Abstract
Purpose
To study the correspondence between fluorescein angiography (FA) and structural en face optical coherence tomography (OCT) in the identification of leaky microaneurysms in diabetic macular edema (DME).
Methods
Fourteen eyes of eight patients with DME (6 males and 2 females, mean age 67.3 ± 8.5) were included. For all eyes, a 6 × 6 mm structural en face image of the middle retina was obtained and superimposed on a FA image. The reflectivity, capsulation, and association with intraretinal cystic fluid (IRCF) of microaneurysms on en face were evaluated depending on their leaky status on FA.
Results
Out of the 320 leaky microaneurysms evaluated, 280 (89.0 ± 8.2%) coincided with those on en face OCT image. Twenty-nine (10.6 ± 6.9%) and 20 (6.5 ± 7.8%) out of all leaky microaneurysms were hyperreflective and demonstrated capsular appearance, respectively. A majority of leaky microaneurysms (97.9 ± 3.2%) were associated with IRCF. From 146 microaneurysms which were found only on en face images, 130 (88.2% ± 15.7%) were hyperreflective, 33 (23.9% ± 15.6%) demonstrated capsular structure, and 13 (9.2% ± 15.0%) demonstrated no associated IRCF. After exclusion of microaneurysms of the inner retina, 95.4 ± 5.4% of leaky microaneurysms were identified on en face image. En face imaging demonstrated 83.5% sensitivity and 89.4% specificity (the area under the curve 0.87) in the identification of leaky microaneurysms.
Conclusions
Structural en face imaging is comparable to FA in identification of leaky microaneurysms in diabetic macular edema. Moderate reflectivity, the absence of capsular structure, and neighboring intraretinal cystic fluid indicate leaky microaneurysms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laser photocoagulation was a standard treatment for diabetic macular edema (DME) until the introduction of intravitreal anti-VEGF therapy, which is a more effective and simple treatment for DME [1,2,3,4]. However, recently developed navigated laser photocoagulation may be a good adjunct to anti-VEGF treatment as a precise and safe procedure [5,6,7]. A key step in preparation for focal laser photocoagulation in DME is the identification of leaky microaneurysms with fluorescein angiography (FA) [8]. However, FA is an invasive procedure with potential adverse reactions and requires additional time and resources to perform [9, 10].
Recently, fine noninvasive analysis of retinal microcirculation has become available with optical coherence tomography angiography (OCTA) [11]. However, visualization of microaneurysms with OCTA is limited [12]. Since blood flow in microaneurysm is turbulent and relatively slow, the decorrelation of the OCT signal is low, and the flow signal within microaneurysms can, therefore, be weak. Additionally, OCTA is not able to detect leakage and does not allow the direct differentiation of leaky microaneurysms [12]. However, the resolution of OCTA imaging is substantially higher than that of FA [11, 13]. We suggest that high-resolution structural en face images produced by OCTA may provide an adequate solution for noninvasive visualization of microaneurysms.
Several studies have already described OCT characteristics of leaky microaneurysms on cross-sectional OCT scans [14, 15]. However, analysis of individual cross-sectional scans is practically useless since a single picture depicting the whole pool of microaneurysms is required for treatment planning. Therefore, the aim of this study was to evaluate the correlation between structural en face imaging and FA in the identification of leaky microaneurysms in DME.
In this study, we have evaluated the association of reflectivity, the presence of capsular structure, and neighboring intraretinal cystic fluid on structural en face image with the leaky status of microaneurysms. Since previous studies showed that microaneurysms of the deep capillary plexus (DCP) are the main contributor to retinal thickening in DME in our study, we used DCP slab to display microaneurysms [16].
Materials and methods
Study population
Only treatment naïve DME patients were included in this prospective cross-sectional study. The study adhered to the tenets of the Declaration of Helsinki and was approved by the Local Ethics Committee of Military Medical Academy. The exclusion criteria included substantial media opacity and a final OCT scan quality of 6/10 or less. All patients underwent a comprehensive ophthalmic examination, including a best-corrected visual acuity (BCVA) examination, FA, and spectral domain OCT, as well as OCT angiography.
Image acquisition
Before image acquisition in all eyes, mydriasis was achieved by the instillation of 1% tropicamide. The OCT examination was performed using RTVue XR Avanti (Optovue, Inc., Fremont, CA) and included a Macular Map protocol and a 6 × 6 mm OCT angiography protocol. The OCT angiography protocol used in this study resulted from the merging of 2 three-dimensional scans acquired in perpendicular dimensions to reduce motion artifacts. A single three-dimensional scan included 400 B-scans, each of 400 repeated A-scans. Therefore, each final cross-sectional scan resulted from quadruplicated A-scans and had a resolution high enough to evaluate even subtle changes in the retinal anatomy. Since microaneurysms originating from DCP play a major contributing role in the pathogenesis of DME [16], we chose the DCP slab (inner plexiform layer = 0 µm, inner nuclear layer = − 70 µm) for the visualization of leaky microaneurysms. The segmentation lines, where needed, were manually corrected, and the resultant structural en face images were exported for further analysis.
FA was performed on the same day with OCT examination using the scanning laser ophthalmoscope F-10 (NIDEK, Gamagori, Japan). For precise identification of microaneurysms, we used standard images acquired at 1 min after dye injection. Additionally, images acquired on the late phase of FA were taken for confirmation of the leaky status of the microaneurysms.
Analysis of the characteristics of microaneurysms
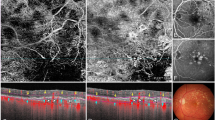
For image analysis, we used Adobe Photoshop CS2 (Adobe Systems, Inc, San Jose, CA). All pairs (early and late phase) of FA images were evaluated randomly by an experienced retinologist to mark all leaky microaneurysms on the first minute image. Leaky microaneurysms were defined as round or oval hyperfluorescent spots with fluorescein extravasation in the late phase of FA. After leaky microaneurysms were marked, a structural en face image was superimposed on the FA image by the alignment of retinal vessels (Fig. 1). Next, each microaneurysm encountered on en face image was evaluated in accordance with the following characteristics: (1) coinciding with leaky microaneurysm on FA image (vs coinciding with non-leaky microaneurysm or non-coinciding with microaneurysms); (2) reflectivity (high vs moderate); (3) the presence of a capsular structure (defined as a hyperreflective contour); (4) association with intraretinal cystic fluid (yes or no) (Fig. 2). Microaneurysms on the structural en face image were defined as round or oval, highly or moderately hyperreflective structures with their own wall. The intraretinal cystic fluid on the en face image was defined as round or elongated hyporeflective structures without their own wall. All leaky microaneurysms not identified on en face image were characterized by (1) localization within the inner retinal layers (yes or no) by retrospective review of cross-sectional scans; and (2) the presence of associated intraretinal cystic fluid (yes or no). All DME cases in this study were characterized as demonstrating focal or diffuse edema and graded in accordance with the number of hard exudates [0-no hard exudates, 1-low (total area less than 1/4 disk area); 2-medium (total area more than 1/4 disk area, but less than 1 disk area); 3-high (total area more than 1 disk area)].
Analysis of en face and fluorescein angiography images in visualization of microaneurysms. a Fluorescein angiography image showing distribution of microaneurysms in the early phase. b Fluorescein angiography image showing leaky microaneurysms in the late phase. c Fluorescein angiography with microaneurysms marked (black circles and white squares indicate leaky and non-leaky microaneurysms, respectively). d Structural en face image (deep capillary plexus slab) showing distribution of microaneurysms, intraretinal cystic fluid, and hard exudates. e En face image superimposed on fluorescein angiography with microaneurysms marked (black circles and white squares indicate leaky and non-leaky microaneurysms, respectively)
Statistics
All data are presented as a mean ± standard deviation. Statistical analysis was performed in MedCalc 18.4.1 (MedCalc Software, Ostend, Belgium). Paired t test was used to compare the percentage of the coincidence of microaneurysms (1) between eyes with focal and diffuse DME and (2) between eyes with no or a low number of hard exudates and eyes with a medium or a high number of hard exudates. The Spearman rank correlation coefficient was calculated for a percentage of the coincidence of microaneurysms and central retinal thickness (CRT). Receiver operating characteristic (ROC) analysis was performed to assess the ability of en face image to identify leaky microaneurysms in comparison with FA, which was taken as the “gold standard.” Every microaneurysm demonstrating hyperreflectivity, capsular appearance or the absence of intraretinal cystic fluid was defined as non-leaky. The sample size for the ROC analysis was calculated with type I and type II errors of 0.05 and a ratio of non-leaky to leaky microaneurysms of 0.5. With the hypothesized area under the ROC curve of 0.8, the required number of microaneurysms was found to be 303. With the assumption that 20–25 microaneurysms may be analyzed in every eye included, the minimum sample size being 12 eyes. p value < 0.05 was considered to be statistically significant.
Results
Four hundred sixty-six microaneurysms of fourteen eyes with DME (8 patients 6 males and 2 females, mean age 67.3 ± 8.5 years) were analyzed in this study. The mean BCVA was 0.51 ± 0.39 LogMAR (9/20, Snellen). Three hundred twenty leaky microaneurysms were identified on FA images, and 146 microaneurysms were found on en face images and were non-identifiable or non-leaky on FA images.
In total, 280 out of 320 (87.5%) leaky microaneurysms were found to coincide with the microaneurysms on en face images. Only 29 (10.4%) and 20 (7.1%) out of all coinciding microaneurysms demonstrated a hyperreflective and a capsular appearance, respectively. A majority of all coinciding microaneurysms (97.5%) were associated with the accumulation of intraretinal cystic fluid. One hundred forty-six microaneurysms which were found on en face images were non-identifiable or non-leaky on FA images. Out of 146 microaneurysms, 130 (89.0%) were hyperreflective, 33 (22.6%) demonstrated a capsular structure, and 13 (8.9%) demonstrated no associated intraretinal cystic fluid.
Eyes with focal macular edema (7 eyes, 115 microaneurysms) demonstrated a statistically significantly higher percentage of coincidence between leaky microaneurysms on FA and microaneurysms on en face compared to eyes with diffuse macular edema (7 eyes, 205 microaneurysms), 93.3 ± 8.6% and 84.6 ± 5.2%, respectively (p = 0.048). There was a negative correlation between CRT and the percentage of coincided microaneurysms (r = − 0.55, p = 0.046). There was no difference in the percentage of the coincidence of microaneurysms between the eyes with no or a low number of hard exudates and the eyes with a medium or high number of hard exudates. From non-coinciding (non-identifiable on the en face image) leaky microaneurysms (n = 40), 23 (57.5%) were found in the inner retina (Fig. 3), and 21 (52.5%) demonstrated no association with intraretinal cystic fluid. After the exclusion of microaneurysms of the inner retinal layers and microaneurysms without associated intraretinal cystic fluid, in total, 95.4% leaky microaneurysms were found to correspond with the en face image. ROC analysis showed 83.5% sensitivity and 89.4% specificity (the area under the curve = 0.87) of en face imaging in the identification of leaky microaneurysms, where FA was taken as the “gold standard.”
Representative example of a microaneurysm in the inner retinal layers. a Fluorescein angiography image in the early phase demonstrating a microaneurysm (arrowhead) in the inner retina. b Fluorescein angiography image in the late phase revealed leakage associated with the microaneurysm (arrowhead). c En face image of deep capillary plexus (DCP) slab demonstrates no visible microaneurysm (arrowhead). d Cross-sectional optical coherence tomography (OCT) scan demonstrating a microaneurysm (arrowhead) in the inner retina outside the boundaries of DCP slab (dashed and solid white lines represent segmentation lines of inner plexiform layer and inner nuclear layer, respectively). e En face image of a customized slab demonstrating a microaneurysm (arrowhead) in the inner retinal layers. f Cross-sectional OCT scan showing the position of the microaneurysm (arrowhead) in the relation to automated segmentation lines of the customized slab (constructed with two segmentation lines of inner limiting membrane)
Discussion
In this study, we confirmed that microaneurysms on the structural en face image correspond precisely with those on the FA image, and the characteristics of these microaneurysms on en face image may predict their leaky status. Specifically, hyperreflective microaneurysms without a capsular structure, demonstrate no leakage during FA. Additionally, the absence of associated intraretinal cystic fluid is indicative of non-leaky microaneurysms. We can, therefore, consider en face imaging as a tool comparable with FA for the identification of leaky microaneurysm.
Although it was known that moderately reflective microaneurysms in the middle retinal layers without a ring sign play a major role in the pathophysiology of DME [14, 15], this data have a limited practical application since individual cross-sectional OCT scans do not show the spatial distribution of microaneurysms. At the same time, the role of en face imaging for laser treatment planning appears to be underestimated because of the absence of data regarding the correspondence between en face and FA imaging.
Hasegawa and coauthors had shown the major contribution in the pathogenesis of DME of microaneurysms, which originate from DCP [16]. Therefore, microaneurysms of the DCP would appear to be the main target for focal laser treatment. It seems reasonable, therefore, to choose the DCP slab for the visualization of microaneurysms in en face mode.
In our study, moderately hyperreflective microaneurysms without capsular structure associated with intraretinal cystic fluid most frequently demonstrated leaky appearance on FA. This agrees with OCT characteristics for leaky microaneurysms on cross-sectional scans, as well as with changes in OCT characteristics of microaneurysms with reduction in leakage after laser photocoagulation [3, 12, 14]. Interestingly, we found a strong association of leaky microaneurysms with intraretinal cystic fluid (up to 98% of leaky microaneurysms). This is to be expected since the intraretinal fluid is a direct consequence of vascular hyperpermeability, including leakage from microaneurysms in DME. This is even more obvious for focal DME since the local accumulation of intraretinal fluid cannot be a result of diffuse vascular leakage, but rather the leakage from a limited number of focal sources [17, 18]. However, in each case, it remains unclear if all the microaneurysms within the area of retinal edema are responsible for the accumulation of intraretinal fluid [19]. In our study, it appears highly unlikely that hyperreflective microaneurysms and those without a capsular structure were responsible for the leakage. Therefore, an algorithm of en face planning for focal laser photocoagulation in DME should include (1) the delineating of the areas of intraretinal fluid accumulation and (2) treatment of all microaneurysms within those areas excluding hyperreflective and those without capsular structure.
As it was shown earlier, the area of localization of leaky microaneurysm can be characterized by retinal thickening [16, 18, 19]. Although it is generally understood that retinal thickening represents the accumulation of intraretinal fluid, color coding does not provide exact delineation of the area where the microaneurysms have to be treated.
Some attention must be given to the partial discrepancy between FA and en face in the visualization of microaneurysms. The absence of microaneurysms on en face image, while they are present on FA, may be explained by the localization of those outside the slab or on its borders (for example, due to local errors in segmentation), as well as the placing of microaneurysm between two consecutive cross-sectional scans. Although we attempted to minimize such errors, we could not exclude them completely. On the other hand, there is a pool of non-leaky microaneurysms on FA which have a leaky appearance on en face (moderately reflective, without capsular structure and associated with intraretinal cystic fluid). We can suggest that these “anomalous” microaneurysms are at the earliest stage of their natural evolution before the leakage appears on FA, or they have very weak leakage hardly detectable on FA. In both cases, en face may be a better tool for treatment planning compared to FA. It should be noted that the obvious reason for such discrepancy is the visualization with FA of microaneurysms in the inner retinal layers, which do not require treatment. We believe that the ability to visualize microaneurysms exclusively in the middle retina (DCP slab) is a strength of en face imaging.
In our study, eyes with focal DME and moderately increased CRT showed a better correspondence between en face and FA in the identification of leaky microaneurysms than eyes with diffuse DME and high CRT. This seems reasonable since focal DME is more likely to be associated with focal leakage from microaneurysms. This fact required some extra precautions for en face-based planning in eyes with severe diffuse DME. Therefore, eyes with focal DME are the best candidates for focal laser photocoagulation using en face planning. Although our study justifies a noninvasive en face OCT-based planning for focal laser photocoagulation, an interventional study is required to confirm the noninferiority of this approach against conventional FA-based planning.
This study has several limitations. Firstly, the sample size is somewhat limited. However, we have been primarily analyzing the characteristics of individual microaneurysms, and, from this point of view, the sample size (466 individual microaneurysms) is sufficient. Secondly, OCTA examination is substantially affected by media opacities and therefore cannot be an adequate tool for planning in such cases. However, it is also highly unlikely that focal laser photocoagulation can be performed in such cases. Thirdly, OCTA compared to FA has a relatively small field of view, which limits its diagnostic value. However, when considering focal laser photocoagulation, there is no obvious need to treat microaneurysms outside the central 6-mm area since these microaneurysms have a little or no impact on the severity of DME.
In conclusion, in this study, we found that hyperreflective microaneurysms or those with a capsular structure on en face OCT image typically have non-leaky status on FA. At the same time, the accumulation of intraretinal cystic fluid is strongly associated with non-hyperreflective microaneurysms without a capsular structure. Cumulatively, these characteristics provide high sensitivity and specificity for en face identification of leaky microaneurysms. Therefore, structural en face imaging provides an adequate noninvasive alternative for the identification of leaky microaneurysms in DME.
References
Early Treatment Diabetic Retinopathy Study Research Group (1995) Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Arch Ophthalmol 113(9):1144–1155
Aiello LP, Edwards AR, Beck RW, Diabetic Retinopathy Clinical Research Network et al (2010) Factors associated with improvement and worsening of visual acuity 2 years after focal/grid photocoagulation for diabetic macular edema. Ophthalmology 117(5):946–953
Lee SN, Chhablani J, Chan CK et al (2013) Characterization of microaneurysm closure after focal laser photocoagulation in diabetic macular edema. Am J Ophthalmol 155(5):905–912
Brown DM, Schmidt-Erfurth U, Do DV et al (2015) Intravitreal Aflibercept for Diabetic Macular Edema: 100-week results from the VISTA and VIVID studies. Ophthalmology 122(10):2044–2052
Boiko EV, Maltsev DS (2017) Combination of navigated macular laser photocoagulation and anti-VEGF therapy: precise treatment for macular edema under dry retinal conditions. J Ophthalmol 2017:7656418
Maltsev DS, Kulikov AN, Uplanchiwar B et al (2018) Direct navigated laser photocoagulation as primary treatment for retinal arterial macroaneurysms. Int J Retina Vitr 4:28
Kozak I, Oster SF, Cortes MA et al (2011) Clinical evaluation and treatment accuracy in diabetic macular edema using navigated laser photocoagulator NAVILAS. Ophthalmology 118(6):1119–1124
Early Treatment Diabetic Retinopathy Study Research Group (1991) Classification of diabetic retinopathy from fluorescein angiograms. ETDRS report number 11. Ophthalmology 98(5 Suppl):807–822
Yannuzzi LA, Rohrer KT, Tindel L et al (1986) Fluorescein angiography complication survey. Ophthalmology 93(5):611–617
Kwiterovich KA, Maguire MG, Murphy RP et al (1991) Frequency of adverse systemic reactions after fluorescein angiography. Results of a prospective study. Ophthalmology 98(7):1139–1142
Nagiel A, Sadda SR, Sarraf D (2015) A promising future for optical coherence tomography angiography. JAMA Ophthalmol 133(6):629–630
Schreur V, Domanian A, Liefers B et al (2019) Morphological and topographical appearance of microaneurysms on optical coherence tomography angiography. Br J Ophthalmol 103:630–635
Spaide RF, Klancnik JM Jr, Cooney MJ (2015) Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol 133(1):45–50
Wang H, Chhablani J, Freeman WR et al (2012) Characterization of diabetic microaneurysms by simultaneous fluorescein angiography and spectral-domain optical coherence tomography. Am J Ophthalmol 153(5):861–867
Ito H, Horii T, Nishijima K et al (2013) Association between fluorescein leakage and optical coherence tomographic characteristics of microaneurysms in diabetic retinopathy. Retina 33(4):732–739
Hasegawa N, Nozaki M, Takase N et al (2016) New insights into microaneurysms in the deep capillary plexus detected by optical coherence tomography angiography in diabetic macular edema. Investig Ophthalmol Vis Sci 57(9):348–355
Soliman W, Sander B, Hasler PW, Larsen M (2008) Correlation between intraretinal changes in diabetic macular oedema seen in fluorescein angiography and optical coherence tomography. Acta Ophthalmol 86(1):34–39
Reznicek L, Kernt M, Haritoglou C et al (2011) Correlation of leaking microaneurysms with retinal thickening in diabetic retinopathy. Int J Ophthalmol 4(3):269–271
Blair NP, Shahidi M, Lai WW, Zelkha R (2008) Correlation between microaneurysms and retinal thickness in diabetic macular edema. Retina 28(8):1097–1103
Funding
The authors have not received any grant support for this study. The authors have no proprietary or financial interest in any aspect of this report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maltsev, D.S., Kulikov, A.N., Burnasheva, M.A. et al. Structural en face optical coherence tomography imaging for identification of leaky microaneurysms in diabetic macular edema. Int Ophthalmol 40, 787–794 (2020). https://doi.org/10.1007/s10792-019-01239-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-019-01239-w