Abstract
Purpose
To investigate the relevance of microaneurysm morphology in optical coherence tomography angiography (OCTA) image averaging and fluorescein leakage in diabetic retinopathy (DR).
Methods
In 38 consecutive patients with DR, ten consecutive 3- × 3-mm fovea-centered OCTA (HS100, Canon Inc., Tokyo, Japan) and fluorescein angiography (FA) were performed, and averaged OCTA images were created based on the 10 images. After detecting all microaneurysms in FA images, the morphology was classified into four types (focal bulge, saccular/pedunculated, fusiform, and mixed) using averaged OCTA images. The correlation between microaneurysm leakage in FA, retinopathy stage, and microaneurysm morphology was estimated.
Results
Thirty-eight eyes (50.0%) of the 33 patients were available for analysis, and 370 (63.5%) of the 583 FA-detected microaneurysms were morphologically classifiable (focal bulge, 46; saccular/pedunculated, 143; fusiform, 29; and mixed, 152) in OCTA. There was a significant correlation between stage and percentage of microaneurysm morphology and between morphology and the presence of leakage (P < 0.0001 and P < 0.01, respectively). The proportion of focal bulges decreased with stage progression, while the other three types increased with stage progression. The percentage of FA leakage for focal bulge, saccular/pedunculated, fusiform, and mixed was 41.3%, 66.4%, 82.8%, and 66.4%, respectively, and the fusiform type showed significant FA leakage.
Conclusion
Microaneurysm morphology is correlated with the DR stage and FA leakage. Microaneurysm morphology recognition using OCTA image averaging may be useful for the clinical evaluation of DR.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microaneurysms are not the only important finding in the diagnosis of diabetic retinopathy (DR) [1], but their number is a predictive marker of progression [2]. Furthermore, vascular leakage from microaneurysms can cause exudative changes in diabetic macular edema (DME) [3, 4]. Therefore, the detection and evaluation of microaneurysm are clinically important in the clinical practice of DR. Previous pathological studies have proposed that there is a stage of microaneurysm formation with morphological changes [5, 6]. Recent high-resolution imaging using adaptive optics has revealed that the microaneurysm morphology is consistent with the pathological image and can be classified [7]. Furthermore, the number of microaneurysms has been shown to increase with the progression of disease [8]. However, the relationship between the retinopathy stage and its morphology remains unclear.
Fluorescein angiography (FA) has been the gold standard for microaneurysm detection for many years, but it is associated with a long examination time and possible allergy to contrast fluorescein dye [9]. Optical coherence tomography angiography (OCTA) is noninvasive and can be performed in a shorter time. It is also useful for microaneurysm detection, although its detection rate is inferior to FA [10,11,12,13]. Uji et al. introduced the utility of image averaging for OCTA image quality due to lower background noise and greater continuity of retinal microvasculature compared with a single unaveraged image [14]. Recently, we reported that image averaging of OCTA images could improve the detection rate of microaneurysms and recognize their morphology [15]. Schreur et al. reported that the morphological appearance in OCTA is correlated with FA leakage and retinal thickness [16]. However, the microaneurysm detection rate was 58% because a single OCTA image was used for evaluation. Spaide et al. [17] previously reported that repeated OCTA scans show different images of microaneurysms in eyes with DR. Furthermore, as we have also shown that the focal bulge type is difficult to detect with a single OCTA imaging [15], certain morphological types of microaneurysms tend to be undetectable, and the possibility of bias in the evaluated microaneurysm forms cannot be denied. In this study, we reevaluated the association of microaneurysm morphology with FA leakage and the distribution of morphology by DR stage using OCTA image averaging.
Materials and methods
This study was approved by the Institutional Ethics Committee of Kyushu University Hospital (Protocol No. 28473, UMIN000028656) and was performed following the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients after providing a detailed explanation of the study.
Patient population
This retrospective study included 76 eyes from 38 consecutive patients with DR who visited Kyushu University Hospital between April 2018 and December 2019. We excluded eyes with any other ocular disease that could cause microvascular disturbances in the retina or choroid (e.g., retinal vascular occlusion, age-related macular degeneration, and glaucoma).
Ophthalmic examination
All patients underwent FA using Spectralis HRA-OCT (Heidelberg Engineering, Heidelberg, Germany) with simultaneous injection of these contrast agent dyes. The FA images for this study were selected from the early phase (within 2 min of dye injection) and late phase (> 7 min after dye injection). We defined microaneurysms as hyperfluorescent dots in the early-phase FA imaging.
OCTA
In this study, OCTA imaging was performed on all patients using a commercial device (OCT-HS100; Canon Inc., Tokyo, Japan). The scanning area was a 3- × 3-mm fovea-centered region. We obtained en face OCTA images of the superficial capillary plexus (SCP) and deep capillary plexus (DCP) from each device, and low-quality images with signal strength less than 5 or images with artifacts were excluded from the study. The OCT-HS100 device had an A-scan rate of 70,000 scans/s and a wavelength of 855 nm. The vertical and axial resolutions were 20 and 3 mm, respectively. The segmentation line for the OCT-HS100 device was defined as follows: For SCP, the inner and outer boundaries were set at the inner limiting membrane and 50 mm below the inner plexiform layer (IPL), respectively. For the DCP, the inner and outer boundaries were set 50 mm below the IPL and at the outer plexiform layer, respectively. OCTA imaging with the OCT-HS100 device was performed over 10 consecutive times for each eye, and we obtained 10 high-quality single images that satisfied the aforementioned acceptance criteria (signal strength ≥ 5). Furthermore, a multiple en face image averaging process was performed on these high-quality single OCTA images using image averaging software installed on the OCT HS-100 device.
Image evaluation and analysis
First, all microaneurysms were detected using FA images of the early phase, and then, microaneurysm-like points in the same location were detected using OCTA in the same patient. We classified microaneurysm-like points into four types (focal bulge, saccular/pedunculated, fusiform, or mixed type) in the averaged two OCTA images (SCP and DCP) based on the report by Dubow et al. [7] (Fig. 1). The morphology of the microaneurysms in OCTA and the leakage of each microaneurysm in the late-phase FA imaging were assessed by two independent retina specialists (YF and SS) using the averaged OCTA images and early phase FA images, respectively. Microaneurysm leakage was defined as the presence of one or more microaneurysms within a surrounding area of leakage, with the fluorescein signal intensity decreasing with increasing distance from the microaneurysm based on a previous report [18]. Leakage from dilated capillaries, veins, and arteries was distinguished from leakage from microaneurysms because it is typically more diffuse than that from microaneurysms and attenuates gradually with increasing distance from the source [18]. When the evaluations of the two observers differed, a third observer (YK) evaluated the morphology of OCTA and leakage in the FA. The k coefficient was 0.89 (95% confidence interval [CI], 0.85 − 0.93; P < 0.0001) and 0.93 (95% CI, 0.89 − 0.97; P < 0.0001) for the evaluation of the morphology in OCTA and the leakage in FA, respectively.
Morphological classification of microaneurysms in representative optical coherence tomography angiography (OCTA) images (65-year-old male with moderate nonproliferative diabetic retinopathy in the left eye). Fluorescein angiography (FA) (a), superficial capillary plexus (b), and deep capillary plexus of OCTA (c) showing multiple microaneurysms in the macula (3 × 3 mm area). d–f Higher magnification of the OCTA images in b and c. Blue and yellow arrowheads indicate focal bulge-type microaneurysms. Blue, pink, and yellow arrows indicate fusiform-, mixed-, and saccular/pedunculated-type microaneurysms, respectively
Statistical analysis
All statistical analyses were performed using a commercial software package (JMP Pro software version 12.0; SAS, Inc., Cary, NC, USA). Descriptive statistics, including mean, standard deviation, median, range, and percentages, were used where appropriate. The correlations between any two of the following variables were analyzed using Fisher’s exact test. All associations were considered statistically significant at P < 0.05.
Results
Patients
Of the 76 eyes with DR in this study, 38 (50.0%) were excluded due to low image quality due to cataracts, vitreous hemorrhage, poor fixation, or image artifacts. A total of 38 eyes (50.0%) from 33 patients could be analyzed (mean age, 60.9 ± 12.2 years; 25 males and eight females; two eyes with mild nonproliferative DR (NPDR), 16 eyes with moderate NPDR, nine eyes with severe NPDR, 11 eyes with proliferative DR, 13 eyes with DME). Seventeen eyes (44.7%) from 15 patients had a history of anti-VEGF therapy.
Microaneurysms
Of the 583 FA-detected microaneurysms, 370 (63.5%) were morphologically classifiable (focal bulge, 46 (12.4%); saccular/pedunculated, 143 (38.6%); fusiform, 29 (7.8%); and mixed, 152 (41.1%)). There was a significant relationship between stage and percentage of microaneurysm morphology and between morphology and presence of fluorescein leakage (P < 0.0001, P < 0.01, respectively; Fig. 2; Tables 1 and 2). The proportion of focal bulges decreased significantly with stage progression, while the other three types increased with stage progression (Table 1). The percentages of FA leakage for focal bulge, saccular/pedunculated, fusiform, and mixed was 41.3%, 66.4%, 82.8%, and 66.4%, respectively, and the fusiform type showed significant FA leakage (Table 2; P < 0.001).
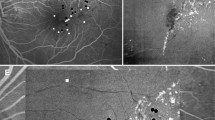
Representative images of optical coherence tomography angiography (OCTA) and fluorescein angiography (FA) images in stages of diabetic retinopathy. Upper: 54-year-old male with mild nonproliferative diabetic retinopathy (NPDR) in the left eye. Middle: 69-year-old male with severe NPDR in the left eye. Lower: 40-year-old male with proliferative diabetic retinopathy (PDR) in the right eye. Blue, yellow, pink, and white dot circles indicate focal bulge type, saccular/pedunculated, fusiform, and mixed types of microaneurysms in OCTA images, respectively. FA images show the late stages. SCP superficial capillary plexus, DCP deep capillary plexus
Discussion
Schreur et al. [16] reported that the morphological appearance of microaneurysms on OCTA is correlated with FA leakage. However, we must be aware that OCTA depicts microaneurysms differently for each acquisition, as observed by Spaide et al. [17]. These facts motivated us to conduct this study using multiple OCTA image averaging. In this study, we reevaluated the microaneurysm morphology in DR stage and the correlation between FA leakage and morphology using 10 × OCTA image averaging. In the early stages of DR, the focal bulge type was observed more frequently, and in the advanced stages, the other forms were observed more frequently. FA leakage tended to be less in the focal bulge type but more in other forms, especially the fusiform type. These results confirmed that focusing on the MA morphology of OCTA images without FA may be useful in understanding the activity of DR vascular lesions, consistent with a previous report by Schreur et al. [16].
Microaneurysms have been used as biomarkers in DR. The presence of microaneurysms has been widely accepted as the most useful finding in the diagnosis of DR on international disease severity scales [18]. This number has also been reported to be useful in predicting the progression of DR [2, 8]. However, the clinical significance of the morphology has not been elucidated because it has been difficult to accurately recognize the morphology due to fluorescein leakage in FA. Our results, along with the results of a previous study by Schreur et al. [16], have shown that the discrimination of morphology has clinical significance in the practice of DR.
In the present study, 63.5% of microaneurysms were recognizable by average OCTA, while 58% of microaneurysms detected by FA were recognizable by OCTA, as also reported by Schreur et al. [16]. Although the focal bulge type may be undetectable on a single OCTA image [15], both studies showed that the percentage of focal bulge type with leakage was low. In contrast, Schreur et al. reported that the irregular type had significantly more leakage, and our results showed that the fusiform type had more leakage [16]. This difference may be due to the presence or absence of an image averaging process.
In this study, we examined the percentage of microaneurysm morphology at each stage of DR. Interestingly, the focal bulge type was frequently observed in the early stages of DR and was not observed in PDR cases. Although Cogan et al. [6] considered the focal bulge type to be a preliminary microvascular change of microaneurysms based on histological observations, recent adaptive optics imaging revealed that the focal bulge type is one of the types recognized as microaneurysms in FA [7]. However, as Cogan et al. [6] predicted in autopsy eyes, the focal bulge may be an early form of microaneurysm that changes to other forms. Future longitudinal studies are required to verify this hypothesis. From a biological perspective, these differences in the morphology might be related to the stability of the vascular wall. It is known that VEGF and angiopoietin 2 increase with the progression of diabetic retinopathy [19]. These may act on pericytes and vascular endothelial cells to cause loss of pericytes and instability of the vessel wall, which may contribute to the formation of capillary aneurysms [20, 21].
We previously reported that turbulence in microaneurysms is associated with OCTA imaging [22]. Our previous observations showed that the focal bulge type was difficult to recognize on a single OCTA scan [15]. These results suggest that other types of microaneurysms are more likely to cause turbulence than focal bulges. This study showed that other types showed more leakage than the focal bulge type. Since several in vitro studies have reported that shear stress is involved in the blood-retinal barrier [23, 24], intramicroaneurysmal hemodynamics may be involved in the impairment of the blood-retinal barrier in DR.
Leakage microaneurysms are an indication of focal photocoagulation; therefore, FA is necessary for their detection. From the results of this study, morphological recognition using multiple OCTA image averaging may be useful for focal photocoagulation, since types other than the focal bulge type, especially fusiform, have more leakage. Uji et al. reported that averaging multiple OCTA images reduces background noise, which may help MA recognition [14]. However, the imaging time is longer than usual and needs to be improved by future technologies.
This study had several limitations. First, the sample size is small. Second, the examined field of view was small in the fovea, although microaneurysms in the DR can be observed anywhere in the retina. Third, patients with previous treatment for DME (e.g., anti-vascular endothelial growth factor therapy despite no recent treatment) were included, although these therapies might affect the morphology [25].
Data availability
Yosuke Fukuda, Shintaro Nakao, and Yoshihiro Kaizu had full access to all study data and took responsibility for the integrity of the data and the accuracy of the data analysis.
References
Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT, Global diabetic retinopathy project G, (2003) Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 110:1677–1682. https://doi.org/10.1016/S0161-6420(03)00475-5
Klein R, Meuer SM, Moss SE, Klein BE (1995) Retinal microaneurysm counts and 10-year progression of diabetic retinopathy. Arch Ophthalmol 113:1386–1391. https://doi.org/10.1001/archopht.1995.01100110046024
Hirano T, Toriyama Y, Iesato Y, Imai A, Hirabayashi K, Nagaoka T, Takamura Y, Sugimoto M, Murata T (2017) Effect of leaking perifoveal microaneurysms on resolution of diabetic macular edema treated by combination therapy using anti-vascular endothelial growth factor and short pulse focal/grid laser photocoagulation. Jpn J Ophthalmol 61:51–60. https://doi.org/10.1007/s10384-016-0483-8
Murakami T, Nishijima K, Sakamoto A, Ota M, Horii T, Yoshimura N (2011) Foveal cystoid spaces are associated with enlarged foveal avascular zone and microaneurysms in diabetic macular edema. Ophthalmology 118:359–367. https://doi.org/10.1016/j.ophtha.2010.03.035
Stitt AW, Gardiner TA, Archer DB (1995) Histological and ultrastructural investigation of retinal microaneurysm development in diabetic patients. Br J Ophthalmol 79:362–367. https://doi.org/10.1136/bjo.79.4.362
Cogan DG, Toussaint D, Kuwabara T (1961) Retinal vascular patterns. IV Diabetic retinopathy Arch Ophthalmol 66:366–378
Dubow M, Pinhas A, Shah N, Cooper RF, Gan A, Gentile RC, Hendrix V, Sulai YN, Carroll J, Chui TYP, Walsh JB, Weitz R, Dubra A, Rosen RB (2014) Classification of human retinal microaneurysms using adaptive optics scanning light ophthalmoscope fluorescein angiography. Invest Ophthalmol Vis Sci 55:1299–1309
Klein R, Meuer SM, Moss SE, Klein BE (1989) The relationship of retinal microaneurysm counts to the 4-year progression of diabetic retinopathy. Arch Ophthalmol 107:1780–1785. https://doi.org/10.1001/archopht.1989.01070020862028
Yannuzzi LA, Rohrer KT, Tindel LJ, Sobel RS, Costanza MA, Shields W, Zang E (1986) Fluorescein angiography complication survey. Ophthalmology 93:611–617. https://doi.org/10.1016/s0161-6420(86)33697-2
Ishibazawa A, Nagaoka T, Takahashi A, Omae T, Tani T, Sogawa K, Yokota H, Yoshida A (2015) Optical coherence tomography angiography in diabetic retinopathy: a prospective pilot study. Am J Ophthalmol 160(35–44):e31. https://doi.org/10.1016/j.ajo.2015.04.021
Hasegawa N, Nozaki M, Takase N, Yoshida M, Ogura Y (2016) New insights into microaneurysms in the deep capillary plexus detected by optical coherence tomography angiography in diabetic macular edema. Invest Ophthalmol Vis Sci 57:OCT348-355
Miwa Y, Murakami T, Suzuma K, Uji A, Yoshitake S, Fujimoto M, Yoshitake T, Tamura Y, Yoshimura N (2016) Relationship between functional and structural changes in diabetic vessels in optical coherence tomography angiography. Sci Rep 6:29064
Hamada M, Ohkoshi K, Inagaki K, Ebihara N, Murakami A (2018) Visualization of microaneurysms using optical coherence tomography angiography: comparison of OCTA en face, OCT B-scan, OCT en face, FA, and IA images. Jpn J Ophthalmol 62:168–175. https://doi.org/10.1007/s10384-018-0570-0
Uji A, Balasubramanian S, Lei J, Baghdasaryan E, Al-Sheikh M, Sadda SR (2017) Impact of multiple en face image averaging on quantitative assessment from optical coherence tomography angiography images. Ophthalmology 124:944–952
Kaizu Y, Nakao S, Wada I, Arima M, Yamaguchi M, Ishikawa K, Akiyama M, Kishimoto J, Hisatomi T, Sonoda KH (2020) Microaneurysm imaging using multiple en face OCT angiography image averaging: morphology and visualization. Ophthalmol Retina 4:175–186. https://doi.org/10.1016/j.oret.2019.09.010
Schreur V, Domanian A, Liefers B, Venhuizen FG, Klevering BJ, Hoyng CB, de Jong EK, Theelen T (2018) Morphological and topographical appearance of microaneurysms on optical coherence tomography angiography. Br J Ophthalmol. https://doi.org/10.1136/bjophthalmol-2018-312258
Spaide RF, Fujimoto JG, Waheed NK (2015) Image artifacts in optical coherence tomography angiography. Retina 35:2163–2180. https://doi.org/10.1097/IAE.0000000000000765
Classification of diabetic retinopathy from fluorescein angiograms. ETDRS report number 11 (1991) Early treatment diabetic retinopathy study research group. Ophthalmology 98:807–822
Watanabe D, Suzuma K, Suzuma I, Ohashi H, Ojima T, Kurimoto M, Murakami T, Kimura T, Takagi H (2005) Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol 139:476–481. https://doi.org/10.1016/j.ajo.2004.10.004
Hammes HP, Lin J, Wagner P, Feng Y, Vom Hagen F, Krzizok T, Renner O, Breier G, Brownlee M, Deutsch U (2004) Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes 53:1104–1110. https://doi.org/10.2337/diabetes.53.4.1104
Arima M, Nakao S, Yamaguchi M, Feng H, Fujii Y, Shibata K, Wada I, Kaizu Y, Ahmadieh H, Ishibashi T, Stitt AW, Sonoda KH (2020) Claudin-5 redistribution induced by inflammation leads to anti-VEGF-resistant diabetic macular edema. Diabetes 69:981–999. https://doi.org/10.2337/db19-1121
Nakao S, Yoshida S, Kaizu Y, Yamguchi M, Wada I, Ishibashi T, Sonoda K-H (2018) Microaneurysm detection in diabetic retinopathy using OCT angiography may depend on intramicroaneurysmal turbulence. Ophthalmology Retina 2:1171–1173
Ishibazawa A, Nagaoka T, Yokota H, Ono S, Yoshida A (2013) Low shear stress up-regulation of proinflammatory gene expression in human retinal microvascular endothelial cells. Exp Eye Res 116:308–311. https://doi.org/10.1016/j.exer.2013.10.001
Molins B, Mora A, Romero-Vazquez S, Pascual-Mendez A, Rovira S, Figueras-Roca M, Balcells M, Adan A, Martorell J (2019) Shear stress modulates inner blood retinal barrier phenotype. Exp Eye Res 187:107751. https://doi.org/10.1016/j.exer.2019.107751
Chen JT, Chen LJ, Chen SN, Chen WL, Cheng CK, Hsu SM, Sheu SJ, Wu WC, Yang CH, Yang CM, Yeung L, Hwang DK, Chen SJ (2020) Management of diabetic macular edema: experts’ consensus in Taiwan. Jpn J Ophthalmol 64:235–242. https://doi.org/10.1007/s10384-020-00741-4
Funding
This study was funded by grants from JSPS KAKENHI, Grant-in-Aid for Scientific Research (C) No. 20K09829 (SN), Alcon Pharma Research Grants (SN), and the Foundation for the Advancement of Clinical Medicine, Fukuoka, Japan (SN).
Author information
Authors and Affiliations
Contributions
Conception and design of the study: YF and SN. Data analysis and interpretation: YF, SN, YK, MA, and SS. Writing of the article: YF and SN. Critical revision of the article: YK, MA, SS, IW, MY, and SK-H. Final approval of the article: YF, SN, YK, MA, SS, IW, MY, and SK-H. Data collection: YF, SN, YK, MA, SS, IW, and MY. Overall responsibility: SN.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the (place name of institution and/or national research committee) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Patient consent
Written informed consent was obtained from all patients after a detailed explanation of the study.
Conflict of interest
Shintaro Nakao has received consulting fee from Kowa and travel reimbursements and speaker fees from Novartis, Bayer Pharma, Canon Inc., Santen Pharmaceutical, Kowa, Senju Pharmaceutical, Ono Pharmaceutical, and MSD in the subject matter or materials discussed in this manuscript. The other authors have no financial disclosures: Yosuke Fukuda, Yoshihiro Kaizu, Mitsuru Arima, Sakurako Shimokawa, Iori Wada, Muneo Yamaguchi, and Atsunobu Takeda. All authors attest that they met the current ICMJE criteria for authorship.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meeting Presentation: the 36th Annual Meeting of the Japanese Society for Ocular Circulation, 2019.
Rights and permissions
About this article
Cite this article
Fukuda, Y., Nakao, S., Kaizu, Y. et al. Morphology and fluorescein leakage in diabetic retinal microaneurysms: a study using multiple en face OCT angiography image averaging. Graefes Arch Clin Exp Ophthalmol 260, 3517–3523 (2022). https://doi.org/10.1007/s00417-022-05713-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05713-7