Abstract
Background
Neuroimaging studies have reported gray matter changes in patients with idiopathic dystonia but with considerable variations. Here, we aimed to investigate the convergence of dystonia-related gray matter changes across studies.
Methods
The whole brain voxel-based morphometry studies comparing idiopathic dystonia and healthy controls were systematically searched in the PubMed, Web of Science and Embase. Meta-analysis of gray matter changes was performed using the anisotropic effect size-based signed differential mapping.
Results
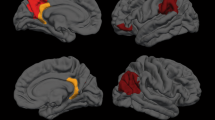
Twenty-eight studies comparing 701 idiopathic dystonia patients and 712 healthy controls were included in the meta-analysis. Compared to healthy controls, idiopathic dystonia patients showed increased gray matter in bilateral precentral and postcentral gyri, bilateral putamen and pallidum, right insula, and left supramarginal gyrus, while decreased gray matter in bilateral temporal poles, bilateral supplementary motor areas, right angular gyrus, inferior parietal gyrus and precuneus, left insula and inferior frontal gyrus. These findings remained robust in the jackknife sensitivity analysis, and no significant heterogeneity was detected. Subgroup analyses of different phenotypes of dystonia were performed to further confirm the above findings.
Conclusion
The meta-analysis showed that consistent widespread gray matter abnormalities were shared in different subtypes of idiopathic dystonia and were not restricted to the corticostriatal circuits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dystonia is a movement disorder characterized by sustained or intermittent muscle contractions that give rise to twisting movements and abnormal postures [2]. Dystonia shows obvious clinical and etiological heterogeneities. The diverse clinical features of dystonia could be reflected in the classifications based on age of onset, body distributions, temporal patterns and associated features. The pathophysiology of idiopathic dystonia remains unknown. Although dystonia was traditionally regarded as a disorder of basal ganglia, microstructural and functional abnormalities outside the basal ganglia have been visualized with the rapid development of new neuroimaging techniques, improving the understanding of neurological pathophysiology of idiopathic dystonia.
Neuroimaging approaches have the potential to define brain structural abnormalities in idiopathic dystonia. Compared with the manual method of drawing regions of interest (ROI) to measure the volume of the brain structures, voxel-based morphometry (VBM) [3] offers an operator independent comprehensive assessment of anatomical differences throughout the brain. VBM is widely used in neuropsychiatric disorders and neurological disorders. VBM studies have explored the gray matter (GM) differences between patients with different types of dystonia and healthy controls, which prompt further understanding of the pathophysiology of idiopathic dystonia. Diverse regions of GM changes differed from healthy controls were reported. Garraux et al. reported increased GM volume in the hand representation area of primary somatosensory and primary motor cortices in patients with focal hand dystonia [30]. In patients with cervical dystonia (CD), one study found a significant increase of GM volume in the right globus pallidus, bilateral orbitofrontal cortex, right medial frontal gyrus, left supplementary motor area (SMA) and left cingulate gyrus [24]. While another study in patients with CD reported decreased GM volume in the left precentral gyrus, left SMA and right somatosensory association cortex [53]. Alternately, there are some studies found no significant difference between dystonia patients and healthy controls [68, 69]. Thus, these studies reported different, partly contradictory results. Differences in design, imaging methodology, small sample sizes, subtypes of dystonia and methodological differences across studies may probably account in large part for these inconsistent and controversial findings. Therefore, it remains unclear what pattern of GM alterations was shared in different subtypes of idiopathic dystonia.
To address this question, we applied meta-analysis of published structural neuroimaging studies in patients with different subtypes of idiopathic dystonia. Although there was one meta-analysis study that included nine studies comparing GM changes between 199 idiopathic focal dystonia and 247 healthy controls in 2011 [72], it only included studies of focal dystonia and a growing number of studies in idiopathic dystonia have been published since then. The primary aim of the current meta-analysis was to identify consistent GM changes across different subtypes of idiopathic dystonia. The anisotropic effect size-based signed differential mapping (AES-SDM), a quantitative voxel-based meta-analytic method based on well-established statistics accounting for within- and between-study variance, has been widely used in various neuropsychiatric diseases [56] and was applied in the current study. The second aim was to perform meta-regression analysis to explore the potential associations between GM changes and clinical features, namely age and disease duration in idiopathic dystonia.
Materials and methods
Searching method
Studies published from January 1990 to November 10th, 2021 were searched systematically and comprehensively in the PubMed, Embase and Web of Science by two researchers respectively using the keywords (“dystonia” OR “focal dystonia” OR “cervical dystonia” OR “blepharospasm” OR “writer's cramp” OR “focal hand dystonia” OR “laryngeal dystonia” OR “spasmodic dysphonia” OR “oromandibular dystonia” OR “musician's dystonia” OR “craniocervical dystonia” OR “meige’s syndrome” OR “segmental dystonia” OR “generalized dystonia”) AND (“voxel*” OR “ VBM” OR “morphometry”). The reference lists of included studies and relevant articles were further searched to obtain potential studies.
Inclusion/exclusion criteria
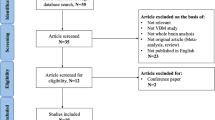
The studies were considered for inclusion if they (1) reported VBM (GM density or volume) comparison between idiopathic dystonia patients and healthy control subjects from an original study; (2) reported whole-brain results of changes in three-dimensional coordinates (x, y, z) (Talairach or Montreal Neurological Institute (MNI)); (3) used thresholds for significance corrected for multiple comparisons or uncorrected with spatial extent thresholds; and (4) published in English with peer review. In such cases, the study with the largest group size and comprehensive information needed was selected if the data of patient group overlapped with the inter-subgroups in another study. The studies were excluded if the stereotactic coordinates were not available even after contacting corresponding authors by emails. The study selection procedures were summarized in Fig. 1.
Data extraction
Peak coordinates and their effect sizes (t-values, z scores, or p-values) with significant differences between idiopathic dystonia and healthy controls in GM changes were extracted in each study according to the SDM tutorial. Two authors conducted the study selection and extracted the data that needed to perform the meta-analysis independently.
Quality assessment
The quality of each included study was assessed using a 15-point checklist (Table S1), which was developed based on previous meta-analytic studies [33, 36] and focused on both the clinical and demographic aspects of each study samples and imaging-specific methodology used in the studies.
Meta-analysis of VBM studies
A voxel-based meta-analytic approach was conducted using SDM software package (www.sdmproject.com) to analyze regional group differences in GM. The approach has been described in detail previously [56, 57]. First, a file containing samples sizes, peak coordinates, effect sizes and clinical characteristics (e.g., mean age and disease duration of idiopathic dystonia patients) were created. Then, a mean analysis was conducted to compare the GM changes between idiopathic dystonia and healthy controls. The default kernel size and statistical thresholds (full width at half maximum (FWHM) = 20 mm, p = 0.005, peak height threshold = 1, extent threshold = 10 voxels) were used, which have been validated to optimize the sensitivity and specificity and to produce a desirable balance between Type I and II error rates.
Q statistics were calculated to assess the heterogeneity between studies. Egger’s tests were carried out to detect potential publication bias (p < 0.05 indicates obvious publication bias), and funnel plots were established for visual inspection. p < 0.005 and an asymmetric plot were recognized as significant. Jackknife sensitivity analysis was conducted to assess the robustness of the main meta-analytical output by removing one study at a time and repeating the analysis. Age and disease duration were used as regressors in the meta-regression to explore whether they were associated with GM changes in patients with idiopathic dystonia, using a stringent threshold (p ≤ 0.005, extent threshold = 10 voxels). Finally, subgroup analyses of several common phenotypes of dystonia, including blepharospasm (BSP), cervical dystonia (CD), focal hand dystonia (FHD), task-specific dystonia (TSD) and non-task-specific dystonia (NTSD), were performed, separately. The subgroup analysis was not conducted for generalized dystonia, as just one included VBM study explored the patients with generalized dystonia as a subgroup in the study.
Results
Twenty-eight studies, including 701 patients with idiopathic dystonia and 712 healthy controls, were included in the meta-analysis. Among the included studies, six studies reported blepharospasm [15, 25, 35, 45, 64, 69], six studies reported cervical dystonia [9, 20, 22, 28, 32, 53], two studies reported spasmodic dysphonia [37, 62], one study reported meige’s syndrome [40], one study reported craniocervical dystonia [51], four studies reported focal hand dystonia [18, 30, 44, 71], one study reported embouchure dystonia [43], seven studies reported a mixed types of dystonia [7, 13, 21, 24, 50, 58, 68]. The demographic and clinical characteristics of the included studies were summarized in Table 1. Quality assessment of each study was done and scores were shown in Table 1. All the studies included in this study got relatively high scores in the quality assessment.
Regional differences in gray matter changes
Compared with healthy controls, patients with idiopathic dystonia showed increased GM in bilateral precentral and postcentral gyri, bilateral putamen and pallidum, right insula, and left supramarginal gyrus, while decreased GM in bilateral temporal poles and bilateral SMAs, right angular gyrus, inferior parietal gyrus and precuneus, left insula and left inferior frontal gyrus (Tables 2 and 3, Figs. 2 and 3).
Analysis of sensitivity, heterogeneity and publication bias
The jackknife sensitivity analyses revealed that the above-mentioned regional differences were highly robust in idiopathic dystonia (Tables 2 and 3). Heterogeneity analyses using Q statistics indicated that there was no variability between studies. The funnel plots showed no obvious asymmetric of all significant brain regions (Figs. S1–S11). The quantitative assessment measured by Egger’s tests revealed publication bias in 2 areas (Table 3).
Meta-regression analysis
Increased GM in the left precentral and postcentral gyrus were positively associated with both age at scanning time and disease duration in patients with idiopathic dystonia. While GM reductions of right precuneus and left inferior frontal gyrus were negatively associated with age at scanning time. GM reduction in left inferior frontal gyrus was also negatively associated with disease duration.
Subgroup analyses
The results of subgroup meta-analyses were presented in the Supplementary Material.
The subgroup analyses of studies reporting GM volume changes between groups included 23 datasets comprising 593 idiopathic dystonia patients and 594 HC (Table S12 and S13 and Figs. S22 and S23), and the results were broadly consistent with the findings when all 28 studies included. The subgroup analyses for all subtypes of dystonia shared clusters of changes with the main results including precentral and postcentral gyri, bilateral striatum, right inferior parietal gyrus and left temporal pole (Tables S2-S11, Figs. S12-S21). In addition, we noted decreased GM in cerebellum in the subgroup analyses of patients with CD, FHD and TSD, respectively (Tables S3, S7 and S9, Figs. S13, S17 and S19).
Discussion
The current meta-analysis revealed consistent widespread GM alterations between idiopathic dystonia and healthy subjects. Patients with idiopathic dystonia had increased GM in bilateral precentral and postcentral gyri and bilateral lentiform nuclei, right insula and left supramarginal gyrus, while decreased GM in bilateral temporal poles and bilateral SMAs, left insula and left inferior frontal gyrus, right angular gyrus, inferior parietal gyrus and precuneus. Our meta-analysis demonstrated that not only GM alterations in sensorimotor cortex-basal ganglia circuits but widespread regions outside were involved in the different subtypes of idiopathic dystonia, which support that dystonia was a network disorder and may provide neuroanatomical basis for the nonmotor symptoms of dystonia.
The current meta-analysis identified that dystonia had consistent increased GM in bilateral lentiform nuclei and precentral cortices. Both structural and functional abnormalities in lentiform nuclei and sensorimotor cortices were also found in dystonia using diffusion tensor imaging and functional MRI techniques [11, 26, 41, 59]. Lentiform nuclei were involved in both the direct striato-pallidal pathway supporting cortically generated movement, and the indirect pathway via lateral globus pallidus which inhibits thalamo-cortical excitation of premotor cortical areas. Alterations of both pathways may result in dystonia. A previous review of clinical reports found that dystonia was the most frequent movement disorder in patients with focal lesions of the basal ganglia, especially lesions confined to the lentiform nucleus which accounted for majority (92%) of these dystonia cases [6]. Dystonia was also reported in the patient after infarction of SMA, a region involved in motor preparation and execution [49]. The inferior frontal gyri are involved in the prevention of unwanted movement by “calling out” or compensating for motor areas responsible for the final motor output [23]. Increased activation in inferior frontal gyri were reported both in genetic and idiopathic dystonia as compensatory to sensorimotor loop dysfunction [29, 54]. GM reductions found in the left inferior frontal gyrus and bilateral SMAs were consistent with previous finding of inhibitory control loss in patients with dystonia [54], which may lead to a lack of movement restriction. The meta-regression revealed GM change of the left inferior frontal gyrus was negatively associated with disease duration and age. Studies revealed that pathophysiological deficits in idiopathic dystonia mainly include reduced inhibition at many levels of the motor system including striatum and motor cortex, and increased plasticity [38, 55]. The findings that abnormal cholinergic transmission and altered dopamine receptors signaling in striatum identified by DYT1 mouse and human, may indicated that loss of mutual control between striatal acetylcholine and dopamine and the subsequent neurochemical imbalance are responsible for the impairment of corticostriatal synaptic plasticity [46, 52, 66].
The GM changes of bilateral postcentral gyri and left supramarginal gyrus were found in current analysis, which were supported by functional neuroimaging studies revealed altered connectivity within the sensorimotor regions in dystonia [39, 42]. Studies in dystonia found that sensorimotor integration was defective, and abnormal central processing of sensory input may contribute to the increased motor cortical excitability in dystonia [1, 16]. Besides abnormality of sensory-motor integration, deficits of sensory system also occur in patients with idiopathic dystonia. Sensory symptoms such as burning sensation, grittiness or dryness of the eyes and photophobia in blepharospasm, pain in cervical dystonia and upper limb dystonia, and sensory deficits such as difficulty in discriminating sensory stimuli in both spatial and temporal domains were reported. Sensory dysfunctions including abnormal proprioceptive, nociceptive and tactile information processing have also been reported in patents with dystonia [63, 67]. In addition, sensory trick, a well-recognized clinical feature in dystonia, demonstrates that the sensory inputs may modulate dystonic symptoms. Studies in patients with dystonia using somatosensory evoked potentials and magnetoencephalography showed a disorganization of somatotopic representations and a lack of intracortical inhibition in sensory cortex in dystonia [47, 65]. Although the neuroanatomical correlates of sensory abnormalities for dystonia may include cortical and subcortical areas, the consistent GM alterations found in the meta-analysis emphasized the involvement of primary sensory cortex in dystonia.
The insula subserves a wide variety of functions ranging from sensorimotor and affective processing, autonomic information to high-level cognition [4]. In the current meta-analysis, GM alterations in insula were detected in idiopathic dystonia, in line with the abnormal functional activity in insula revealed by neuroimaging studies in dystonia [4]. Cortical thickness and functional activity of insula were correlated with symptom severity in patients with spasmodic dysphonia [62]. The reorganized structural segregation of insular-subregions identified in spasmodic dysphonia was found to be associated with the different aspects of sensorimotor and cognitive control of speech production [5]. As the insula has an integrative role in linking information from diverse functional systems, we hypothesize that the insula may be involved in the pathophysiology of both motor and nonmotor symptoms of idiopathic dystonia, which needs further study. We also noticed the asymmetric deficits of insula found in the current meta-analysis. One possible reason for the asymmetry finding may be the asymmetric functions of insula itself, such as the insula asymmetry was associated with lateralized of gesture and language, and different forms of empathy [8, 27]; another one may be the clinical heterogeneity of included patients and different insular subregions may be involved; lastly, the spatial reorganization of insula and asymmetry of insula-sensorimotor circuit may occur in disease conditions [5, 31].
GM reductions in bilateral temporal poles, right precuneus, inferior parietal gyrus and angular gyrus were also detected in the current meta-analysis. The temporal pole is a complex structure with high-order brain functions including multimodal sensory integration, autobiographic memory, motion and social cognition and semantic memory [34]. Precuneus is a critical structure involved in the visuospatial system and plays a central role in highly integrated tasks including visuospatial imagery and cognitive functions including episodic memory, self-related information processing and consciousness [12]. Decreased GM within these regions may relate to visuospatial and cognitive dysfunction reported in patients with idiopathic dystonia [14, 19]. The parietal cortex provides a sensorimotor interface for the control of higher-order and multimodal integration processes, which are necessary to inform and guide movement execution [61]. While the GM atrophy in right inferior parietal gyrus may be associated with dysfunction of supramodal spatial processing and perception of sound movements [10]. The right angular gyrus is associated with movement awareness and involved in the inhibition of the inappropriate response across a variety of go/no-go tasks [48]. GM reduction in right angular gyrus in current analysis is in line with a previous study [17], which used interleaved transcranial magnetic stimulation (TMS)/fMRI when subjects performed right-hand tasks successively in executive, imagery or rest mode, and detected reduction of cortical activation in right angular gyrus, indicating that the awareness for movement execution was decreased after TMS in CD patients. The right angular gyrus also has a role in semantic processing, word reading and comprehension, number processing, default mode network, memory retrieval, attention and spatial cognition, reasoning and social cognition [60]. Whether GM alteration within this region is associated with cognition dysfunction in patients with dystonia needs further investigations.
Some limitations exist in the current meta-analysis. First, unpublished international studies, studies not published in English, and studies that the stereotactic coordinates were not available even after we contacted the authors were excluded. Second, the heterogeneity of the methodologies of the included studies, such as various preprocessing protocols, smoothing kernels, and statistical thresholding methods could not be totally ruled out. Third, the current meta-analysis was based on the pooling of stereotactic coordinates with significant differences rather than on raw data from the included studies, which may lower the accuracy of the results. Fourth, the gray matter volume or density was not specified in the results as we included studies exploring either gray matter volume or density as previous studies did[70, 72], although most (23/28) of the included studies reported gray matter volume changes. The subgroup analyses of the 23 studies reporting gray matter volume changes between idiopathic dystonia patients and healthy controls were conducted, and the results were largely remained when compared with those when 28 studies included.
Conclusions
In conclusion, the current meta-analysis revealed shared abnormal gray matter pattern in different types of idiopathic dystonia, which includes sensorimotor-basal ganglia circuits and regions that may associated with nonmotor symptoms. These findings may prompt the understanding of key pathophysiology of idiopathic dystonia.
References
Abbruzzese G, Marchese R, Buccolieri A, Gasparetto B, Trompetto C (2001) Abnormalities of sensorimotor integration in focal dystonia: a transcranial magnetic stimulation study. Brain J Neurol 124:537–545
Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, Hallett M, Jankovic J, Jinnah HA, Klein C, Lang AE, Mink JW, Teller JK (2013) Phenomenology and classification of dystonia: a consensus update. Mov Disord Off J Mov Disord Soc 28:863–873
Ashburner J, Friston KJ (2000) Voxel-based morphometry–the methods. Neuroimage 11:805–821
Augustine JR (1996) Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 22:229–244
Battistella G, Kumar V, Simonyan K (2018) Connectivity profiles of the insular network for speech control in healthy individuals and patients with spasmodic dysphonia. Brain Struct Funct 223:2489–2498
Bhatia KP, Marsden CD (1994) The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain J Neurol 117(Pt 4):859–876
Bianchi S, Fuertinger S, Huddleston H, Frucht SJ, Simonyan K (2019) Functional and structural neural bases of task specificity in isolated focal dystonia. Mov Disord Off J Mov Disord Soc 34:555–563
Biduła SP, Króliczak G (2015) Structural asymmetry of the insula is linked to the lateralization of gesture and language. Eur J Neurosci 41:1438–1447
Burciu RG, Hess CW, Coombes SA, Ofori E, Shukla P, Chung JW, McFarland NR, Wagle Shukla A, Okun MS, Vaillancourt DE (2017) Functional activity of the sensorimotor cortex and cerebellum relates to cervical dystonia symptoms. Hum Brain Mapp 38:4563–4573
Bushara KO, Weeks RA, Ishii K, Catalan MJ, Tian B, Rauschecker JP, Hallett M (1999) Modality-specific frontal and parietal areas for auditory and visual spatial localization in humans. Nat Neurosci 2:759–766
Carbon M, Kingsley PB, Su S, Smith GS, Spetsieris P, Bressman S, Eidelberg D (2004) Microstructural white matter changes in carriers of the DYT1 gene mutation. Ann Neurol 56:283–286
Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain J Neurol 129:564–583
Cerasa A, Nisticò R, Salsone M, Bono F, Salvino D, Morelli M, Arabia G, Quattrone A (2014) Neuroanatomical correlates of dystonic tremor: a cross-sectional study. Parkinsonism Relat Disord 20:314–317
Chillemi G, Calamuneri A, Morgante F, Terranova C, Rizzo V, Girlanda P, Ghilardi MF, Quartarone A (2017) Spatial and temporal high processing of visual and auditory stimuli in cervical dystonia. Front Neurol 8:66
Chirumamilla VC, Dresel C, Koirala N, Gonzalez-Escamilla G, Deuschl G, Zeuner KE, Muthuraman M, Groppa S (2019) Structural brain network fingerprints of focal dystonia. Ther Adv Neurol Disord. https://doi.org/10.1177/1756286419880664
Cuny E, Ghorayeb I, Guehl D, Escola L, Bioulac B, Burbaud P (2008) Sensory motor mismatch within the supplementary motor area in the dystonic monkey. Neurobiol Dis 30:151–161
de Vries PM, de Jong BM, Bohning DE, Hinson VK, George MS, Leenders KL (2012) Reduced parietal activation in cervical dystonia after parietal TMS interleaved with fMRI. Clin Neurol Neurosurg 114:914–921
Delmaire C, Vidailhet M, Elbaz A, Bourdain F, Bleton JP, Sangla S, Meunier S, Terrier A, Lehéricy S (2007) Structural abnormalities in the cerebellum and sensorimotor circuit in writer’s cramp. Neurology 69:376–380
Delnooz CC, Pasman JW, Beckmann CF, van de Warrenburg BP (2013) Task-free functional MRI in cervical dystonia reveals multi-network changes that partially normalize with botulinum toxin. PLoS ONE 8:e62877
Delnooz CC, Pasman JW, van de Warrenburg BP (2015) Dynamic cortical gray matter volume changes after botulinum toxin in cervical dystonia. Neurobiol Dis 73:327–333
Draganski B, Schneider SA, Fiorio M, Klöppel S, Gambarin M, Tinazzi M, Ashburner J, Bhatia KP, Frackowiak RS (2009) Genotype-phenotype interactions in primary dystonias revealed by differential changes in brain structure. Neuroimage 47:1141–1147
Draganski B, Thun-Hohenstein C, Bogdahn U, Winkler J, May A (2003) “Motor circuit” gray matter changes in idiopathic cervical dystonia. Neurology 61:1228–1231
Duann JR, Ide JS, Luo X, Li CS (2009) Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci Off J Soc Neurosci 29:10171–10179
Egger K, Mueller J, Schocke M, Brenneis C, Rinnerthaler M, Seppi K, Trieb T, Wenning GK, Hallett M, Poewe W (2007) Voxel based morphometry reveals specific gray matter changes in primary dystonia. Mov Disord Off J Mov Disord Soc 22:1538–1542
Etgen T, Mühlau M, Gaser C, Sander D (2006) Bilateral grey-matter increase in the putamen in primary blepharospasm. J Neurol Neurosurg Psychiatry 77:1017–1020
Fabbrini G, Pantano P, Totaro P, Calistri V, Colosimo C, Carmellini M, Defazio G, Berardelli A (2008) Diffusion tensor imaging in patients with primary cervical dystonia and in patients with blepharospasm. Eur J Neurol 15:185–189
Fan Y, Duncan NW, de Greck M, Northoff G (2011) Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev 35:903–911
Filip P, Gallea C, Lehéricy S, Bertasi E, Popa T, Mareček R, Lungu OV, Kašpárek T, Vaníček J, Bareš M (2017) Disruption in cerebellar and basal ganglia networks during a visuospatial task in cervical dystonia. Mov Disord Off J Mov Disord Soc 32:757–768
Gallea C, Herath P, Voon V, Lerner A, Ostuni J, Saad Z, Thada S, Solomon J, Horovitz SG, Hallett M (2018) Loss of inhibition in sensorimotor networks in focal hand dystonia. NeuroImage Clin 17:90–97
Garraux G, Bauer A, Hanakawa T, Wu T, Kansaku K, Hallett M (2004) Changes in brain anatomy in focal hand dystonia. Ann Neurol 55:736–739
Goelman G, Dan R, Růžička F, Bezdicek O, Jech R (2021) Asymmetry of the insula-sensorimotor circuit in Parkinson’s disease. Eur J Neurosci 54:6267–6280
Gracien RM, Petrov F, Hok P, van Wijnen A, Maiworm M, Seiler A, Deichmann R, Baudrexel S (2019) Multimodal quantitative mri reveals no evidence for tissue pathology in idiopathic cervical dystonia. Front Neurol. https://doi.org/10.3389/fneur.2019.00914
Han Q, Hou Y, Shang H (2018) A voxel-wise meta-analysis of gray matter abnormalities in essential tremor. Front Neurol 9:495
Herlin B, Navarro V, Dupont S (2021) The temporal pole: from anatomy to function-A literature appraisal. J Chem Neuroanat 113:101925
Horovitz SG, Ford A, Najee-Ullah MA, Ostuni JL, Hallett M (2012) Anatomical correlates of blepharospasm. Trans Neurodegener 1:12–12
Iwabuchi SJ, Krishnadas R, Li C, Auer DP, Radua J, Palaniyappan L (2015) Localized connectivity in depression: a meta-analysis of resting state functional imaging studies. Neurosci Biobehav Rev 51:77–86
Kirke DN, Battistella G, Kumar V, Rubien-Thomas E, Choy M, Rumbach A, Simonyan K (2017) Neural correlates of dystonic tremor: a multimodal study of voice tremor in spasmodic dysphonia. Brain Imaging Behav 11:166–175
Kojovic M, Pareés I, Kassavetis P, Palomar FJ, Mir P, Teo JT, Cordivari C, Rothwell JC, Bhatia KP, Edwards MJ (2013) Secondary and primary dystonia: pathophysiological differences. Brain J Neurol 136:2038–2049
Li Z, Prudente CN, Stilla R, Sathian K, Jinnah HA, Hu X (2017) Alterations of resting-state fMRI measurements in individuals with cervical dystonia. Hum Brain Mapp 38:4098–4108
Liu J, Li L, Chen L, Liu R, Jiang Y, Fang J, Wang D, Liu Z, Ouyang J (2020) Grey matter changes in Meige syndrome: a voxel-based morphology analysis. Sci Rep 10:14533
Løkkegaard A, Herz DM, Haagensen BN, Lorentzen AK, Eickhoff SB, Siebner HR (2016) Altered sensorimotor activation patterns in idiopathic dystonia-an activation likelihood estimation meta-analysis of functional brain imaging studies. Hum Brain Mapp 37:547–557
Ma LY, Wang ZJ, Ma HZ, Feng T (2021) Hyper- and hypo-connectivity in sensorimotor network of drug-naïve patients with cervical dystonia. Parkinsonism Relat Disord 90:15–20
Mantel T, Altenmüller E, Li Y, Meindl T, Jochim A, Lee A, Zimmer C, Dresel C, Haslinger B (2019) Abnormalities in grey matter structure in embouchure dystonia. Parkinsonism Relat Disord 65:111–116
Mantel T, Meindl T, Li Y, Jochim A, Gora-Stahlberg G, Kräenbring J, Berndt M, Dresel C, Haslinger B (2018) Network-specific resting-state connectivity changes in the premotor-parietal axis in writer’s cramp. NeuroImage Clin 17:137–144
Martino D, Di Giorgio A, D’Ambrosio E, Popolizio T, Macerollo A, Livrea P, Bertolino A, Defazio G (2011) Cortical gray matter changes in primary blepharospasm: a voxel-based morphometry study. Mov Disord Off J Mov Disord Soc 26:1907–1912
Mazere J, Dilharreguy B, Catheline G, Vidailhet M, Deffains M, Vimont D, Ribot B, Barse E, Cif L, Mazoyer B, Langbour N, Pisani A, Allard M, Lamare F, Guehl D, Fernandez P, Burbaud P (2021) Striatal and cerebellar vesicular acetylcholine transporter expression is disrupted in human DYT1 dystonia. Brain J Neurol 144:909–923
Meunier S, Garnero L, Ducorps A, Mazières L, Lehéricy S, du Montcel ST, Renault B, Vidailhet M (2001) Human brain mapping in dystonia reveals both endophenotypic traits and adaptive reorganization. Ann Neurol 50:521–527
Nee DE, Wager TD, Jonides J (2007) Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci 7:1–17
Nishimura K, Uehara T, Toyoda K (2014) Early-onset dystonia after supplementary motor area infarction. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc 23:1267–1268
Obermann M, Yaldizli O, De Greiff A, Lachenmayer ML, Buhl AR, Tumczak F, Gizewski ER, Diener HC, Maschke M (2007) Morphometric changes of sensorimotor structures in focal dystonia. Mov Disord Off J Mov Disord Soc 22:1117–1123
Piccinin CC, Piovesana LG, Santos MCA, Guimarães RP, de Campos BM, Rezende TJR, Campos LS, Torres FR, Amato-Filho AC, França MC, Lopes-Cendes I, Cendes F, D’Abreu A (2015) Diffuse decreased gray matter in patients with idiopathic craniocervical dystonia: a voxel-based morphometry study. Front Neurol. https://doi.org/10.3389/fneur.2014.00283
Pisani A, Martella G, Tscherter A, Bonsi P, Sharma N, Bernardi G, Standaert DG (2006) Altered responses to dopaminergic D2 receptor activation and N-type calcium currents in striatal cholinergic interneurons in a mouse model of DYT1 dystonia. Neurobiol Dis 24:318–325
Prell T, Peschel T, Köhler B, Bokemeyer MH, Dengler R, Günther A, Grosskreutz J (2013) Structural brain abnormalities in cervical dystonia. BMC Neurosci 14:123
Premi E, Diano M, Gazzina S, Cauda F, Gualeni V, Tinazzi M, Fiorio M, Liberini P, Lazzarini C, Archetti S, Biasiotto G, Turla M, Bertasi V, Cotelli M, Gasparotti R, Padovani A, Borroni B (2016) Functional connectivity networks in asymptomatic and symptomatic DYT1 carriers. Mov Disord Off J Mov Disord Soc 31:1739–1743
Prescott IA, Dostrovsky JO, Moro E, Hodaie M, Lozano AM, Hutchison WD (2013) Reduced paired pulse depression in the basal ganglia of dystonia patients. Neurobiol Dis 51:214–221
Radua J, Mataix-Cols D, Phillips ML, El-Hage W, Kronhaus DM, Cardoner N, Surguladze S (2012) A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry J Assoc Eur Psychiatr 27:605–611
Radua J, Rubia K, Canales-Rodríguez EJ, Pomarol-Clotet E, Fusar-Poli P, Mataix-Cols D (2014) Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front Psych 5:13
Ramdhani RA, Kumar V, Velickovic M, Frucht SJ, Tagliati M, Simonyan K (2014) What’s special about task in dystonia? A voxel-based morphometry and diffusion weighted imaging study. Mov Disord 29:1141–1150
Schmidt KE, Linden DE, Goebel R, Zanella FE, Lanfermann H, Zubcov AA (2003) Striatal activation during blepharospasm revealed by fMRI. Neurology 60:1738–1743
Seghier ML (2013) The angular gyrus: multiple functions and multiple subdivisions. Neurosci Rev J Bring Neurobiol Neurol Psychiatry 19:43–61
Sereno MI, Huang RS (2014) Multisensory maps in parietal cortex. Curr Opin Neurobiol 24:39–46
Simonyan K, Ludlow CL (2012) Abnormal structure-function relationship in spasmodic dysphonia. Cereb Cortex 22:417–425
Suttrup I, Oberdiek D, Suttrup J, Osada N, Evers S, Marziniak M (2011) Loss of sensory function in patients with idiopathic hand dystonia. Mov Disord Off J Mov Disord Soc 26:107–113
Suzuki Y, Kiyosawa M, Wakakura M, Mochizuki M, Ishii K (2011) Gray matter density increase in the primary sensorimotor cortex in long-term essential blepharospasm. Neuroimage 56:1–7
Tamura Y, Matsuhashi M, Lin P, Ou B, Vorbach S, Kakigi R, Hallett M (2008) Impaired intracortical inhibition in the primary somatosensory cortex in focal hand dystonia. Mov Disord Off J Mov Disord Soc 23:558–565
Tassone A, Martella G, Meringolo M, Vanni V, Sciamanna G, Ponterio G, Imbriani P, Bonsi P, Pisani A (2021) Vesicular acetylcholine transporter alters cholinergic tone and synaptic plasticity in DYT1 dystonia. Mov Disord Off J Mov Disord Soc. https://doi.org/10.1002/mds.28698
Trompetto C, Currà A, Buccolieri A, Suppa A, Abbruzzese G, Berardelli A (2006) Botulinum toxin changes intrafusal feedback in dystonia: a study with the tonic vibration reflex. Mov Disord Off J Mov Disord Soc 21:777–782
Waugh JL, Kuster JK, Levenstein JM, Makris N, Multhaupt-Buell TJ, Sudarsky LR, Breiter HC, Sharma N, Blood AJ (2016) Thalamic volume is reduced in cervical and laryngeal dystonias. PLoS ONE 11:e0155302
Yang J, Luo C, Song W, Chen Q, Chen K, Chen X, Huang X, Gong Q, Shang H (2013) Altered regional spontaneous neuronal activity in blepharospasm: a resting state fMRI study. J Neurol 260:2754–2760
Yang J, Pan P, Song W, Huang R, Li J, Chen K, Gong Q, Zhong J, Shi H, Shang H (2012) Voxelwise meta-analysis of gray matter anomalies in Alzheimer’s disease and mild cognitive impairment using anatomic likelihood estimation. J Neurol Sci 316:21–29
Zeuner KE, Knutzen A, Granert O, Götz J, Wolff S, Jansen O, Dressler D, Hefter H, Hallett M, Deuschl G, van Eimeren T, Witt K (2015) Increased volume and impaired function: the role of the basal ganglia in writer’s cramp. Brain Behav 5:e00301
Zheng Z, Pan P, Wang W, Shang H (2012) Neural network of primary focal dystonia by an anatomic likelihood estimation meta-analysis of gray matter abnormalities. J Neurol Sci 316:51–55
Acknowledgements
The authors thank all authors of the included studies.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81971071), and the Applied Basic Research Programs of Science and Technology Department of Sichuan Province (No. 2021YJ0447).
Author information
Authors and Affiliations
Contributions
XH: conception, organization, execution, data acquisition, statistical analysis and manuscript preparation. JYL: project execution and data acquisition, manuscript review and critique. HFS: manuscript review and critique. JY: conception, organization, statistical analysis, manuscript review and critique, responsible for the overall content as the guarantor.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there are no financial or personal relationships with other people showing conflict of interest in this paper.
Ethical standards
All studies in this review have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, X., Lin, J., Shang, H. et al. Voxel-based meta-analysis of gray matter abnormalities in idiopathic dystonia. J Neurol 269, 2862–2873 (2022). https://doi.org/10.1007/s00415-022-10961-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-10961-y