration—measurement and evaluation of human exposure to hand-transmitted vibration. Part 1: General requirements. Geneva, ISO 5349-1
Luo J, Sakakibara H, Zhu S-H, Kondo T, Toyoshima H (2000) Effect of vibration magnitude and repetitive exposure on finger blood flow in healthy subjects. Int Arch Occup Environ Health 73:281–284
The European Parliament and the Council of the European Union (2002) On the minimum health and safety requirements regarding the exposure of workers to the risks arising from physical agents (vibration). Directive 2002/44/EC. Official Journal of the European Communities, 6 July 2002, L177/13-19, Brussels
Welsh CL (1980) The effect of vibration on digital blood flow. Br J Surg 67:708–710
Acknowledgement
This research was supported by the European Commission under the Quality of Life and Management of Living Resources programme—project no. QLK4-2002-02650 (VIBRISKS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bovenzi, M., Welsh, A.J.L. & Griffin, M.J. Acute effects of continuous and intermittent vibration on finger circulation. Int Arch Occup Environ Health 77, 255–263 (2004). https://doi.org/10.1007/s00420-004-0507-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-004-0507-4