Abstract
Purpose
The effect of combination therapy using intravitreal ranibizumab (IVR) injections and short pulse focal/grid laser photocoagulation was evaluated for the treatment of diabetic macular edema (DME).
Methods
The current investigation was a preliminary single-arm, open-label, prospective clinical study conducted on 21 eyes at 4 sites in Japan. Treatment protocol consisted of two phases. The induction IVR phase included two monthly IVRs followed by PRN IVR phase in which additional IVR was administered if the central macular thickness (CMT) exceeded 300 μm. One week after each IVR in both phases, short pulse focal/grid laser was delivered to treat residual leakage outside of the fovea (>500 μm) and reduce edema fluid influx. At the 6-month endpoint, the effects of treatment were examined in terms of best corrected visual acuity (BCVA), CMT, and required number of IVR injections in eyes with or without perifoveal leaking microaneurysms (MAs).
Results
In eyes with initial BCVA ≤70 letters, mean BCVA was significantly ameliorated by 7.0 ± 7.4 letters (P = 0.0324) and mean CMT improved significantly by 174.8 ± 105.0 µm (P = 0.0005). Both BCVA improvement (P = 0.8693) and CMT reduction (P = 0.9336) were comparable between MA(−) and MA(+) groups. The MA(−) group required significantly fewer PRN-IVR injections than did the MA(+) group over the 6-month study period (mean 3.4 ± 1.6 vs. 5.3 ± 0.9, median 3.0 vs. 5.5; P = 0.0229).
Conclusions
Short pulse focal/grid laser photocoagulation could reduce the number of IVR injections required to resolve macular edema and increase BCVA in a possible mechanism of reduced influx of edema fluid into the foveal area in eyes without apparent perifoveal microaneurysms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR) is the leading cause of vision loss among working aged individuals in industrialized countries, wherein diabetic macular edema (DME) is the main culprit in so-called legal blindness defined as a best corrected visual acuity (BCVA) of 0.1 or less in the better seeing eye [1, 2]. The number of patients affected with DR and DME is expected to increase drastically since the worldwide prevalence of diabetes mellitus will rise to 5.4 % in 2025 from 4.0 % in 1995 [3], with the number of patients projected to reach 430 million by 2030 [4].
Based on reports from the Early Treatment Diabetic Retinopathy Study (ETDRS) research group, the standard of care for DME was focal/grid laser photocoagulation until the advent of anti-vascular endothelial growth factor (VEGF) agents. Grid laser is defined as laser delivered to areas of thickened retina (macular edema) showing capillary dropout in early phase fluorescein angiography (FA) and/or diffuse leakage in late phase FA. Any focal leaks within thickened retina were treated by focal laser, which was defined as direct laser delivered to discrete points of retinal hyperfluorescence or focal leakage (most of which are microaneurysms). Thus, focal/grid laser means both focal and grid laser, but not either focal or grid laser. However, only 3 % of patients improved by 15 letters or more [5]. Therefore, anti-VEGF monotherapy has become the first-line treatment for DME due to its rapid and prominent effects on vision improvement in numerous multicenter trials [4–18]. Intravitreal injection of anti-VEGF agents is effective in suppressing leakage both in the fovea and elsewhere in the retina. Focal/grid laser treatment cannot be applied directly to areas of foveal leakage, but has been found to resolve fovea-threatening clinically significant diabetic macular edema [5, 11, 12, 19].
In the REVEAL study that investigated the effects of intravitreal ranibizumab (IVR) in 396 Asian DME patients including 103 Japanese patients, IVR monotherapy showed comparable results to IVR and focal/grid laser combination treatment. The percentage of patients in the combination therapy arm who had gained ≥15 ETDRS letters at 12 months was 17.8 % and it was 18.8 % of patients in IVR monotherapy arm, but with photocoagulation it was limited to 7.8 %. Consequently, it appears that IVR played the primary role in BCVA improvement during combination therapy. IVR monotherapy and combination therapy provided very similar vision gains in most other multicenter studies [4–18]. These results are reasonable since vision improvement depends on the resolution of foveal edema consistently achieved by intravitreal injections of anti-VEGF agents. In contrast, focal/grid laser photocoagulation can be applied only to leakage areas outside the fovea (>500 µm), which may indirectly contribute to foveal edema resolution by reducing edema fluid influx to the fovea. This may be why vision gains following focal/grid laser treatment have been generally modest while anti-VEGF agents provided prompt and significant improvement.
In planning the current study, we hypothesized two potential reasons why focal/grid laser photocoagulation did not produce any additive effects on BCVA or resolution of macular edema fluid despite the reduction of focal leakage by focal burns and local VEGF overproduction by ablation of capillary dropout by grid laser [5]. One is that focal/grid laser treatment induces inflammation and a subsequent increase in macular edema and the other is a lack of panretinal photocoagulation (PRP) required to reduce overall VEGF production in the retina. We, therefore, adopted two key modifications in our treatment protocol. First, to minimize laser-induced inflammation, we employed a modern short pulse (0.02–0.03 s.) focal/grid laser instead of the conventional ETDRS focal/grid laser (0.1 s.). This method markedly reduces total energy in photocoagulation [19–21] which decreases the inflammation leading to foveal edema as measured by central subfield macular thickness (CMT). In the REVEAL study [13], combination therapy with conventional focal/grid laser demonstrated a reduction in CMT thickness less than IVR monotherapy throughout the study period. Another problem that has limited the use of macular focal/grid laser photocoagulation is potential “atrophic laser creep” [22], which may damage visual acuity in the long term. Recently introduced pattern scan lasers that employ short pulse (0.02–0.03 s.), high power burns that do not enlarge and cause creeping over time help solve this issue [23]. The effect and safety of this method have been evaluated in eyes with DR [19, 21]. PRP reaching the ora serrata was also performed to mend hypoxia in all areas of retinal capillary nonperfusion since VEGF overproduction in these regions has been implicated in macular edema pathogenesis [24–26]. We completed PRP by one month after the second IVR of the induction phase to avoid PRP-induced exacerbation of macular edema. In earlier clinical trials on DME, the precise status of PRP was not described in the data analysis. This study also included sub-analyses on improvements in vision and CMT, as well as on the reduction of IVR injections, depending on the presence or absence of leaking microaneurysms (MAs) in the perifoveal capillary network (PCN) within 500 µm of the fovea. We recently reported that leaking MAs in branch retinal vein occlusion represented markers of capillary damage and remodeling in the PCN, which could result in foveal leakage, and that patients with these manifestations required significantly more IVR injections to sustain foveal edema resolution (i.e., CMT < 300 µm) [27]. Thus, in DME, capillary damage in the PCN could also be a strong determining factor of IVR injections necessary to sustain a suitable CMT. A CMT of 300 µm has been regarded as the cutoff thickness to diagnose fovea-involving DME in previous trials on DME using spectral-domain optical coherence tomography (SD-OCT) [6, 15–17, 28]. Accordingly, we compared BCVA, CMT, and the required number of IVR injections administered in a pro re nata (PRN) regimen to maintain CMT <300 µm in the treatment of fovea-involving DME between eyes with and without leaking MAs in the PCN.
Materials and methods
Study objectives
This study evaluated the effect of combination therapy using IVR and focal/grid laser photocoagulation on MAs and foveal leakage in the PCN during the treatment of foveal edemas. The primary outcome measure was improvement in BCVA from baseline values. Secondary outcome measures included reduction in CMT from baseline and total number of IVR injections needed to sustain CMT <300 µm.
Study design
The current investigation was a preliminary single-arm, open-label, prospective clinical study conducted at four sites in Japan following approval by the institutional review board of Shinshu University, Matsumoto, Japan (Registration No. 3060). The study was performed in accordance with the ethical tenets outlined in the Declaration of Helsinki as well as the current Good Clinical Practice Guidelines in Japan (J-GCP). Written informed consent was obtained from all participants. This investigation is registered at the University Hospital Medical Information Network under the identifier UMIN00012549 and at ClinicalTrials.gov under the identifier NCT02131350.
Patient eligibility and exclusion criteria
Eligible participants were at least 20 years of age, afflicted with type 2 diabetes mellitus diagnosed according to the guidelines of the Japanese Diabetes Association (JDA), and able to visit the study sites as scheduled. The inclusion criteria were: (1) visual impairment due to DME in at least 1 eye; (2) study eye BCVA letter score ≥24 letters based on ETDRS VA testing charts that approximate Snellen equivalent ≥20/320; (3) fovea-involving macular edema defined as CMT ≥300 µm measured as mean retinal thickness in the central 1 mm diameter circle by SD-OCT (Cirrus® OCT; Carl Zeiss Meditec, Inc., Dublin, California); and (4) no history or presence of other ocular diseases causing vision deterioration, such as age-related macular degeneration and severe proliferative DR. The exclusion criteria were: (1) any retinal photocoagulation treatment in the study eye within 3 months preceding the initial IVR; (2) treatment by injection of any anti-VEGF agent into either eye within 2 months preceding the initial IVR; (3) history of vitreous surgery; (4) glaucoma or intraocular pressure (IOP) >24 mmHg; (5) history of cataract surgery in the study eye within the previous 6 months; (6) opaque optic media through which high quality fundus photographs or OCT images could not be obtained; (7) history of cerebrovascular accident, myocardial infarction, or other systemic disease requiring medications that could affect the results of the study; (8) poorly controlled diabetes mellitus (i.e., HbA1c >12.0 %); (9) renal failure requiring hemodialysis; and (10) poorly controlled hypertension [i.e., systolic blood pressure (BP) ≥160 mmHg or diastolic BP ≥95 mmHg]. Patients who were judged as ineligible for any other reason by the investigators were excluded.
Treatment regimen
To detect leaking MAs and capillaries in the PCN and capillary nonperfusion in the macula, all patients underwent baseline fluorescein angiography (FA) examination using confocal scanning laser ophthalmoscopy (HRA-2; Heidelberg Engineering, Inc., Dossenheim, Germany) before IVR and focal/grid laser photocoagulation. Patients received 2 initial consecutive monthly injections of ranibizumab (Lucentis, Genentech, Inc., South San Francisco, CA, USA; 0.5 mg in 0.05 ml) in the treatment induction phase (Fig. 1). Thereafter, participants were evaluated each month for additional IVR injections in a PRN regimen when CMT was ≥300 µm and the physician judged that edema fluid accumulation was causative of vision deterioration. Based on FA findings, short pulse focal/grid laser photocoagulation was performed 1 week after the initial IVR injection using an MC-500 Vixi® multicolor pattern scan laser (NIDEK, Inc., Gamagori, Japan) following ETDRS coagulation guidelines with minor modifications for short pulse coagulation conditions [5]. Focal burns were delivered to leaking MAs at the settings of: (1) spot size of 50 µm; (2) duration of 0.02–0.03 s.; and (3) power ranging 100–250 mW to achieve mild whitening of MAs. Grid laser photocoagulation was delivered to thickened retinal areas with capillary nonperfusion or diffuse leakage within the vascular arcades at the settings of: (1) spot size of 50 µm; (2) duration of 0.03 s.; and (3) power ranging from 100 to 250 mW to achieve vaguely visible laser burns. Additional short pulse focal/grid laser treatment was administered at monthly visits when a recurrence of macular edema was detected by OCT macular map images. If CMT exceeded 300 µm, laser was performed one week after IVR to obtain adequate burns with minimal laser power. Even when CMT was less than 300 µm, retinal thickening greater than 400 µm outside of the fovea was treated with short pulse focal/grid laser without additional IVR. We considered such recurrence suggesting existence of residual capillary nonperfusion or persistent MAs within the vascular arcades. When an FA examination revealed capillary nonperfusion in the mid to peripheral retina, a new or additional session of panretinal photocoagulation was performed to reduce VEGF overproduction by mending retinal hypoxia at the settings of 200 µm in size and 0.02–0.03 s. of duration to achieve burns with mild retinal whitening within the treatment induction phase to avoid PRP-associated worsening of macular edema. Although most examinations used the above-described SD-OCT, confocal scanning laser ophthalmoscopy, and laser devices, comparable machines were used at some sites.
Flowchart of patient population. Dagger Short pulse focal/grid laser photocoagulation was performed 1 week after the first intravitreal ranibizumab (IVR) injection. Within the first 2 months, panretinal photocoagulation was carried out if required based on fluorescein angiography results. Double dagger Subsequent IVR injections were performed according to a pro re nata (PRN) regimen when central subfield macular thickness was ≥300 µm and the physician judged that edema fluid accumulation was causative of vision deterioration. Additional focal/grid laser treatment was given if needed, with a minimum 1-month interval following the previous session
Patient demographics
We assessed at baseline the parameters of patient age, sex, HbA1c, hemoglobin, diastolic/systolic BP, creatinine, previous PRP, and DR severity. At baseline and monthly follow-ups, all patients underwent complete ophthalmic examinations that included BCVA with an ETDRS vision chart, IOP, slit-lamp biomicroscopy, indirect ophthalmoscopy, and fundus photography.
Evaluation of foveal leakage using FA
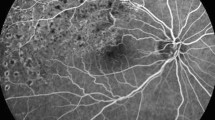
The patients were divided into MA(−) and MA(+) groups depending on the absence or presence, respectively, of leaking MAs in the PCN. The eyes in the MA(−) group did not display leaking MAs in early phase FA (Fig. 2a). Foveal hypofluorescence was vague, but maintained in late phase FA, at which time it was surrounded by the hyperfluorescence of fluid derived from leaks outside of the fovea (Fig. 2b). These FA findings indicated mild damage in the PCN. Reflecting such leakage patterns, OCT macular map images disclosed temporally located focal DME involving the fovea at its nasal edge. In OCT cross-sectional images, the foveal retina was relatively thin as compared with the markedly thickened temporal half of the retina (Fig. 2c). Foveal thickening of CMT ≥300 µm was mainly due to serous retinal detachment and not retinal swelling itself.
Fluorescein angiography (FA) and optical coherence tomography (OCT) images of eyes with foveal involvement of macular edema without microaneurysms (MAs) (a–c) and with MAs (d–f) in the perifoveal capillary network (PCN). a Early phase FA of an eye without MA in the PCN. Many MAs are present, but not in the PCN. b Late phase FA of the same eye demonstrated in Fig. 1a. Vague hypofluorescence of the fovea is preserved, although surrounded by diffuse leakage. c OCT macular thickness map image of the MA(−) eye shows temporally located focal diabetic macular edema (DME) involving the fovea in its nasal edge (blue arrow), and a corresponding horizontal cross-sectional image demonstrates serous retinal detachment and a swollen retina in the temporal half. d Early phase FA of an eye with MA in the PCN. Many leaking MAs are present in the PCN (white arrows). e Late phase FA of the same eye demonstrated in Fig. 1d. Prominent pooling of hyperfluorescence is observed. f OCT macular thickness map image of the MA(+) eye shows diffuse DME centered on the fovea. A corresponding cross-sectional image demonstrates that retinal swelling is most prominent in the fovea
The eyes in the MA(+) group displayed leaking MAs in the PCN in early phase FA (Fig. 2d). Physiological foveal hypofluorescence was replaced by hyperfluorescence pooling due to leakage from the damaged PCN in late phase FA (Fig. 2e). Reflecting these changes, OCT map images depicted DME centered on the fovea, and cross-sectional images revealed a markedly swollen foveal retina with cystoid spaces (Fig. 2f).
Even in the eyes of the MA(−) group, foveal hypofluorescence was eventually replaced with hyperfluorescence in the very late phase as long as there was foveal edema. All FA results were evaluated by 2 masked graders (AI, YI).
Safety assessments
Safety was assessed by the incidence of adverse events (AEs) and serious adverse events (SAEs), ophthalmic examination results (slit lamp and indirect ophthalmoscopy), and changes in IOP, vital signs, and laboratory evaluations over the 6-month assessment period. All ocular and nonocular AEs and SAEs were recorded along with information on their relationship to IVR and laser photocoagulation.
Statistical analysis
Statistical analysis was carried out using Graph Pad Prism version 6.0 software for Windows (Graph Pad Software, San Diego, CA, USA). Continuous variables were expressed as the mean ± standard deviation or median and interquartile range. Categorical variables were presented as number and percentage. In evaluations of BCVA and CMT, changes from baseline and differences between groups during the study period were analyzed using two-way repeated-measures ANOVA and post hoc Dunnett correction. Paired t tests or the Mann–Whitney U test were employed for continuous variables. For categorical analysis, Chi square or Fisher’s exact tests were adopted. A P value of <0.05 was judged as statistically significant.
Results
Baseline characteristics and patient demographics
Of the 21 patients with DME enrolled in this study, 17 completed the 6-month follow-up. Two patients who did not respond to IVR in the induction phase declined to undergo PRN IVR and were successfully treated by vitrectomy. One patient failed to visit the outpatient clinic on a scheduled date and was excluded from the study, although the treatment itself continued and one patient was removed due to an SAE (Fig. 2). Among the remaining 17 patients, 9 were classified into the leaking MA(−) group and 8 were placed into the leaking MA(+) group based on FA findings. There were no statistically significant differences among baseline characteristics, including BCVA and CMT, between the test groups (Table 1).
Improvement in BCVA
After 2 IVR injections in the induction phase followed by PRN IVR according to the treatment regimen, mean BCVA was improved by 4.2 ± 7.4 letters, from 67.4 ± 11.7 letters to 71.6 ± 11.1 letters, at 6 months (P = 0.2341) (Fig. 3a). Among eyes with baseline BCVA ≤70 letters (approximately equivalent to 20/40 vision), mean BCVA had ameliorated significantly by 7.0 ± 7.4 letters, from 58.6 ± 8.3 letters to 65.6 ± 10.6 letters at the study end point (P = 0.0324) (Fig. 3b).
Changes in visual acuity over 6 months. a Mean best corrected visual acuity (BCVA) letter score in all subjects. b Mean BCVA letter score among the 9 eyes with pre-treatment BCVA ≤70 letters. BCVA significantly improved (P = 0.0324). *P < 0.05 compared with baseline values. Dunnett’s multiple comparison tests were used. SD standard deviation, ETDRS Early Treatment Diabetic Retinopathy Study
Improvement in CMT
The mean baseline CMT of 470.8 ± 95.6 µm had decreased significantly by 132.9 ± 114.4 µm at 6 months (P < 0.0001) (Fig. 4a). Among eyes with initial BCVA ≤70 letters, mean CMT was significantly reduced by 174.8 ± 105.0 µm, from 495.2 ± 109.4 to 320.4 ± 85.9 µm, at the final follow-up (P = 0.0005) (Fig. 4b).
Changes in central subfield macular thickness (CMT) over 6 months. a Mean CMT in all subjects. b Mean CMT among the 9 eyes with pre-treatment best corrected visual acuity (BCVA) ≤70 letters. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with baseline values. Dunnett’s multiple comparison tests were used. SD standard deviation
Mean number of IVR injections
Additional IVR injections were given when CMT was ≥300 µm and macular edema was judged as causative for vision deterioration. Overall, the subjects underwent 4.3 ± 1.6 IVR injections over 6 months.
Laser photocoagulation therapy
The participants underwent an average of 2.5 ± 1.3 sessions of short pulse focal/grid laser photocoagulation. Concomitant PRP was given to 12 subjects with proliferative changes who were PRP naïve or who had received insufficient prior PRP based on FA results.
Representative changes in response to combination therapy of IVR and focal/grid laser photocoagulation
Leaking MA(+) group
A 73-year-old man presented with vision deterioration OS. Early phase FA disclosed prominently leaking MAs with dilated capillaries in the PCN and prior PRP appeared insufficient (Online Resource 1a). Hyperfluorescence pooling was noted in the fovea in late phase FA (Online Resource 1b). An OCT macular map image revealed fovea-involving macular edema, and a corresponding cross-sectional image demonstrated cystoid spaces in the fovea responsible for foveal thickening (Online Resource 1c). Six months later, despite 6 IVR injections, focal/grid laser photocoagulation of the thickened macular area with capillary nonperfusion >500 μm outside of the fovea, and PRP, foveal leakage persisted in early phase FA (Online Resource 1d) and foveal pooling of hyperfluorescence was not resolved in the late phase (Online Resource 1e). Moreover, there were no remarkable improvements noted in OCT findings on macular map or cross-sectional images (Online Resource 1f) as compared with those obtained before treatment (Online Resource 1c).
Leaking MA(−) group
A 78-year-old man complained of vision deterioration OD. FA showed no apparent leakage in the PCN but many leaking points outside of the fovea, within the vascular arcades (Online Resource 2a). Foveal hypofluorescence was largely preserved, even in late phase FA (Online Resource 2b). An OCT macular map image demonstrated diffuse macular edema encompassing the entire macula, and a cross-sectional image showed serous retinal detachment in the fovea (Online Resource 2c). Over 6 months, he was treated with 2 IVR injections, focal/grid laser photocoagulation, and PRP. The number of leakage points became decreased and resolved in early phase FA (Online Resource 2d). Foveal hypofluorescence was well preserved in the late phase (Online Resource 2e). An OCT macular map image showed that the diffuse DME (Online Resource 2c) had become divided into 2 areas of focal DME, and resolution of the serous detachment was apparent in a cross-sectional image (Online Resource 2f). CMT was decreased from 426 to 265 µm.
Comparisons of BCVA, CMT, total number of IVR injections, and focal/grid laser photocoagulation between MA(+) and MA(−) groups
There was no significant difference in BCVA improvement between the MA(−) and MA(+) groups (4.3 ± 6.7 vs. 4.1 ± 8.5 letters, respectively; P = 0.8693) (Fig. 5a), nor was there one for CMT reduction (125.0 ± 76.9 vs. 141.8 ± 151.6 µm, respectively; P = 0.9336) (Fig. 5b). However, the MA(−) group required significantly fewer IVR injections than did the MA(+) group over the 6-month study period [3.4 ± 1.6 vs. 5.3 ± 0.9, median (25–75 % interquartile range) 3.0 (2.0–5.0) vs. 5.5 (4.3–6.0), respectively; P = 0.0229] (Fig. 5c). On the other hand, there was no significant difference in the number of focal/grid laser photocoagulation between the MA(−) and MA(+) groups [2.6 ± 1.3 vs. 2.4 ± 1.3, median (25–75 % interquartile range) 3.0 (1.0–4.0) vs. 2.5 (1–3.8), respectively; P = 0.8221] (Fig. 5d).
Comparison of outcomes between microaneurysm (MA) (+) and MA(−) groups. a Comparison of best corrected visual acuity (BCVA) between MA(−) (triangle) and MA(+) (shaded square) groups; 4.3 ± 6.7 letters vs. 4.1 ± 8.5 letters, respectively. No significant difference is seen (P = 0.8693). b Comparison of central subfield macular thickness (CMT) between MA(−) (triangle) and MA(+) (shaded circle) groups; 125.0 ± 76.9 vs. 141.8 ± 151.6 µm, respectively. No significant difference is seen (P = 0.9336). c The number of intravitreal ranibizumab (IVR) injections required to sustain CMT < 300 µm over the 6-month study period. MA(−) group (shaded circle) required significantly fewer intravitreal IVR injections than did the MA(+) group (shaded square); 3.4 ± 1.6 vs. 5.3 ± 0.9, respectively (P = 0.0229). d The number of focal/grid laser photocoagulation between the MA(−) and MA(+) groups over the 6-month study period. No significant difference is seen for the number of focal/grid laser sessions between the MA(−) (shaded circle) and MA(+) (shaded square) groups; 2.6 ± 1.3 vs. 2.4 ± 1.3, respectively (P = 0.8221) *P < 0.05. Mann–Whitney U tests were used. SD standard deviation, ETDRS Early Treatment Diabetic Retinopathy Study
Safety outcomes
No ocular or systemic AEs were observed during the trial period apart from occasional minor subconjunctival hemorrhage. One patient, who was excluded from the study, experienced a cerebral infarction without evidence suggesting a direct relation to IVR; the incident was recorded as an SAE.
Discussion
In the treatment of fovea-involving DME, combination therapy of IVR in a PRN regimen and short pulse focal/grid laser photocoagulation provided an overall mean of 4.2 ± 7.4 ETDRS letters of vision gain. Among eyes with initial BCVA ≤70 letters, mean BCVA improved by 7.0 ± 7.4 letters, which was comparable to outcomes reported in previous multicenter clinical trials, such as 7.2 letters in the READ-2 study [16], 6.4 letters in the REVEAL study [13], 6.4 letters in the RESTORE study [14], and 7 letters in DRCR.net protocol I [8]. There was no significant difference in vision gain in comparisons between MA(+) (4.1 ± 8.5 letters) and MA(−) groups (4.3 ± 6.7 letters) (P = 0.8693). The total required number of IVR injections was significantly higher in the MA(+) group [3.4 ± 1.6 vs. 5.3 ± 0.9, median (25–75 % interquartile range) 3.0 (2.0–5.0) vs. 5.5 (4.3–6.0), respectively; P = 0.0229], likely because only IVR, but not focal/direct laser, could halt leakage from the MAs and dilated capillaries in the PCN. Without such leakage points in the MA(−) group, edema fluid in the fovea was derived mainly from leakage outside the fovea (>500 µm). Since these leakage points were treatable with focal/grid laser photocoagulation, the eyes in MA(−) group presumably required fewer PNR IVR injections. However, as capillary injuries in diabetic patients are caused by glucotoxicity, damage exists ubiquitously in all retinal vessels and there is always some extent of foveal capillary damage from DR. Consequently, in the present study, none of the eyes was successfully treated by focal/grid laser treatment without PRN IVR. We consider this to be the reason why in most multicenter clinical trials on DME, focal/grid laser failed to produce synergetic effects with IVR in the resolution of foveal edema and vision recovery and did not meaningfully impact the number of IVR injections. Unfortunately, the ratio of patients with or without leaking MAs in the PCN was not stated in earlier studies.
Although the majority of clinical investigations on DME did not detect therapeutic benefits for focal/grid laser photocoagulation in conjunction with IVR, subanalyses by DME type, such as focal or diffuse DME in the REVEAL study [13], uncovered some benefits: combination therapy provided significantly better vision improvement (7.8 letters) than IVR monotherapy alone (4.9 letters) in eyes with focal DME. Furthermore, the proportion of patients with a maximum treatment-free interval of 3 months or more was 38.3 % in the IVR monotherapy arm, which increased to 50.8 % by the addition of photocoagulation. This subanalysis suggests that focal/grid laser treatment was significantly more effective when used in conjunction with PRN-IVR in specific cases of focal DME. The precise definition of focal edema is unclear [29], but usually refers to a restricted area of macular thickening that evades the fovea or involves the fovea in its periphery. On the other hand, diffuse edema typically encompasses the entire macula and contains the fovea in its center. In this regard, we consider that the focal edema group in the REVEAL study corresponds to our MA(−) group and the diffuse edema group to our MA(+) patients, because if there are leaking MAs in the PCN, the ensuing macular edema should be centered over the fovea and diffusely encompass the macula. Together with those of the REVEAL study, our results indicate that focal/grid laser photocoagulation of leakage points outside of the fovea at least partly reduces edema fluid influx to the fovea and may be key to decreasing the number of required IVR injections, especially in cases where leaking capillaries and MAs are mainly localized outside of the PCN and manifest as focal edema.
We delivered grid laser burns to all capillary nonperfusion areas, which were sources of ischemia-induced VEGF, regardless of size if located within the vascular arcades and especially when located proximal to the fovea and outside the 500 µm safety margin. We believed that this treatment was critical since leaking MAs and dilated capillaries were often formed at the boundaries between nonperfused and perfused retinae. Grid laser burns to capillary nonperfusion areas suppressed pathologic VEGF overproduction and preempted the formation of leakage points in the PCN.
In diffuse edema with leaking MAs in the PCN, leakage outside the fovea could still be treated by focal/grid laser treatment, but only sustained IVR injections could halt the leakage from the PCN. The relatively high ratio of MA(−) eyes (9/17, 53 %) demonstrated a successful reduction in IVR injections by focal/grid laser photocoagulation over MA(+) eyes. Our selection criteria included BCVA ≥24 ETDRS letters, whose approximate Snellen equivalent was ≥20/320. We did not exclude eyes with BCVA >20/40, which would have led to a high ratio of MA(−) eyes. In our daily clinical experience, most eyes with DME have foveal leakage points, including leaking MAs, unless DR is still in very early stage. This also suggests that leaking MAs in the PCN may represent markers of severe capillary damage and subsequent remodeling of the PCN, leading to local leakage that adversely affects the resolution of foveal edema.
In our treatment-naïve patients, PRP reaching the ora serrata was performed to inhibit the progression of proliferative changes, which may also diminish macular edema since VEGF overproduction in the mid to peripheral retina has been implicated in the pathogenesis of DME [24–26].
This investigation had several limitations. It contained a limited sample size, lacked an IVR-monotherapy control arm, and had a relatively high rate of subjects lost to follow-up, all of which might have affected results. To the best of our knowledge, however, this study is the first investigator-initiated ophthalmological clinical trial in Japan performed in accordance with the strict J-GCP guidelines. All data were recorded in HOPE eACReSS, created by the University Hospital Clinical Trial Alliance, and 1 patient was excluded from the follow-up despite presenting several days late for a scheduled monthly hospital visit. The present study did not include a ranibizumab monotherapy control mainly because it aimed to optimize the applicability of high cost IVR with that of concomitant focal/grid laser photocoagulation. Lastly, we employed focal, grid, and PRP in this investigation, while most prior studies did not describe PRP status, which has been found to adversely affect DME. Thus, we could not pinpoint the exact benefits of each laser modality. Longer-term follow-up will also be needed to examine the precise changes in BCVA and CMT over time in this cohort.
In conclusion, combination therapy of IVR in a PRN regimen with focal/grid laser photocoagulation is effective in the treatment of DME, with or without leaking MAs in the PCN. However, since MA leakage can be considered a marker of capillary damage and remodeling, combination treatment prior to the appearance of MAs in the PCN may significantly reduce the number of IVR injections to sustain resolution of foveal edema in DME.
References
Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–8.
Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14:179–83.
King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31.
Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–39.
Early Treatment Diabetic Retinopathy Study Research G. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Ophthalmology. 1987;94:761–74.
Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013–22.
Diabetic Retinopathy Clinical Research N. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–1449, 1449e1–10.
Diabetic Retinopathy Clinical Research N, Elman MJ, Aiello LP, Beck RW, Bressler NM, SB Bressler, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(1064–77):e35.
Diabetic Retinopathy Clinical Research N, Elman MJ, Qin H, Aiello LP, Beck RW, Bressler NM, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119:2312–8.
Do DV, Nguyen QD, Khwaja AA, Channa R, Sepah YJ, Sophie R, et al. Ranibizumab for edema of the macula in diabetes study: 3-year outcomes and the need for prolonged frequent treatment. JAMA Ophthalmol. 2013;131:139–45.
Early Treatment Diabetic Retinopathy Study Research G. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806.
Early Treatment Diabetic Retinopathy Study Research G. Photocoagulation for diabetic macular edema: early Treatment Diabetic Retinopathy Study report no. 4. Int Ophthalmol Clin. 1987;27:265–72.
Ishibashi T, Li X, Koh A, Lai TY, Lee FL, Lee WK, et al. The REVEAL study: ranibizumab monotherapy or combined with laser versus laser monotherapy in asian patients with diabetic macular edema. Ophthalmology. 2015;122:1402–15.
Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25.
Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801.
Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D, et al. Primary End Point (Six Months) Results of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) Study. Ophthalmology. 2009;116(2175–81):e1.
Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, et al. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117:2146–51.
Stewart MW. The clinical utility of aflibercept for diabetic macular edema. Diabetes Metab Syndr Obes. 2015;8:473–82.
Hirano T, Iesato Y, Toriyama Y, Imai A, Murata T. Detection of fovea-threatening diabetic macular edema by optical coherence tomography to maintain good vision by prophylactic treatment. Ophthalmic Res. 2014;52:65–73.
Hirano T, Iesato Y, Imai A, Toriyama Y, Kikushima W, Murata T. Effect of Laser wavelength on delivering appropriate laser burns through the opaque lens using a pattern scan laser. Ophthalmic Res. 2014;51:204–9.
Hirano T, Iesato Y, Murata T. Multicolor pattern scan laser for diabetic retinopathy with cataract. Int J Ophthalmol. 2014;7:673–6.
Morgan CM, Schatz H. Atrophic creep of the retinal pigment epithelium after focal macular photocoagulation. Ophthalmology. 1989;96:96–103.
Muqit MM, Denniss J, Nourrit V, Marcellino GR, Henson DB, Schiessl I, et al. Spatial and spectral imaging of retinal laser photocoagulation burns. Invest Ophthalmol Vis Sci. 2011;52:994–1002.
Takamura Y, Tomomatsu T, Matsumura T, Arimura S, Gozawa M, Takihara Y, et al. The effect of photocoagulation in ischemic areas to prevent recurrence of diabetic macular edema after intravitreal bevacizumab injection. Invest Ophthalmol Vis Sci. 2014;55:4741–6.
Patel RD, Messner LV, Teitelbaum B, Michel KA, Hariprasad SM. Characterization of ischemic index using ultra-widefield fluorescein angiography in patients with focal and diffuse recalcitrant diabetic macular edema. Am J Ophthalmol. 2013;155:1038–1044e2.
Wessel MM, Nair N, Aaker GD, Ehrlich JR, D’Amico DJ, Kiss S. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96:694–8.
Iesato Y, Imai A, Hirano T, Toriyama Y, Murata T. Effect of leaking capillaries and microaneurysms in the perifoveal capillary network on resolution of macular edema by anti-vascular endothelial growth factor treatment. Jpn J Ophthalmol. 2016;. doi:10.1007/s10384-016-0425-5.
Brown DM, Schmidt-Erfurth U, Do DV, Holz FG, Boyer DS, Midena E, et al. Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results From the VISTA and VIVID Studies. Ophthalmology. 2015;122:2044–52.
Browning DJ, Altaweel MM, Bressler NM, Bressler SB, Scott IU, Diabetic Retinopathy Clinical Research N. Diabetic macular edema: what is focal and what is diffuse? Am J Ophthalmol. 2008;146:649–655, 55 e1–6.
Acknowledgments
The authors thank Drs. Shigeo Yoshida, Fumiki Okamoto and Kousuke Noda for supporting this study. Funding was provided by Novartis Pharma.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
T. Hirano, Grants (Novartis), Lecture fees (Bayer, Novartis); Y. Toriyama, Grants (Novartis); Y. Iesato, Grants (Novartis), Lecture fees (Novartis); A. Imai, Grants (Novartis); K. Hirabayashi, Grants (Novartis); T. Nagaoka, Grants (Novartis); Y. Takamura, Grants (Novartis), Lecture fees (Alcon, Bayer, Kowa, Novartis, Santen, Senju); M. Sugimoto, Grants (Novartis), Lecture fees (Bayer, Kowa, Novartis, Santen, Wakamoto); T. Murata, Grants (Novartis), Lecture fees (Alcon, Bayer, Kowa, Novartis, Santen).
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Hirano, T., Toriyama, Y., Iesato, Y. et al. Effect of leaking perifoveal microaneurysms on resolution of diabetic macular edema treated by combination therapy using anti-vascular endothelial growth factor and short pulse focal/grid laser photocoagulation. Jpn J Ophthalmol 61, 51–60 (2017). https://doi.org/10.1007/s10384-016-0483-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-016-0483-8