Abstract
Tremor, affecting a dystonic body part, is a frequent feature of adult-onset dystonia. However, our understanding of dystonic tremor pathophysiology remains ambiguous as its interplay with the main co-occurring disorder, dystonia, is largely unknown. We used a combination of functional MRI, voxel-based morphometry and diffusion-weighted imaging to investigate similar and distinct patterns of brain functional and structural alterations in patients with dystonic tremor of voice (DTv) and isolated spasmodic dysphonia (SD). We found that, compared to controls, SD patients with and without DTv showed similarly increased activation in the sensorimotor cortex, inferior frontal (IFG) and superior temporal gyri, putamen and ventral thalamus, as well as deficient activation in the inferior parietal cortex and middle frontal gyrus (MFG). Common structural alterations were observed in the IFG and putamen, which were further coupled with functional abnormalities in both patient groups. Abnormal activation in left putamen was correlated with SD onset; SD/DTv onset was associated with right putaminal volumetric changes. DTv severity established a significant relationship with abnormal volume of the left IFG. Direct patient group comparisons showed that SD/DTv patients had additional abnormalities in MFG and cerebellar function and white matter integrity in the posterior limb of the internal capsule. Our findings suggest that dystonia and dystonic tremor, at least in the case of SD and SD/DTv, are heterogeneous disorders at different ends of the same pathophysiological spectrum, with each disorder carrying a characteristic neural signature, which may potentially help development of differential markers for these two conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tremor, affecting a dystonic body part, is a frequent feature of adult-onset dystonia with prevalence of up to 70 % in patients with dystonia (Defazio et al. 2015; Jankovic et al. 1991; Deuschl et al. 1998). Clinically, dystonic tremor (DT) is characterized by involuntary rhythmic oscillatory movements and may resemble symptoms of dystonia, essential tremor (ET), or Parkinson tremor, oftentimes complicating the diagnosis and management of these disorders. Despite the existing consensus statement on tremor (Deuschl et al. 1998), there is an ongoing debate as to whether DT is a disorder distinct from dystonia and ET or whether these disorders are more heterogeneous conditions within the same clinical spectrum (Defazio et al. 2015; Elble 2013; Shaikh et al. 2015).
Several studies have lent support to the concept that DT and ET are pathophysiologically distinct disorders by showing that patients with DT, compared to ET, have characteristic EMG patterns that are dependent on age of onset of disorder and symptom progression (Munchau et al. 2001), which may be associated with increased brainstem excitability (Nistico et al. 2012) and abnormal temporal discrimination (Tinazzi et al. 2013). A recent neuroimaging study examining microstructural brain organization in a mixed group of patients with head and limb DT has reported increased gray matter volume and cortical thickness in the left sensorimotor cortex as opposed to ET patients who showed atrophy in the anterior cerebellar cortex (Cerasa et al. 2014). Collectively, these studies proposed that DT and ET may have different courses and pathophysiology and therefore may be considered as clinically separate conditions.
Nevertheless, our understanding of DT phenomenology still remains ambiguous, as its interplay with the main co-occurring disorder, dystonia, is largely unknown. Considerable progress has been recently made in identification of brain alterations in different forms of dystonia, pointing to abnormal function and structure within the basal ganglia-thalamo-cortical and cerebello-thalamo-cortical circuits (Neychev et al. 2011; Ramdhani and Simonyan 2013). However, the available studies contributed little to complete the understanding of DT, which is an integral clinical feature of the large number of dystonia cases, as they fell short of examining brain organization in patients with dystonia with and without DT, directly.
Here, we used multimodal neuroimaging, including functional MRI (fMRI) during symptomatic task production as well as structural voxel-based morphometry (VBM) and diffusion-weighted imaging (DWI), to investigate similar and distinct patterns of brain function and structure in patients with DT of voice (DTv) and isolated spasmodic dysphonia (SD). SD is a form of laryngeal dystonia, which predominantly affects speech production, leading to the inability to produce certain vowels (adductor form, ADSD) or voiceless consonants (abductor form, ABSD) due to involuntary, uncontrolled spasms in the laryngeal muscles. In about one-third of SD patients, dystonic symptoms are associated with DTv, characterized by rhythmic alterations in voice pitch and loudness and by the inability to sustain a vowel for more than a few seconds, which additionally challenges already impaired vocal communication in SD patients (White et al. 2011; Gurey et al. 2013). Based on earlier studies in DT vs. ET, as well as on similarities and differences in clinical features between SD and DTv, we hypothesized that while SD patients with and without DTv would have similar structural and functional alterations within the sensorimotor cortical regions, SD patients would have additional abnormalities in the subcortical regions (e.g., putamen) and SD/DTv patients would show distinct abnormalities in the cerebellum.
Methods
Subjects
Twenty patients with combined SD and DTv (hereafter defined as SD/DTv; age 60.0 ± 10.1 years; 18 females/2 males; 10 ADSD/10 ABSD), 20 patients with isolated SD (age 54.4 ± 8.3 years; 16 females/4 males; 10 ADSD/10 ABSD), and 20 healthy controls (age 53.8 ± 9.9 years; 16 females/4 males) participated in the study (Table 1). All subjects were monolingual native English speakers and right-handed as determined by the Edinburgh Handedness Inventory. No participant had any past or present history of any neurological (except SD and SD/DTv in patient groups), psychiatric or laryngeal problems. All SD and SD/DTv subjects were fully symptomatic and did not have dystonia or tremor in other body parts. The diagnosis of DTv was made at the same time as the diagnosis of SD, although there is a possibility that these two disorders might not have started concurrently. Patients receiving botulinum toxin treatment participated in the study only at the end of their treatment cycle, when fully symptomatic, at least 3 months post injection. There were no significant differences between subject groups in their age, gender, disorder onset, duration, or severity (all p ≥ 0. 06) (Table 1). Prior to participation, all subjects gave written informed consent, which was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai.
Data acquisition
All scans were acquired on a 3.0 T Philips MRI scanner equipped with an 8-channel head coil. Whole-brain fMRI data were obtained using a gradient-weighted echo planar imaging (EPI) pulse sequence (sparse-sampling event-related design: TR = 2 s per volume and 10.6 s between volumes, TE = 30 ms, FA = 90°, FOV = 240 mm, voxel size =3.75 × 3.75 mm, 36 slices with 4-mm slice thickness) and blood oxygen level dependent (BOLD) contrast. Experimental design included ten SD- and DTv-symptom provoking sentences (for example, “Are the olives large?”, “He is hiding behind the house”), which were produced by all subjects one at a time. Subjects’ speech production during the fMRI session was audio recorded for later analysis, which revealed that production of all sentences was symptomatic in all patients. To minimize head movements during scanning, subjects’ head was tightly cushioned, and they were instructed to remain motionless throughout the scan; possible movements were monitored online. High-order shimming was performed before EPI acquisition to minimize EPI distortions and ensure homogeneity of the magnetic field within the scanner.
A structural high-resolution T1-weighted MRI was acquired in each subject using 3D magnetization prepared rapid acquisition gradient echo sequence (3D-MPRAGE: TR = 7.5 ms, TE = 3.4 ms, TI = 819 ms, FA = 8°, FOV = 210 mm, 172 slices with 1 mm slice thickness) and used as an anatomical reference for fMRI data and for VBM analysis. Diffusion-weighted images (DWI) were acquired using a single-shot spin-echo EPI sequence with 54 contiguous axial slices along 60 noncollinear directions, in addition to one volume without diffusion encoding (b0 image) (TR = 13,000 ms, FOV = 240 mm, matrix =96 × 96 mm zero-filled to 256 × 256 mm, slice thickness 2.4 mm, b = 1000s/mm2).
Data analysis
Data analysis was performed using a combination of AFNI, FSL and SPM8 software packages. For functional datasets, the first four volumes in each subject’s time series were discarded to account for the magnetization equilibrium. All images were visually inspected for motion artifacts. To assess head motion in each subject, we calculated the root mean square (RMS) for each individual across the entire fMRI session as well as during speech production and resting, separately. Two-sample t-tests between the subject groups showed no significant differences in head motion at p ≥ 0.43 as well as no significant differences between the motion during task production and resting at p ≥ 0.94. Therefore, no data were excluded from the study due to the motion artifacts. Following the standard pre-processing and spatial smoothing of EPI data with a 4-mm Gaussian filter, a single regressor for each task was convolved with a canonical hemodynamic response function and entered into a multiple regression model to predict the observed BOLD response. To correct for possible residual head motions, six motion parameter estimates were included as covariates of no interest, and quadratic polynomials in time were used to model baseline drifts for each imaging run. The resultant images were spatially normalized to the AFNI standard Talairach-Tournoux space using the TT_N27 template and submitted to a two-way analysis of variance (ANOVA) with subject as a random factor and the group and task as fixed factors. Statistical analyses were performed to identify (1) shared abnormalities in SD and SD/DTv patients vs. controls, and (2) distinct abnormalities between SD and SD/DTv patient groups at family-wise (FWE) corrected p ≤ 0.05.
The VBM8 toolbox in SPM8 software running on MATLAB version 8.4 (Mathworks, MA) was used to identify gray matter volumetric abnormalities between the groups. Whole-brain gray matter segmentation was performed using the unified segmentation approach, which was further refined by applying adaptive a posteriori estimations and a hidden Markov Random Field model. Gray matter probability maps were non-linearly registered to the SPM standard Montreal Neurological Institute (MNI) space using the diffeomorphic nonlinear registration tool (DARTEL) to improve inter-subject registration through spatial normalization. To assess voxelwise statistical differences between all patients and controls, as well as between SD and SD/DTv patients directly, we performed two independent t-tests, including age and gender as covariates, at FWE-corrected p ≤ 0.05.
Diffusion-weighted data were pre-processed with FSL FMRIB Diffusion Toolbox (FDT), which corrected eddy current distortion and subject movements via an affine registration to b0 reference. Fractional anisotropy (FA) values were calculated using nonlinear fitting and submitted to a Tract-Based Spatial Statistics (TBSS) pipeline to perform statistical comparisons of group FA values in patients and controls. Individual FA maps were registered to the standard MNI template using b-spline nonlinear registration, and an alignment-invariant tract representation known as the mean FA skeleton (threshold 0.2) was generated. Each subject’s FA map was projected onto the mean FA skeleton, resulting in a 4D skeletonised FA volume. Inferential statistics was performed using t-tests between the examined groups at FWE-corrected p ≤ 0.05.
In order to assess the relationships between abnormal gray matter structure and function in SD and SD/DTv patients, we performed a conjunction analysis using the significant clusters identified in the VBM and fMRI datasets at a group level. Similar to the procedures described earlier (Casanova et al. 2007; Simonyan and Ludlow 2012; Termsarasab et al. 2016), each patient’s VBM dataset (i.e., a smoothed modulated for the non-linear components DARTEL warped segmented gray matter image) was transformed from the MNI standard space into the Talairach-Tournoux standard space using @auto_tlrc program of AFNI software to match the space transformation of the fMRI dataset (i.e., beta coefficients of functional activation during symptomatic sentence production). To create a single 3D + subjects volume for each of the VBM and fMRI datasets, the respective images were concatenated across all subjects in each group. The resultant single volumes of VBM datasets were resampled to match the same grid spacing and orientation of the fMRI dataset. To identify the relationships between VBM and fMRI abnormalities, Pearson’s correlation coefficients were computed between the corresponding voxels of VBM and fMRI datasets in each patient group using AFNI’s 3dTcorrelate program. The resultant maps were thresholded at an FWE-corrected p ≤ 0.05.
Clinical characteristics of SD and SD/DTv were evaluated using the patients’ speech recorded during the fMRI session. These recordings were anonymized, randomized, and blindly rated by an experienced speech-language pathologist. SD symptoms were assessed by counting the number of SD-characteristic voice breaks in each sentence; DTv symptoms were evaluated using a visual analog scale of severity that used three gradation indicators along a 100-mm line (normal, modulates voice, offsets voice), with distance in mm used to describe the degree of deviancy from normal. Information on the disorder onset and duration was obtained during history and physical examination in each patient. Relationships between SD and SD/DTv symptom severity, disorder onset and duration were assessed in each patient group separately using Pearson’s correlation coefficients at p ≤ 0.05.
Results
Shared brain abnormalities in SD and SD/DTv
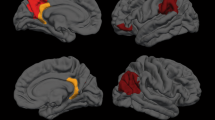
During symptomatic speech production, both SD and SD/DTv groups, compared to healthy controls, showed significantly increased cortical activation in the bilateral primary sensorimotor cortex, left premotor cortex, inferior frontal (IFG) and superior temporal (STG) gyri, right insula, parietal operculum and supplementary motor area (SMA), as well as subcortically in the left putamen and bilateral ventral thalamus (Fig. 1a-I, Table 2). Conversely, deficient activation in all patients compared to healthy controls was found in the right inferior parietal cortex (IPC) and middle frontal gyrus (MFG) (Fig. 1a-I, Table 2). Structurally, SD and SD/DTv groups, compared to controls, showed gray matter volumetric increases in the left IFG, bilateral putamen and left pallidum (Fig. 1a-II, Table 2), as well as increased fractional anisotropy in the white matter underlying the left IFG (Fig. 1a-III, Table 2).
a Common functional and structural brain differences in SD and SD/DTv patients compared to healthy controls. Regions of abnormal functional activation during symptomatic speech production (I), gray matter volume (II) and white matter integrity (III) in SD and SD/DTv patients compared with controls are shown on a series of axial, coronal and sagittal slices in the AFNI standard Talairach-Tournoux space. b Distinct alterations in brain function (I) and white matter integrity (II) between SD and SD/DTv patients are shown on a series of axial slices in the standard space. The color bars represent the t-score. PT – patients; HV – healthy volunteers; SD – spasmodic dysphonia; SD/DTv – spasmodic dysphonia with dystonic tremor of voice
Distinct brain abnormalities between SD and SD/DTv
Direct comparisons between the two patient groups showed that SD/DTv had additional functional alterations in the right MFG and right cerebellum (lobule VIIa) and structural white matter abnormalities in the right posterior limb of the internal capsule (Fig. 1b-I,II; Table 2). The only SD-specific difference was found in the white matter integrity of the right genu of the internal capsule, which was in line with the previous finding in SD patients (Simonyan et al. 2008) (Fig. 1b-II, Table 2). No statistically significant differences in gray matter volume were found between SD and SD/DTv patients.
Structure-function interactions in SD and SD/DTv
Both SD and SD/DTV patients groups showed an overlap between the significant clusters of increased activation and increased gray matter volume in the bilateral putamen and left IFG (Fig. 2a-I, II). In the SD group, volumetric changes were correlated positively with the activation in the right putamen (r = 0.64, p = 0.003) and negatively with the activation in the left IFG (r = −0.45, p = 0.047) (Fig. 2b-I). In addition, activation of the left putamen was positively correlated with the age of SD onset (r = 0.61, p = 0.004) (Fig. 2c-I). There were no significant correlations between structural/functional alterations and SD severity or duration (all r < 0.07, p > 0.77).
Correlations between abnormal function, structure and clinical characteristics of SD (a) and SD/DTv (b). Panels I-a and II-a depict the overlap between significant clusters of functional and gray matter volumetric abnormalities identified in the comparison of SD and SD/DTv with healthy controls on a series of axial and sagittal slices in the AFNI standard Talairach-Tournoux space. Panels II-b-c and II-b-d show correlations of the disorder onset and severity with the significant cluster overlap
In the SD/DTv group, gray matter volume showed significant negative relationship with abnormal activation in the left putamen (r = −0.59, p = 0.006) and significant positive relationship with abnormal activation in the left IFG (r = 0.56, p = 0.01) (Fig. 2b-II). Furthermore, gray matter volumetric abnormalities in the right putamen were negatively correlated with the age of SD/DTv onset (r = −0.48, p = 0.032) (Fig. 2c-II); abnormal volume of the left IFG showed a negative relationship with DTv (r = −0.47, p = 0.037) (Fig. 2d-II). Again, no significant relationships were observed between structure/function alterations, SD/DTv severity (i.e., voice breaks and tremor) and its duration (all r < −0.20, p > 0.39).
Discussion
Our study demonstrated that the majority of functional and structural alterations in SD and SD/DTv compared to healthy controls are shared between these two patient groups (Fig. 3). However, each of SD and SD/DTv groups also exhibited a set of focal disorder-specific alterations. These findings suggest that dystonia and dystonic tremor, at least in the case of SD and SD/DTv, are heterogeneous disorders at different ends of the same pathophysiological spectrum, with each disorder carrying a characteristic neural signature, which may potentially help development of differential markers for these two conditions.
A schematic diagram summarizing the main findings of functional and structural abnormalities in patients with SD and SD/DTv. The middle panel shows the alterations of brain function and structure that are common between the two disorders; the right and left panels show abnormalities that are characteristic to dystonia and dystonic tremor, respectively. The arrow signifies the presence of shared abnormalities by SD and SD/DTv as well as the presence of disorder-specific alterations. L – left; R – right; F – functional abnormality; G – abnormal gray matter volume; W – abnormal white matter integration
Common to both SD and SD/DTv, neural abnormalities were observed within the basal ganglia and thalamus as well as in the cortical regions responsible for sensorimotor processing, planning and execution of motor commands. Specific to SD and SD/DTv symptomatology, these brain regions are also known to control different stages of speech production, which is affected in patients with both disorders. Alterations in the majority of these brain regions have been previously reported across different forms of dystonia and are thought to contribute to dystonia pathophysiology (Neychev et al. 2011; Ramdhani and Simonyan 2013). Therefore, our findings point to likely shared pathophysiological mechanisms related to impaired speech control in SD and SD/DTv.
Among these, the IFG and putamen were the only regions that showed strong associations between abnormally increased activity, gray matter volume, and underlying white matter alterations. The putamen has been historically known to play an important role in the pathophysiology of dystonia (Berardelli et al. 1985; Marsden 1984), and its functional, structural and dopaminergic abnormalities have been previously reported in SD (Haslinger et al. 2005; Simonyan et al. 2013; Simonyan and Ludlow 2010, 2012; Simonyan et al. 2008). The putamen receives heavy projections from the laryngeal motor cortex (Simonyan et al. 2009), and its lesioning has been associated with SD symptom manifestation (Lee et al. 1996). In SD patients, we observed a positive structure-function relationship in the right putamen, whereas the later onset of isolated SD was associated with the greater extent of abnormal increases of left putaminal activation. In SD/DTv patients, these relationships were somewhat reversed, with the left putamen showing a positive correlation between abnormal structure and function and younger SD/DTv patients exhibiting greater gray matter volume in the right putamen. Such a left/right dissociation of putaminal involvement in SD and SD/DTv may be due to the different roles this structure plays in the control of speech production. The left putamen was shown to have greater activity during initiation and execution of overt vs. silent speech (Rosen et al. 2000) with increased demands on the articulatory system (Klein et al. 1994) and slower production rates (Wildgruber et al. 2001). On the other hand, the right putamen was found to suppress right frontal activation to reduce interference during speech production, leading to left lateralized activation in a number of brain regions, including the IFG (Crosson et al. 2003). These findings, although regionally similar but physiologically disparate, indicate the critical involvement of the putamen in the development of both SD and SD/DTv symptoms, while also suggesting a presence of somewhat different mechanisms underlying the progression of these two disorders. Although neuroimaging modalities generally lack the ability to clearly distinguish between primary and compensatory brain alterations, our findings of associations between the disorder onset and putaminal abnormalities speak to the importance of this structure from the early stages of symptom manifestation.
Another structure that showed correlations between functional and structural abnormalities in both SD and SD/DTv patients was the left IFG. This region is known to be involved in an array of preparatory processes to speech motor execution, including sound categorization, discrimination and phonological decisions on sound content of words (Burton et al. 2005; Husain et al. 2006), processing of syllabic order (Moser et al. 2009), semantic retrieval (Rodd et al. 2005; Noppeney and Price 2002), covert articulatory planning and translating speech into articulatory code (Paulesu et al. 1997; Sidtis 2012). In our patient cohorts, coupling between functional and structural abnormalities in the IFG may reflect symptomatology of SD and SD/DTv, which is associated with continuous struggles with the choice of words in order to avoid or minimize SD-characteristic voice breaks and to accurately map articulatory planning to speech motor output due to both voice breaks and tremor. Furthermore, the observed association between the left IFG gray matter volumetric changes and DTv severity suggests that evaluation of this structure may be considered for the assessment of dystonic tremor severity and possible treatment responses. Taken together, our data show that SD and SD/DTv may share common pathophysiological mechanisms related to impaired speech control.
However, direct comparisons between the SD and SD/DTv groups revealed specific, disorder-characteristic alterations present in each group (Fig. 3). While both disorders appeared to follow a similar pathophysiological trajectory, brain abnormalities in SD/DTv enhanced and complimented those present in SD alone. Compared to SD patients, SD/DTv patients exhibited greater extent of functional abnormalities in the right MFG and cerebellar lobule VII as well as white matter changes in the right posterior limb of the internal capsule at the junction of the corticospinal/corticopontine tracts and superior thalamic radiation. The right MFG is known to be involved in processing and control of such executive functions as searching the semantic network and increased word retrieval effort (Sachs et al. 2011; Basho et al. 2007). Structurally, the MFG establishes a direct link with the cerebellum via the thalamus (Middleton and Strick 1997), and its activity is accompanied by cerebellar activation, including of the lobule VII (Krienen and Buckner 2009; O’Reilly et al. 2010). It is therefore not surprising that SD/DTv patients had distinct abnormalities in both MFG and cerebellar lobule VII. Instead, this finding suggests that patients with DTv component had abnormality along the entire cerebello-thalamo-prefrontal executive pathway in addition to widespread abnormalities within the sensorimotor system.
The presence of cerebellar changes in SD/DTv patients may also raise the possibility of a similarity with essential tremor (ET), where this structure is thought to be dysfunctional (Nicoletti et al. 2015; Pinto et al. 2003; Lin et al. 2014; Louis et al. 2014). Current hypotheses of tremor pathogenesis include histopathological changes in the cerebellum, aberrant oscillations within the cerebello-thalamo-cortical network, and/or impaired GABAergic discharge. Some have suggested that DT may be distinct to ET, based on phenotypic and neurophysiological differences between the two disorders (Defazio et al. 2015), while others proposed that these disorders may be more intermixed (Elble 2013; Louis 2014; Helmich et al. 2013). As our current findings in SD and SD/DTv patients demonstrate that dystonia and dystonic tremor may be more heterogeneous than separate disorders (Fig. 3), DTv is likely positioned in-between ET and dystonia based on its clinical symptomatology resembling both disorders and brain abnormalities common to both ET and dystonia. Yet, distinct brain functional and structural alterations that were observed in patients with DTv may be important for future development of neuroimaging biomarkers of this disorder.
References
Basho, S., Palmer, E. D., Rubio, M. A., Wulfeck, B., & Muller, R. A. (2007). Effects of generation mode in fMRI adaptations of semantic fluency: paced production and overt speech. Neuropsychologia, 45(8), 1697–1706. doi:10.1016/j.neuropsychologia.2007.01.007.
Berardelli, A., Rothwell, J. C., Day, B. L., & Marsden, C. D. (1985). Pathophysiology of blepharospasm and oromandibular dystonia. Brain, 108 (Pt 3), 593–608.
Burton, M. W., Locasto, P. C., Krebs-Noble, D., & Gullapalli, R. P. (2005). A systematic investigation of the functional neuroanatomy of auditory and visual phonological processing. NeuroImage, 26(3), 647–661. doi:10.1016/j.neuroimage.2005.02.024.
Casanova, R., Srikanth, R., Baer, A., Laurienti, P. J., Burdette, J. H., Hayasaka, S., et al. (2007). Biological parametric mapping: a statistical toolbox for multimodality brain image analysis. NeuroImage, 34(1), 137–143. doi:10.1016/j.neuroimage.2006.09.011.
Cerasa, A., Nistico, R., Salsone, M., Bono, F., Salvino, D., Morelli, M., et al. (2014). Neuroanatomical correlates of dystonic tremor: a cross-sectional study. Parkinsonism & Related Disorders, 20(3), 314–317. doi:10.1016/j.parkreldis.2013.12.007.
Crosson, B., Benefield, H., Cato, M. A., Sadek, J. R., Moore, A. B., Wierenga, C. E., et al. (2003). Left and right basal ganglia and frontal activity during language generation: contributions to lexical, semantic, and phonological processes. Journal of the International Neuropsychological Society, 9(7), 1061–1077. doi:10.1017/S135561770397010X.
Defazio, G., Conte, A., Gigante, A. F., Fabbrini, G., & Berardelli, A. (2015). Is tremor in dystonia a phenotypic feature of dystonia? American academy of neurology(84), 1053–1059.
Deuschl, G., Bain, P., & Brin, M. (1998). Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Movement Disorders, 13 Suppl 3, 2–23.
Elble, R. (2013). Defining Dystonic Tremor. Current Neuropharmacology, 11, 48–52.
Gurey, L. E., Sinclair, C. F., & Blitzer, A. (2013). A new paradigm for the management of essential vocal tremor with botulinum toxin. Laryngoscope, 123(10), 2497–2501. doi:10.1002/lary.24073.
Haslinger, B., Erhard, P., Dresel, C., Castrop, F., Roettinger, M., & Ceballos-Baumann, A. O. (2005). "Silent event-related" fMRI reveals reduced sensorimotor activation in laryngeal dystonia. Neurology, 65(10), 1562–1569. doi:10.1212/01.wnl.0000184478.59063.db.
Helmich, R. C., Toni, I., Deuschl, G., & Bloem, B. R. (2013). The pathophysiology of essential tremor and parkinson’s tremor. Current Neurology and Neuroscience Reports, 13(9), 378. doi:10.1007/s11910-013-0378-8.
Husain, F. T., Fromm, S. J., Pursley, R. H., Hosey, L. A., Braun, A. R., & Horwitz, B. (2006). Neural bases of categorization of simple speech and nonspeech sounds. Human Brain Mapping, 27(8), 636–651. doi:10.1002/hbm.20207.
Jankovic, J., Leder, S., Warner, D., & Schwartz, K. (1991). Cervical dystonia: clinical findings and associated movement disorders. Neurology, 41(7), 1088–1091.
Klein, D., Zatorre, R. J., Milner, B., Meyer, E., & Evans, A. C. (1994). Left putaminal activation when speaking a second language: evidence from PET. Neuroreport, 5(17), 2295–2297.
Krienen, F. M., & Buckner, R. L. (2009). Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cerebral Cortex, 19(10), 2485–2497. doi:10.1093/cercor/bhp135.
Lee, M. S., Lee, S. B., & Kim, W. C. (1996). Spasmodic dysphonia associated with a left ventrolateral putaminal lesion. Neurology, 47(3), 827–828.
Lin, C. Y., Louis, E. D., Faust, P. L., Koeppen, A. H., Vonsattel, J. P., & Kuo, S. H. (2014). Abnormal climbing fibre-purkinje cell synaptic connections in the essential tremor cerebellum. Brain, 137(Pt 12), 3149–3159. doi:10.1093/brain/awu281.
Louis, E. D. (2014). ‘Essential tremor’ or ‘the essential tremors’: is this one disease or a family of diseases? Neuroepidemiology, 42(2), 81–89. doi:10.1159/000356351.
Louis, E. D., Lee, M., Babij, R., Ma, K., Cortes, E., Vonsattel, J. P., et al. (2014). Reduced purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain, 137(Pt 12), 3142–3148. doi:10.1093/brain/awu314.
Marsden, C. D. (1984). Motor disorders in basal ganglia disease. Human Neurobiology, 2(4), 245–250.
Middleton, F. A., & Strick, P. L. (1997). Cerebellar output channels. International Review of Neurobiology, 41, 61–82.
Moser, D., Baker, J. M., Sanchez, C. E., Rorden, C., & Fridriksson, J. (2009). Temporal order processing of syllables in the left parietal lobe. The Journal of Neuroscience, 29(40), 12568–12573. doi:10.1523/JNEUROSCI.5934-08.2009.
Munchau, A., Schrag, A., Chuang, C., MacKinnon, C. D., Bhatia, K. P., Quinn, N. P., et al. (2001). Arm tremor in cervical dystonia differs from essential tremor and can be classified by onset age and spread of symptoms. Brain, 124(Pt 9), 1765–1776.
Neychev, V. K., Gross, R. E., Lehericy, S., Hess, E. J., & Jinnah, H. A. (2011). The functional neuroanatomy of dystonia. Neurobiology of Disease, 42(2), 185–201. doi:10.1016/j.nbd.2011.01.026.
Nicoletti, V., Cecchi, P., Frosini, D., Pesaresi, I., Fabbri, S., Diciotti, S., et al. (2015). Morphometric and functional MRI changes in essential tremor with and without resting tremor. Journal of Neurology, 262(3), 719–728. doi:10.1007/s00415-014-7626-y.
Nistico, R., Pirritano, D., Salsone, M., Valentino, P., Novellino, F., Condino, F., et al. (2012). Blink reflex recovery cycle in patients with dystonic tremor: a cross-sectional study. Neurology, 78(17), 1363–1365. doi:10.1212/WNL.0b013e3182518316.
Noppeney, U., & Price, C. J. (2002). Retrieval of visual, auditory, and abstract semantics. NeuroImage, 15(4), 917–926. doi:10.1006/nimg.2001.1016.
O’Reilly, J. X., Beckmann, C. F., Tomassini, V., Ramnani, N., & Johansen-Berg, H. (2010). Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cerebral Cortex, 20(4), 953–965. doi:10.1093/cercor/bhp157.
Paulesu, E., Goldacre, B., Scifo, P., Cappa, S. F., Gilardi, M. C., Castiglioni, I., et al. (1997). Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. Neuroreport, 8(8), 2011–2017.
Pinto, A. D., Lang, A. E., & Chen, R. (2003). The cerebellothalamocortical pathway in essential tremor. Neurology, 60(12), 1985–1987.
Ramdhani, R. A., & Simonyan, K. (2013). Primary dystonia: conceptualizing the disorder through a structural brain imaging lens. Tremor Other Hyperkinet Mov (N Y), 3.
Rodd, J. M., Davis, M. H., & Johnsrude, I. S. (2005). The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cerebral Cortex, 15(8), 1261–1269. doi:10.1093/cercor/bhi009.
Rosen, H. J., Ojemann, J. G., Ollinger, J. M., & Petersen, S. E. (2000). Comparison of brain activation during word retrieval done silently and aloud using fMRI. Brain and Cognition, 42(2), 201–217. doi:10.1006/brcg.1999.1100.
Sachs, O., Weis, S., Zellagui, N., Sass, K., Huber, W., Zvyagintsev, M., et al. (2011). How different types of conceptual relations modulate brain activation during semantic priming. Journal of Cognitive Neuroscience, 23(5), 1263–1273. doi:10.1162/jocn.2010.21483.
Shaikh, A. G., Zee, D. S., & Jinnah, H. A. (2015). Oscillatory head movements in cervical dystonia: dystonia, tremor, or both? Movement Disorders, 30(6), 834–842. doi:10.1002/mds.26231.
Sidtis, J. J. (2012). Performance-based connectivity analysis: a path to convergence with clinical studies. NeuroImage, 59(3), 2316–2321. doi:10.1016/j.neuroimage.2011.09.037.
Simonyan, K., & Ludlow, C. L. (2010). Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an fMRI study. Cerebral Cortex, 20(11), 2749–2759. doi:10.1093/cercor/bhq023.
Simonyan, K., & Ludlow, C. L. (2012). Abnormal structure-function relationship in spasmodic dysphonia. Cerebral Cortex, 22(2), 417–25. doi: 10.1093/cercor/bhr120.
Simonyan, K., Tovar-Moll, F., Ostuni, J., Hallett, M., Kalasinsky, V. F., Lewin-Smith, M. R., et al. (2008). Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain, 131(Pt 2), 447–459. doi:10.1093/brain/awm303.
Simonyan, K., Ostuni, J., Ludlow, C. L., & Horwitz, B. (2009). Functional but not structural networks of the human laryngeal motor cortex show left hemispheric lateralization during syllable but not breathing production. The Journal of Neuroscience, 29(47), 14912–14923. doi:10.1523/JNEUROSCI.4897-09.2009.
Simonyan, K., Berman, B. D., Herscovitch, P., & Hallett, M. (2013). Abnormal striatal dopaminergic neurotransmission during rest and task production in spasmodic dysphonia. The Journal of Neuroscience, 33(37), 14705–14714. doi:10.1523/JNEUROSCI.0407-13.
Termsarasab, P., Ramdhani, R. A., Battistella, G., Rubien-Thomas, E., Choy, M., Farwell, I. M., et al. (2016). Neural correlates of abnormal sensory discrimination in laryngeal dystonia. Neurologic Clinics, 10, 18–26. doi:10.1016/j.nicl.2015.10.016.
Tinazzi, M., Fasano, A., Di Matteo, A., Conte, A., Bove, F., Bovi, T., et al. (2013). Temporal discrimination in patients with dystonia and tremor and patients with essential tremor. Neurology, 80(1), 76–84. doi:10.1212/WNL.0b013e31827b1a54.
White, L. J., Klein, A. M., Hapner, E. R., Delgaudio, J. M., Hanfelt, J. J., Jinnah, H. A., et al. (2011). Coprevalence of tremor with spasmodic dysphonia: a case-control study. Laryngoscope, 121(8), 1752–1755. doi:10.1002/lary.21872.
Wildgruber, D., Ackermann, H., & Grodd, W. (2001). Differential contributions of motor cortex, basal ganglia, and cerebellum to speech motor control: effects of syllable repetition rate evaluated by fMRI. NeuroImage, 13(1), 101–109. doi:10.1006/nimg.2000.0672.
Acknowledgments
We thank Amanda Pechman, BA, Ian M. Farwell, MSG, and Heather Alexander, BA, for help with patient recruitment and data acquisition.
Author contributions
KS designed the study; DNK, GB, ERT, MC collected the data; DNK, GB, VK, ERT, MC, AR and KS analyzed the data; DNK wrote the first draft of the manuscript; KS, GB, VK, ERT, MC and AR revised and critiqued the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Diana N. Kirke declares that she has no conflict of interest. Diana N. Kirke was supported by a research fellowship grant from the Foundation for Surgery Reg Worcester Research Fellowship Scholarship, Royal Australasian College of Surgeons.
Giovanni Battistella declares that he has no conflict of interest.
Veena Kumar declares that she has no conflict of interest.
Estee Rubien-Thomas declares that she has no conflict of interest.
Melissa Choy declares that she has no conflict of interest.
Anna Rumbach declares that she has no conflict of interest.
Kristina Simonyan declares that she has no conflict of interest. Kristina Simonyan received grants from National Institute on Deafness and Other Communication Disorders, National Institutes of Health (R01DC011805, R01DC012434, R01DC007658), National Institute of Neurological Disorders and Stroke, National Institutes of Health (R01NS088160). Kristina Simonyan serves on the Medical and Scientific Advisory Council of the Dystonia Medical Research Foundation.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Funding
This study was funded by the National Institute on Deafness and Other Communication Disorders, National Institutes of Health (grant number R01DC012545) to KS. DNK was supported by a research fellowship grant from the Foundation for Surgery Reg Worcester Research Fellowship Scholarship, Royal Australasian College of Surgeons.
Rights and permissions
About this article
Cite this article
Kirke, D.N., Battistella, G., Kumar, V. et al. Neural correlates of dystonic tremor: a multimodal study of voice tremor in spasmodic dysphonia. Brain Imaging and Behavior 11, 166–175 (2017). https://doi.org/10.1007/s11682-016-9513-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-016-9513-x